Abstract
Objective
The present study aimed to evaluate the association between serum ferritin and vitamin D levels in fibroid uterus cases presenting with anemia.
Methods
Sixty premenopausal women with uterine fibroids (30 associated with anemia and 30 without anemia) were enrolled as cases and control. All participants were evaluated on the basis of a questionnaire, which included queries related to obstetric, medical, and sociodemographic history. Peripheral blood smear, complete blood count (CBC), hemoglobin (Hb), and serum ferritin concentration were measured by a fully automated analyzer, and 25(OH) vitamin D level was measured by enzyme-linked immunosorbent assay (ELISA).
Results
There was a significant difference in ferritin levels between cases and control (p<0.001). The exposure to sunlight was moderate (one-hour exposure) in all subjects, eliminating the confounding effect of sunlight exposure influencing vitamin D levels. The median vitamin D level in cases was 5.0 ng/ml [interquartile range (IQR): 4.8], and that in control was 18.4 ng/ml (IQR: 7.9; p<0.001). A strong positive correlation of (r)=0.616 (p<0.001) was found between serum ferritin and vitamin D levels.
Conclusion
Fibroid uterus cases with anemia are more prone to vitamin D deficiency as compared to cases without anemia. Vitamin D estimation in fibroid uterus cases presenting with anemia would be useful for better patient management.
Keywords: fibroid uterus, anaemia, ferritin, vitamin d deficiency
Introduction
Uterine fibroid (leiomyoma) is the most common benign tumor in the uterus, and there is a growing prevalence of fibroid uterus in India [1], which has been associated with major health and economic burden in women of reproductive age group. Fibroid uterus occurs in 20-40% of women of reproductive age, and one-third of gynecology admissions happen to be fibroids with menorrhagia, anemia, and lump in the abdomen with pain [2] severe enough to significantly impact women’s quality of life. Submucosal fibroids cause severe bleeding per vagina followed by intramural type resulting in anemia [3]. Studies have highlighted vitamin D deficiency as a major risk factor for the development of uterine fibroids [4,5]. Though factors like race, ethnicity, tradition, culture, seasonal variation, exposure to sunlight, and nutritional status play a major role in regulating vitamin D levels, the cause of hypovitaminosis D in fibroids is still a topic of active research. Recently, the co-existence of anemia and hypovitaminosis D has emerged as a new causal association [6] in fibroid uterus cases, but many studies have also reported vitamin D deficiency without anemia [7,8]. Vitamin D influences iron metabolism and erythropoiesis, whereas iron is essential for vitamin D synthesis as well [9]. In terms of anemia, some studies have reported that ferritin levels are positively associated with serum vitamin D [10-12]. Though the existing scientific data indicates the role of vitamin D in fibroid uterus cases, the co-existence of anemia with vitamin D deficiency and the association between ferritin and vitamin D levels in premenopausal women with fibroid uterus are still not fully understood. The present study was conducted to estimate serum ferritin, vitamin D, and hemoglobin (Hb) levels in fibroid uterus cases presenting with anemia and to compare these values with premenopausal fibroid uterus cases presenting without anemia. Further evaluation was done to determine the association between ferritin and serum vitamin D levels in anemic and non-anemic fibroid uterus women to ascertain the correlation.
Materials and methods
A hospital-based case-control study was conducted in the Department of Biochemistry, All India Institute of Medical Sciences, Bhubaneswar after obtaining approval from the Institute Ethical Committee. Sixty clinically diagnosed premenopausal women with uterine fibroids (30 cases with anemia and 30 cases without anemia) within the age group of 25-45 years, irrespective of their parity, who were attending the outpatient section of the Obstetrics and Gynaecology Department, and who satisfied the inclusion criteria, were enrolled randomly in this study. Post-menopausal women, women with inflammatory disorders, acute infections, and chronic liver and kidney diseases, and those with autoimmune conditions were excluded from the study.
All participants were evaluated on the basis of a questionnaire prepared pertaining to the study, which included queries related to parity, age at menarche, last menstrual period, breastfeeding, type of delivery, history of intake of oral contraceptive pills (OCPs), hormone replacement therapy, miscarriage, duration of exposure to sunlight (<1 hour/day, one hour/day, >1 hour/day), race, ethnicity, history of chronic diseases like diabetes, hypertension, renal disorders, inflammatory conditions, autoimmune disorders, history of recent blood transfusion, history of intake of multivitamin/iron supplements, and the type of nutrition. The blood pressure of the participants was recorded, and height and weight were measured to calculate their body mass index (BMI).
