Abstract
BACKGROUND AND PURPOSE: Three-dimensional phase-contrast (3DPC) is limited by long imaging times, limited coverage, flow artifacts, and the need to perform multiple additional 2D examinations (2DPC) to measure flow. A highly undersampled 3D radial acquisition (isotropic-voxel radial projection imaging [PCVIPR]) makes it possible to increase the product of volume coverage and spatial resolution by a factor of 30 for the same imaging time as conventional Cartesian 3DPC. This provides anatomic information over a large volume with high isotropic resolution and permits retrospective measurement of average flow rates throughout the volume.
METHODS: PCVIPR acquires a reference and three flow-encoded acquisitions for each VIPR projection. Complex difference images were formed by combining information from all flow directions. Following retrospective definition of planes perpendicular to selected vessels, volume flow rates were determined by using phase-difference information. The accuracy of average flow measurement was investigated in a phantom and in six volunteers. Anatomic PCVIPR images acquired in three patients and three volunteers by using a 3843 matrix were compared with conventional Cartesian 3DPC.
RESULTS: The flow validation produced R2 = 0.99 in vitro and R2 = 0.97 in vivo. PCVIPR produced minimal streak and pulsatile flow artifacts. PCVIPR produced far higher resolution and volume coverage in comparable imaging times. The highest acceleration factors relative to 3DPC were achieved by using gadolinium-contrast material. Ultimately, acceleration factors are limited by signal-to-noise ratio.
CONCLUSION: PCVIPR rapidly provides isotropic high-resolution angiographic images and permits retrospective measurement of average flow rate throughout the volume without the need to prescribe multiple 2D acquisition planes.
Three-dimensional phase-contrast (3DPC) imaging has several shortcomings that limit its use. Because of the need to acquire a reference and three flow-encoded excitations for each phase-encoding view, spatial resolution, especially in the section-encoding direction, must be limited to prevent the imaging time from being prohibitively long (1). Because of the anisotropic resolution nature of the 3D data sets, 3DPC is not typically used for obtaining flow rates. Instead a 2D phase contrast (2DPC) is prescribed perpendicular to the vessel of interest. If the flow rate is to be determined in multiple vessels, multiple 2DPC images must be obtained. Another shortcoming is the artifacts that result from pulsatile flow. Because PC MR has not been generally used clinically for measuring flow velocity, radiologists have in general relied on other methods. For example, in the evaluation of carotid stenosis, anatomic data are now acquired with MR and physiologic data with Doppler sonography. MR images show the relative size of the lumen, and Doppler sonography shows whether it has a significant hemodynamic effect.
The aim of this study was to develop a 3DPC method that could rapidly acquire enough data to produce images of a large volume with high isotropic spatial resolution to determine anatomic information and permit retrospective measurement of flow rates in vessels of arbitrary orientation. With this PC technique, both the size of the lumen and the velocity of flow can be determined within a single short imaging time.
MR image acquisition can be greatly accelerated by sampling k-space with vastly undersampled isotropic-voxel radial projection imaging (VIPR; 2, 3). The VIPR sequence samples data along radial lines evenly spaced through a spherical volume each intersecting the origin of k-space (4). VIPR permits short acquisition times, large volume coverage, and small isotropic voxels. It has long been known, for example in conventional radiographic CT, that undersampling with radially oriented projections results in streak artifacts. The surprising result of the 3D undersampled VIPR approach is that when undersampling is performed in 3D, the artifacts spread out over the 3D space and are far less noticeable than in 2D (2, 3).
Whereas acceptable acceleration factors relative to conventional Cartesian acquisition were about four in undersampled 2D radial projection acquisitions (5), in 3D VIPR, even at acceleration factor on the order of 50, the streak artifacts result in a generally acceptable diffuse background “fog.” This background “fog” arises from any signal intensity–generating source within the imaged volume. In the application discussed here, VIPR is used to implement a 3D radially undersampled phase-contrast sequence called PCVIPR. All stationary background anatomy is subtracted in the formation of the phase-contrast images. This limits the artifacts to those generated by the vessels. Therefore, phase contrast is well suited for VIPR acquisition.
Acceleration factors between 17 and 60 relative to conventional Cartesian 3DPC were implemented, depending on whether contrast material was present. Contrast material increases signal-to-noise ratio (SNR) and permits an increase in the readout resolution that can be supported by the available SNR, thus permitting the acceleration factor to be increased.
Accurate average flow-rate measurements were obtained both in vitro and in vivo by using measurement planes retrospectively selected from the acquired isotropic image volume. We report here on the comparison of these measurements with those obtained in separate 2DPC images.
Methods
Data Acquisition and Reconstruction
The PCVIPR sequence, implemented by using the 4-point excitation method (6, 7), was designed so that each VIPR projection was excited four times. One excitation was a reference with no flow-encoding gradients applied. This was followed by flow-encoded excitations for each of three orthogonal directions. The excitation data were saved separately for off-line reconstruction. Complex-difference and phase-difference reconstruction were performed for each flow direction to reconstruct angiographic images or to calculate quantitative flow rate. Three sets of data were reconstructed, one for each flow direction. Composite speed images were generated by taking the square root of the sum of the squares of the three flow-direction images. Through phase difference reconstruction, velocity in each pixel in each flow direction was calculated. Flow rates of each vessel were calculated from the phase-difference images by multiplying the average velocity of the blood vessel by the area of the vessel. As generally practiced in PC MR flow measurements (8), a threshold equal to 30% of the peak amplitude in the magnitude image was adopted to define the vessel cross-section. The acquisition time for PCVIPR is typically several minutes and is determined by the number of projections. The reconstruction time per flow direction for PCVIPR is 30 seconds for a 2563 data set and about 60 seconds for a 3843 data set.
The PCVIPR sequence was implemented on a GE 1.5T Signa system (GE Medical Systems, Milwaukee, WI). The sequence was set as a protocol in the system. For this article, the images were all generated off-line. When imaging was finished, the raw data were transferred automatically to a host computer for image reconstruction. The reconstructed images were then transferred automatically back to the reading room for the radiologists. We are currently working to make the PC VIPR sequence a routine practice with on-line image reconstruction.
PCVIPR High-Spatial-Resolution Angiography with and without Contrast Material
Three volunteers and three patients were imaged for the purpose of comparing the anatomic images produced by PCVIPR with those produced with the 3DPC sequence available on our system. One volunteer was imaged without administration of contrast material. Another was imaged without and then with administration of contrast material; the third was imaged with administration of contrast material. Three patients were imaged with residual contrast material present. The PC VPR and 3DPC examinations were done in rapid succession. When contrast material was used, the order of the 3DPC and PCVIPR images was alternated in sequential patients. Forty milliliters of contrast material were injected in about 2 minutes. Imaging was started at the same time as the contrast material injection was started.
All imaging was performed with field of view (FOV) = 24 cm, flip angle = 15, and velocity to be encoded = 20 cm/s. For 3D Cartesian PC, only 256 readout was available on the GE 1.5T system, so the number of phase-encoded samples was matched to the number of readout samples, resulting in an in-plane resolution of 0.94 × 0.94 mm2. The through-plane resolution was 2 mm. Imaging time was 7 minutes, 22 seconds. The section thickness was 4 cm. The TR/TE for 3DPC was 18/6.6 ms, and the receiver bandwidth was 16 kHz.
For PCVIPR a 384 readout was used, providing an isotropic spatial resolution of 0.63 × 0.63 × 0.63 mm3. The TR/TE for a 384 readout matrix was 17.34/7.57 ms at a bandwidth of 16 kHz and 11.6/ 5.14 ms at 32 kHz.
An imaging speed factor S was defined as the acquired volume divided by the product of voxel size and imaging time. The acceleration factor A for PCVIPR relative to 3DPC was defined as the ratio of the speed factors for each. PCVIPR was implemented with three different acceleration factors: 17, 30, and 61. For A = 17, the PCVIPR volume was 24 × 24 × 10, the number of acquired projections was 6400, the receiver bandwidth was 16 kHz, and the imaging time was 7 minutes, 30 seconds. For A = 30, the PCVIPR volume was 24 × 24 × 18, the number of acquired projections was 6400, the receiver bandwidth was 16 kHz, and the imaging time was 7 minutes, 30 seconds. For A = 61, the PCVIPR volume was 24 × 24 × 18, the number of acquired projections was 5000, the receiver bandwidth was 32 kHz, and imaging time was 3 minutes, 50 seconds.