Biochemical analysis
After obtaining informed written consent, a 5-ml blood sample was collected from all participants. The serum was stored at -20 °C until it was used for biochemical evaluation. Peripheral blood smear, complete blood count (CBC), and Hb concentration were measured by a fully automated analyzer (AU platform) using system-compatible kits. The serum ferritin levels were estimated by three-site sandwich immunoassay using direct chemiluminometric technology in chemiluminescence immunoassay analyzer (ADVIA Centaur®, Siemens Healthineers, Erlangen, Germany). Serum vitamin D levels were estimated by enzyme-linked immunosorbent assay (ELISA) method (sandwich ELISA) using a commercial 25(OH) vitamin D ELISA Kit (Elabscience, Houston, TX) as per the manufacturer's recommendations.
Based on the data obtained from the questionnaire as well as the biochemical analysis, the patients were divided into two groups: group A: fibroid with anemia (treated as cases); group B: fibroid without anemia (treated as the control group). Subjects in the anemic group (group A) were further classified into iron deficiency anemia (Hb: <11g/dl, ferritin: <12ng/ml) and iron deficiency state (Hb: >11g/dl, ferritin: <12ng/ml). Subjects in the non-anemic group (group B) were those with Hb of >11 g/dl and ferritin of >12 ng/ml. Vitamin D status analyzed by estimating 25(OH) vitamin D levels (circulating active metabolite) in the serum was categorized as vitamin D deficiency, with 25(OH)D levels of <20ng/ml; vitamin D insufficiency, with 25(OH)D levels of 20-30 ng/ml; and vitamin D sufficiency, with 25(OH)D levels of >30 ng/ml.
Statistical analysis
The data were analyzed using the SPSS Statistics software (IBM, Armonk, NY). The Mann-Whitney U test was used for comparing the two groups. We investigated the Pearson’s correlations between the hematologic parameters, i.e., ferritin and 25(OH)D levels. The odds ratio was calculated to determine the strength of association between the variables and uterine fibroids. To evaluate the odds ratio between iron deficiency anemia, iron deficiency state, and vitamin D deficiency/insufficiency subjects, the iron deficiency anemia and iron deficiency state groups, as well as the vitamin D deficiency and vitamin D insufficiency groups, were combined to form two separate groups for further analysis. Multivariate logistic regression analysis was also performed.
Results
The clinical characteristics of the study groups were evaluated (Table 1). The data was found to be non-parametric in the Kolmogorov-Smirnov test. The median ages of fibroid subjects with anemia and those without anemia were more or less the same. The median BMI of the anemic group was 22.7 kg/m2 [interquartile range (IQR): 3.6], and that of the control group was 20.3 kg/m2 (IQR-4.7, p=0.008). There was a significant difference in Hb levels between the two groups. The mean corpuscular volume (MCV), mean corpuscular Hb (MCH), and mean corpuscular Hb concentration (MCHC) also showed a significant difference between the groups (p<0.001 for each), so did the packed cell volume (PCV) (p<0.001) and red cell distribution width (p=0.037). There was no significant difference in RBC counts between cases and control (p=0.717). The median ferritin level in cases was 8.0 ng/ml (IQR: 4.1), and that in control was 47.1 ng/ml (IQR: 59), and the difference was found to be statistically significant (p<0.001). The median vitamin-D level in fibroid cases with anemia was 5.0 ng/ml (IQR: 4.8), and that in cases without anemia was 18.4 ng/ml (IQR-7.9), which also indicated a significant difference (p<0.001).
Table 1. Clinical characteristics of the study population.
Data was found to be non-parametric in the Kolmogorov-Smirnov test. P-values were calculated using the Mann-Whitney U test
*Significant p-value (<0.05)
IQR: interquartile range; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; RBC: red blood cells; WBC: white blood cells; PCV: packed cell volume; RDW: red cell distribution width
| Parameters | Fibroid subjects without anemia, median (IQR) | Fibroid subjects with anemia, median (IQR) | P-value |
| Age, years | 42.5 (10.5) | 44.0 (7.5) | 0.736 |
| BMI, kg/m2 | 20.3 (4.7) | 22.7 (3.6) | 0.008* |
| SBP, mmHg | 110.5 (10) | 113.0 (10) | 0.804 |
| DBP, mmHg | 76.0 (10) | 73.0 (10) | 1.000 |
| Hemoglobin, g/dl | 12.5 (0.8) | 9.8 (2.0) | <0.001* |
| MCV, fl/cell | 86.5 (6.1) | 75.0 (10) | <0.001* |
| MCH, pg/cell | 28.2 (1.9) | 22.5 (5.2) | <0.001* |
| MCHC, g/dl | 31.7 (1.6) | 30.2 (1.5) | <0.001* |
| RBC, million/mm3 | 4.5 (0.9) | 4.4 (1.1) | 0.717 |
| WBC, thousand cells/mm3 | 7.1 (2.7) | 7.6 (2.3) | 0.762 |
| Platelets, lakhs/mm3 | 2.8 (1.0) | 3.3 (1.4) | 0.070 |
| PCV, % | 38.1 (7.1) | 32.3 (6.3) | <0.001* |
| RDW, % | 15.2 (5.8) | 17.2 (3.0) | 0.037* |
| Urea, mg/dl | 16.5 (5.0) | 18.0 (4.0) | 0.427 |
| Creatinine, mg/dl | 0.7 (0.2) | 0.7 (0.2) | 0.694 |
| Ferritin, ng/ml | 47.1 (59.0) | 8.0 (4.1) | <0.001* |
| Vitamin D (ng/ml) | 18.4 (7.9) | 5.0 (4.8) | <0.001* |
Table 2 compares the baseline characteristics of the two groups. The comparison was done using the chi-square test. The number of fibroids or type of fibroids was more or less the same in the two groups. Obstetric parameters such as parity, history of breastfeeding, and the use of oral OCPs did not differ much between cases and controls; 70% of the cases had a microscopic hypochromic appearance in the peripheral smear, which was less for controls. Only nine (30%) had a normocytic normochromic appearance in the anemic group, but 28 (93.3%) subjects in the control group had a normal appearance. This difference was significant (p<0.001). The exposure to sunlight was moderate (one-hour exposure) in all subjects, thereby eliminating the confounding effect of sunlight exposure influencing vitamin D levels in fibroid uterus cases.
Table 2. Baseline characteristics of the study population.
*Significant p-value (<0.05) (calculated by chi-square test)
DM: diabetes mellitus
| Parameters | Fibroid subjects without anemia, n (%) | Fibroid subjects with anemia, n (%) | P-value | |
| Associated type 2 DM | 3 (10) | 3 (10) | 1.000 | |
| Associated hypertension | 0 (0) | 3 (10) | 0.237 | |
| Associated thyroid disorder | 6 (20) | 4 (13.3) | 0.551 | |
| Number of fibroids | 1 | 23 (76.7) | 17 (56.7) | 0.104 |
| 2 | 3 (10) | 1 (0.3) | ||
| 3 | 0 (0) | 1 (0.3) | ||
| Multiple | 4 (13.3) | 11 (36.7) | ||
| Type of fibroid | Submucosal | 7 (23.3) | 7 (23.3) | 0.072 |
| Intramural | 13 (43.3) | 20 (66.7) | ||
| Subserosal | 10 (30.3) | 3 (10) | ||
| Size of fibroid | <7 cm | 17 (56.7) | 18 (60) | 0.739 |
| >7 cm | 13 (43.3) | 12 (40) | ||
| Menarche | <13 years | 6 (20) | 11 (36.7) | 0.252 |
| >13 years | 24 (80) | 19 (63.3) | ||
| Parity | Nulliparous | 8 (26.7) | 4 (13.3) | 0.290 |
| Uniparous | 5 (16.7) | 9 (30) | ||
| Multiparous | 17 (56.7) | 17 (56.7) | ||
| History of breastfeeding | Yes | 21 (70) | 26 (86.7) | 0.209 |
| No | 9 (30) | 4 (13.3) | ||
| History of miscarriage | Yes | 8 (26.7) | 6 (20) | 0.761 |
| No | 22 (73.3) | 24 (80) | ||
| History of oral contraceptive pill use | Yes | 1 (3.3) | 2 (6.7) | 1.000 |
| No | 29 (96.7) | 28 (93.3) | ||
| Peripheral smear | Microcytic hypochromic | 2 (6.7) | 21 (70) | <0.001* |
| Normocytic normochromic | 28 (93.3) | 9 (30) | ||
| Exposure to sunlight | <1 hour/day, 1 hour/day, >1 hour/day | 0 (0), 30 (100), 0 (0) | 0 (0), 30 (100), 0 (0) | 0.639 |
Table 3 compares the characteristics of the study population between the groups according to their vitamin D concentration. The Kruskal-Wallis test was used for comparison. In the anemic group, 28 subjects were found to be with vitamin D deficiency with two participants having vitamin D insufficiency. In the control group, the distribution was 19 subjects with vitamin D deficiency, nine with vitamin D insufficiency, and two with vitamin D sufficiency. There was no significance between most of the parameters according to vitamin D levels. But serum ferritin showed a significant difference (p=0.001) between the three subgroups based on vitamin D levels in the control group. Among the cases, serum ferritin showed a significant difference (p=0.007) between the vitamin D subgroups.