For the A = 61 angiographic image comparison described above, the acceleration factor is calculated as (3:50 = 230 s, 7:22 = 442 s)
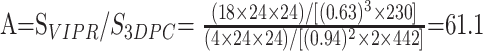 |
Complex difference images of both PCVIPR and 3D Cartesian PC MR were generated. The coronal and axial maximum intensity projections (MIPs) of the two acquisitions were compared.
Quantitative In Vitro and In Vivo Flow Measurement
In this section, the ability of PCVIPR to provide accurate quantitative average flow measurements were validated both in vitro and in vivo. These comparisons with 2DPC were carried out without contrast material present and with a readout resolution of 256 for 2DPC and PCVIPR.
In Vitro Quantitative Flow Measurement
A phantom was built by attaching a straight polyethylene tube, 6 mm inside diameter and 3 m length, to a pump (Quest Image; London, Ontario, Canada) that provides a series of flow waveforms and flow rates. The tube was filled with distilled water and aligned along the bore in a GE 1.5T system equipped with high-speed gradients. From the choices resident in the microprocessor of the pump, a programmed waveform (carotid2) was selected to provide phasic flow with 72 beats per minute simulating blood flow in the carotid artery. Corresponding to each peak flow rate that was selected, the average flow rate was provided by the pump and used as a standard value to be compared with the value measured by using PCVIPR. PCVIPR images centered on the tube were acquired by using the following parameters: 3000 projections; flip angle, 15°; FOV, 24 cm; 256-pixel readout resolution; section thickness, 2 mm; flow-encoding direction, S/I; and imaging time, about 3 minutes. Ten sets of images were acquired with peak flow rates varying between 3 and 18 mL/s. PCVIPR was repeatedly applied five times for each flow rate for statistical purpose. To calculate flow, voxels in the PCVIPR with signal intensity exceeding 30% of the peak signal intensity of the complex difference image were identified. In this area, average flow rate was computed as the sum of velocity in each selected voxel times the pixel area. At each flow rate, the mean and standard deviation of the five measurements were calculated. Average flow calculated from the PCVIPR acquisition was compared with the actual flow by least squares regression analysis.
In Vivo Quantitative Flow Measurement
Six adult volunteers, who gave written consent to participate in this institutional review board–approved study, were imaged with PCVIPR and 2D cine PC MR. 2D cine PC MR is the method in our clinic to obtain quantitative flow rate measurements in vivo, so it was selected as the method to validate the accuracy of the quantitative flow measurements obtained with PCVIPR. Because of the limited available imaging time, only two vessels were imaged for this study: the right internal carotid artery and the basilar artery.
The 2D cine PC MR images were acquired first and were repeated five times on each vessel. The imaging plane was chosen to be perpendicular to the direction of the blood flow in each vessel. Twenty cardiac phases were imaged during each exam, which took about 2 minutes. The in-plane resolution was 0.9 × 0.9 mm2. The section thickness was 3 mm. Phase difference images of each image were analyzed. The flow rate during each cardiac phase was measured. The average flow rate over the cardiac cycle of each image was then calculated. For each blood vessel, five flow rates were obtained. The mean and standard deviation of these five measurements were calculated for each blood vessel.
For PCVIPR, a 10-cm-thick axial section was prescribed, to include the circle of Willis. This section was imaged by using the following parameters: 3,000 projections; flip angle, 15°; voxel size, 0.9 × 0.9 × 0.9 mm3; and imaging time, about 3 minutes. From the volume of acquired data, a plane perpendicular to the flow direction in each vessel was retrospectively selected at a location close to where the 2D cine PC MR was performed. For statistical purposes, the same section was imaged five times with the same location being measured to calculate the flow rate of each vessel. The values measured from each blood vessel of each volunteer were compared with those obtained from the 2D cine PC MR.
Besides the average flow validation on the two blood vessels, additional quantitative flow measurement consistency checks could be made from the 3D data set from the PCVIPR acquisition. Without the presence of the posterior communicating arteries, the basilar artery is the only blood supplier to the left and right posterior cerebral arteries (PCAs). For the five volunteers without posterior communicating arteries, the flow rates from the left and right PCAs were measured and compared with the flow rate measured from the basilar artery. Flows in the PCAs of the five volunteers were not measured by using 2D PC MR for validation, because of the limit of imaging times.