Table 3. Characteristics of the study population based on serum vitamin D concentrations between the groups.
*Significant p-value (<0.05) (calculated using the Kruskal-Wallis test)
SD: standard deviation; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; RBC: red blood cells; WBC: white blood cells; PCV: packed cell volume; RDW: red cell distribution width
| Parameters | Fibroid subjects without anemia, mean ± SD | Fibroid subjects with anemia, mean ± SD | |||||
| Vitamin D deficiency (<20 ng/ml) | Vitamin D insufficiency (20-30 ng/ml) | Vitamin D sufficiency (>30 ng/ml) | P-value | Vitamin D deficiency (<20 ng/ml) | Vitamin D insufficiency (20-30 ng/ml) | P-value | |
| Age, years | 41.6 ± 6.7 | 40.0 ± 8.2 | 42.5 ± 2.1 | 0.992 | 41.9 ± 6.3 | 44.0 ± 0.7 | 0.737 |
| BMI, kg/m2 | 20.1 ± 3.6 | 20.4 ± 4.8 | 22.0 ± 4.4 | 0.823 | 23.1 ± 3.7 | 23.7 ± 3.1 | 0.868 |
| SBP, mmHg | 113.0 ± 9.8 | 117.2 ± 10.3 | 116.0 ± 2.8 | 0.548 | 114.9 ± 9.1 | 115.0 ± 21.2 | 0.899 |
| DBP, mmHg | 75.7 ± 8.4 | 74.8 ± 7.0 | 67.0 ± 4.2 | 0.303 | 74.5 ± 6.7 | 80.0 ± 14.1 | 0.445 |
| Hemoglobin, g/dl | 12.6 ± 0.5 | 12.9 ± 1.0 | 12.4 ± 0.1 | 0.702 | 9.7 ± 1.5 | 9.0 ± 1.3 | 0.339 |
| MCV, fl/cell | 85.3 ± 6.1 | 88.3 ± 3.9 | 84.9 ± 1.8 | 0.221 | 74.5 ± 7.2 | 72.5 ± 13.3 | 0.739 |
| MCH, pg/cell | 27.6 ± 1.9 | 28.6 ± 1.7 | 27.7 ± 0.9 | 0.282 | 22.6 ± 3.0 | 21.3 ± 5.6 | 0.678 |
| MCHC, g/dl | 31.6 ± 1.0 | 32.2 ± 1.4 | 32.4 ± 1.5 | 0.541 | 30.1 ± 1.4 | 29.2 ± 2.4 | 0.739 |
| RBC, million/mm3 | 4.5 ± 0.5 | 4.3 ± 1.0 | 3.5 ± 0.4 | 0.131 | 4.4 ± 0.6 | 4.3 ± 0.5 | 0.934 |
| WBC, thousand cells/mm3 | 7.5 ± 1.8 | 9.2 ± 4.3 | 7.5 ± 0.6 | 0.823 | 8.3 ± 3.3 | 7.6 ± 1.6 | 0.739 |
| Platelets, lakhs/mm3 | 2.7 ± 0.7 | 3.1 ± 1.2 | 2.4 ± 0.2 | 0.503 | 3.3 ± 0.9 | 3.6 ± 0.7 | 0.533 |
| PCV, % | 38.2 ± 3.1 | 35.5 ± 7.4 | 30.1 ± 4.3 | 0.079 | 32.3 ± 4.5 | 30.6 ± 2.1 | 0.360 |
| RDW, % | 16.7 ± 3.8 | 17.6 ± 6.2 | 20.6 ± 8.4 | 0.664 | 17.8 ± 3.5 | 22.8 ± 12.2 | 1.000 |
| Urea, mg/dl | 17.3 ± 5.2 | 15.6 ± 2.7 | 20.5 ± 5.0 | 0.344 | 17.3 ± 5.1 | 20.0 ± 5.7 | 0.558 |
| Creatinine, mg/dl | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.344 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.965 |
| Ferritin, ng/ml | 40.0 ± 19.0 | 107.2 ± 25.2 | 126.3 ± 28.6 | 0.001* | 10.6 ± 8.6 | 28.5 ± 3.3 | 0.007* |
Table 4 compares the distribution of fibroid subjects with iron deficiency anemia and those with iron deficiency state based on their vitamin D levels; 28 (93.3%) subjects were found to have vitamin D deficiency, and two (6.7%) had vitamin D insufficiency in the iron deficiency anemia group. Five subjects in the study were found to have an iron deficiency state and all of them had vitamin D deficiency.
Table 4. Distribution of fibroid subjects with iron deficiency anemia and subjects with iron deficiency state according to serum vitamin D levels.
| Vitamin D levels | Fibroid subjects with iron deficiency anemia, n (%) | Fibroid subjects with iron deficiency, n (%) |
| Vitamin D deficiency | 28 (93.3) | 5 (100) |
| Vitamin D insufficiency | 2 (6.7) | 0 |
The odds ratio with 95% CI between the serum vitamin D groups (vitamin D deficiency and vitamin D insufficiency) and serum ferritin groups (low and normal ferritin levels) is shown in Table 5. The odds ratio was found to be 9.6 (95% CI: 1.1-80.9, p=0.019). This indicates that low ferritin levels are associated with vitamin D deficiency in fibroid subjects.
Table 5. Odds ratio and 95% CI for low and normal ferritin groups according to serum vitamin D levels.
CI: confidence interval
| Vitamin D levels | Ferritin of <12 ng/ml | Ferritin of >12 ng/ml | Odds ratio |
| Vitamin D deficiency | 23 | 24 | 9.6 (95% CI: 1.1–80.9, p=0.019) |
| Vitamin D insufficiency | 3 | 10 |
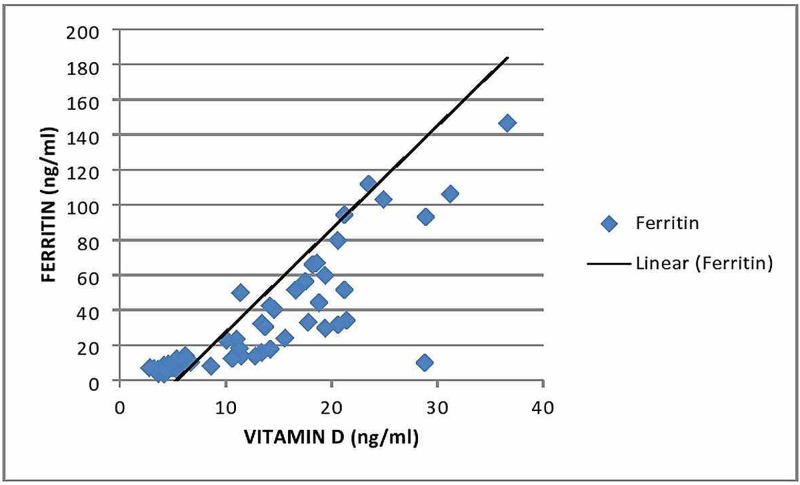
Figure 1 shows the Pearson correlation (r) between the serum ferritin levels and serum vitamin D levels among the study groups. A strong positive correlation of (r)=0.616 (p<0.001) was obtained.
Figure 1. Scatter plot of serum ferritin against serum vitamin D levels.
Pearson correlation coefficient (r)=0.616 (p<0.001)
Discussion
Uterine fibroids (submucosal, intramural, and subserosal fibroids) have different effects; submucosal and intramural types are presumed to cause heavy menstrual bleeding leading to anemia [13]. Factors like estrogen and progesterone, black ethnicity, and obesity are well-known risk factors for fibroids. Reproductive factors such as nulliparity, early menarche, and the use of oral contraceptives before the age of 16 years also contribute to its causation [14]. Hypovitaminosis D is believed to be a major risk factor for the development of uterine fibroids.
Recently, vitamin D has been found to have a protective effect, and both the initiation and growth of uterine fibroid might be inhibited with vitamin D supplementation [15]. Serum ferritin indicates the total body iron storage, and it represents iron status. Ferritin is decreased in subjects with iron deficiency anemia but may be increased in subjects with anemia of chronic disease and inflammation [16]. In the present study, most of the cases had microcytic hypochromic anemia; low levels of serum ferritin could be due to iron deficiency resulting from an inadequate intake of dietary iron or a negative iron balance. Katsumata et al. [17], in a rat model, have reported that iron deficiency decreased the levels of 1,25-dihydroxycholecalciferol and osteocalcin concentrations, suggesting that hydroxylation of vitamin D is dependent on iron, and hence iron-deficient rats had lower concentrations of the active form of vitamin D. In the present study, ferritin level and Hb level were the most significant factors affecting vitamin D levels. This result is consistent with previous studies [18,19]. Exposure to sunlight was more or less adequate in both the study groups, further suggesting the interrelationship of ferritin and vitamin D levels.
In terms of anemia, some studies have reported that vitamin D is positively associated with ferritin levels [20,21]. In the present study, vitamin D levels were reduced in patients with uterine fibroid and it was statistically significant in fibroid cases with anemia. Low serum vitamin D levels in fibroid cases could be due to the fact that vitamin D reduces the effect of transforming growth factor β3 (TGFβ3)-mediated expression of collagen type-1, and the expression and activity of metalloproteinase (MMP-2, MMP-9) that degrades the extracellular matrix [22]. Earlier studies have also reported the role of vitamin D in regulating the cellular signaling pathways associated with cell growth and proliferation, i.e., PCNA, CDK1, CDK2, CDK4, apoptosis, i.e., BCL2, BAD, caspase 3 as well as steroid hormone receptors. We found significantly low levels of vitamin D in fibroid cases with anemia. Serum ferritin levels are regulated by hepcidin, which plays a role in reducing iron absorption from the intestine. Vitamin D regulates the hepcidin-ferroportin axis in macrophages and the increase of vitamin D is known to reduce systemic hepcidin levels that ameliorate anemia [23]. Globally, various studies have been conducted to analyze the association between vitamin D and ferritin. Seong et al. [24] and Andıran et al. [25] reported that serum 25(OH)D was positively correlated with serum ferritin levels in patients in Korea and the US, respectively. However, Monlezun et al. [26] and de Castro et al. [27] reported that serum 25(OH)D was not associated with serum ferritin levels in adults from the US and Portugal, respectively. These inconsistent results may be due to the different populations, ethnic groups/countries, and the differences among the subjects of the studies (e.g., gender, absence or presence of diseases).
A strong positive correlation between serum ferritin and vitamin D levels was observed in the present study, which is in accordance with earlier findings. The odds ratios for iron deficiency and iron-deficiency anemia in subjects with vitamin D deficiency [25(OH)D of <15 ng/ml] were 1.86 (95% CI: 1.07-3.22) and 2.59 (95% CI: 1.11-6.07) in healthy Korean women as reported by Lee et al. [28]. In addition, Suh et al. [29] reported that 25(OH)D levels were lower in Korean women with iron deficiency anemia than in those without iron deficiency anemia (p<0.001). In our study, the finding of an odds ratio of 9.6 (1.1-80.9, p=0.019) with a 95% CI suggested that low ferritin levels are associated with vitamin D deficiency in fibroid uterus subjects. As reported, female gender, winter season, lack of sunlight exposure, residence in high latitudes, ethnicity (dark skin), clothing (clothing with low exposure), obesity, low socioeconomic status, and malnutrition are the factors affecting vitamin D levels. The present study found serum ferritin levels significantly affecting serum vitamin D levels in fibroid uterus cases with anemia.
Conclusions
Fibroid uterus cases with microcytic hypochromic anemia were found more prone to have vitamin D deficiency in the population of the Eastern Zone of India as compared to fibroid uterus women presenting without anemia. Serum ferritin and Hb levels were the important factors affecting serum vitamin D values, as exposure to sunlight was more or less adequate in both the study groups.
A strong positive correlation was observed between serum ferritin and vitamin D levels, suggesting that fibroid uterus cases with microcytic hypochromic anemia should also be evaluated for vitamin D deficiency, as timely corrective measures would improve the treatment outcomes in women with fibroid uterus.
Acknowledgments
We express our appreciation for the study participants, and hospital staff members of the Department of Obstetrics and Gynaecology, Department of Biochemistry AIIMS, Bhubaneswar for assisting in this project.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Institute Ethical Committee, All India Institute of Medical Sciences, Bhubaneswar issued approval IEC/AIIMS BBSR/STS/2019-20/11. The research proposal was submitted to the Institute Ethical Committee, AIIMS, Bhubaneswar for full board review. The study started only after obtaining approval from the Institute Ethical Committee, All India Institute of Medical Sciences, Bhubaneswar.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Prevalence of fibroids: a study in a semiurban area in Telangana, India. Srilatha J, Malathi V. Int J Reprod Contracept Obstet Gynecol. 2017;6:5247–5250. [Google Scholar]
- 2.Current medical treatment of uterine fibroids. Sohn GS, Cho S, Kim YM, Cho CH, Kim MR, Lee SR; Working Group of Society of Uterine Leiomyoma. Obstet Gynecol Sci. 2018;61:192–201. doi: 10.5468/ogs.2018.61.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uterine fibroids - what's new? Williams ARW. F1000Res. 2017;6:2109. doi: 10.12688/f1000research.12172.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Can vitamin D reduce the risk of uterine fibroids? Al-Hendy A, Badr M. Womens Health (Lond) 2014;10:353–358. doi: 10.2217/whe.14.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hypovitaminosis D and "small burden" uterine fibroids: opportunity for a vitamin D supplementation. Ciavattini A, Delli Carpini G, Serri M, et al. Medicine (Baltimore) 2016;95:0. doi: 10.1097/MD.0000000000005698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ability of serum ferritin to diagnose iron deficiency anemia in an elderly cohort. Babaei M, Shafiei S, Bijani A, Heidari B, Hosseyni SR, Vakili Sadeghi M. Rev Bras Hematol Hemoter. 201;39:223–228. doi: 10.1016/j.bjhh.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The prevalence of vitamin D deficiency in iron-deficient and normal children under the age of 24 months. Jin HJ, Lee JH, Kim MK. Blood Res. 2013;48:40–45. doi: 10.5045/br.2013.48.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The association between serum ferritin and 25-hydroxyvitamin D and metabolic syndrome in Korean women: the Korea National Health and Nutrition Examination Survey 2010-2012. Yoon H, Young Bae N, Young Gi M, Yeon Park B, Min Seong J. J Clin Biochem Nutr. 2017;61:60–66. doi: 10.3164/jcbn.16-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The association between iron and vitamin D status in female elite athletes. Malczewska-Lenczowska J, Sitkowski D, Surała O, Orysiak J, Szczepańska B, Witek K. Nutrients. 2018;10:167. doi: 10.3390/nu10020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The role of 25-hydroxy vitamin D deficiency in iron deficient children of North India. Sharma S, Jain R, Dabla PK. Indian J Clin Biochem. 2015;30:313–317. doi: 10.1007/s12291-014-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Association between vitamin D deficiency and anemia in inflammatory bowel disease patients with ileostomy. Fialho A, Fialho A, Kochhar G, Shen B. J Coloproctol. 2015;35:139–145. [Google Scholar]
- 12.Effects of iron on vitamin D metabolism: a systematic review. Azizi-Soleiman F, Vafa M, Abiri B, Safavi M. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5159690/ Int J Prev Med. 2016;7:126. doi: 10.4103/2008-7802.195212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Extreme anemia (hemoglobin 1.8 g/dL) secondary to abnormal uterine bleeding. Panse K, Regn R, May J. Case Rep Obstet Gynecol. 2017;2017:5179265. doi: 10.1155/2017/5179265. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Large prolapsing uterine fibroid and severe anemia in a teenager: a case report. Evans M, Prokai D, Wilson EE. http://www.jscholaronline.org/articles/JWHG/Large-Prolapsing.pdf J Womens Health Gynecol. 2019;6:1–4. [Google Scholar]
- 15.The effect of vitamin D supplementation on the size of uterine leiomyoma in women with vitamin D deficiency. Hajhashemi M, Ansari M, Haghollahi F, Eslami B. Caspian J Intern Med. 2019;10:125–131. doi: 10.22088/cjim.10.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Management of elevated serum ferritin levels. Adams P. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3093720/ Gastroenterol Hepatol (N Y) 2008;4:333–334. [PMC free article] [PubMed] [Google Scholar]
- 17.Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Katsumata N, Yasuda M, Isonishi S, et al. Lancet Oncol. 2013;14:1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 18.Ferritin and Vitamin D levels and its relation to bone diseases in thalassemic adults: a hospital-based retrospective cohort study. Tharwat RJ, Balilah S, Habib H, et al. J Appl Hematol. 2019;10:15. [Google Scholar]
- 19.The effect of vitamin D supplementation on hemoglobin concentration: a systematic review and meta-analysis. Arabi SM, Ranjbar G, Bahrami LS, Vafa M, Norouzy A. Nutr J. 2020;19:11. doi: 10.1186/s12937-020-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Effect of vitamin D3 supplementation on iron status: a randomized, double-blind, placebo-controlled trial among ethnic minorities living in Norway. Madar AA, Stene LC, Meyer HE, Brekke M, Lagerløv P, Knutsen KV. Nutr J. 2016;15:74. doi: 10.1186/s12937-016-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Effect of calcitriol on serum hepcidin in individuals with chronic kidney disease: a randomized controlled trial. Panwar B, McCann D, Olbina G, Westerman M, Gutiérrez OM. BMC Nephrol. 2018;19:35. doi: 10.1186/s12882-018-0823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.High-dose vitamin D 3 reduces circulating hepcidin concentrations: a pilot, randomized, double-blind, placebo-controlled trial in healthy adults. Smith EM, Alvarez JA, Kearns MD, et al. Clin Nutr. 2017;36:980–985. doi: 10.1016/j.clnu.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Association of vitamin D, calcium and phosphate with uterine fibroid in premenopausal women of coastal Odisha. Kumari S, Babu BD, Singh S. Int J Sci Res. 2019;8:27–29. [Google Scholar]
- 24.Gender difference in relationship between serum ferritin and 25-hydroxyvitamin D in Korean adults. Seong JM, Yoon YS, Lee KS, Bae NY, Gi MY, Yoon H. PLoS One. 2017;31:0. doi: 10.1371/journal.pone.0177722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitamin D deficiency in children and adolescents. Andıran N, Çelik N, Akça H, Doğan G. J Clin Res Pediatr Endocrinol. 2012;4:25–29. doi: 10.4274/jcrpe.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitamin D status and the risk of anemia in community-dwelling adults: results from the National Health and Nutrition Examination Survey 2001-2006. Monlezun DJ, Camargo CA Jr, Mullen JT, Quraishi SA. Medicine (Baltimore) 2015;94:0. doi: 10.1097/MD.0000000000001799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.P298 Vitamin D status and inflammatory bowel disease - the role in disease activity and quality of life. de Castro FD, Magalhães J, Carvalho PB, Moreira MJ, Mota P, Cotter J. J Crohns Colitis. 2014;8:0. [Google Scholar]
- 28.Low vitamin D levels are associated with both iron deficiency and anemia in children and adolescents. Lee JA, Hwang JS, Hwang IT, Kim DH, Seo JH, Lim JS. Pediatr Hematol Oncol. 2015;32:99–108. doi: 10.3109/08880018.2014.983623. [DOI] [PubMed] [Google Scholar]
- 29.Prevalence and relationships of iron deficiency anemia with blood cadmium and vitamin d levels in Korean women. Suh YJ, Lee JE, Lee DH, et al. J Korean Med Sci. 2016;31:25–32. doi: 10.3346/jkms.2016.31.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]