Results
PCVIPR high-spatial-resolution angiography with and without contrast material
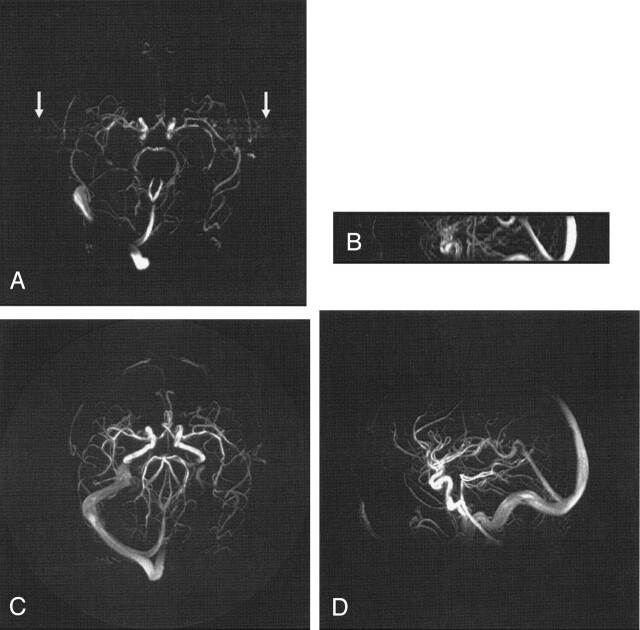
Example axial and coronal PCVIPR and 3DPC MIP images are shown for A = 17, 30, and 61 in Figures 1, 2, and 3. Figure 1 illustrates the case of A = 17, in which both examinations were performed without contrast material. The most notable features are the absence of pulsatility artfacts and the greater coverage provided by the PCVIPR examination. Pulsatile blood flow in the arteries resulted in ghosting artifacts appearing along the phase encoding direction of the conventional 3DPC axial MIP image as indicated by the arrows on Figure 1A. Projection acquisition is insensitive to pulsatile flow (9). Therefore, it is not necessary to apply ECG gating to remove pulsatile ghosting artifacts in a PCVIPR examination.
Fig 1.
Comparison of 3DPC (A, B) with a PCVIPR (C, D) acquisition having an acceleration factor of 17. In this case, comparable imaging times were used and the acceleration is due to a factor of 2.5 in volume coverage and a factor of 7 reduction in voxel volume. No pulsatile artifact appears on the PCVIPR image (C) as it does on the 3DPC image (A, arrows).
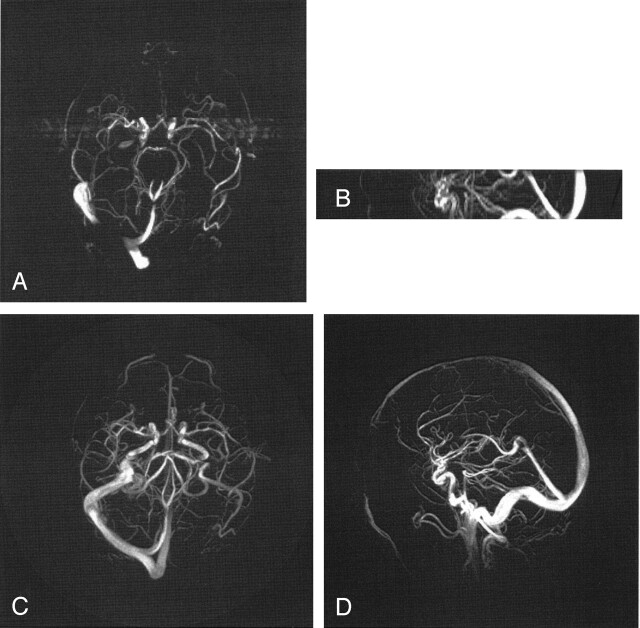
Fig 2.
Comparison of 3DPC (A, B) with a PCVIPR (C, D) acquisition having an acceleration factor of 30. In this case residual contrast was present and the acceleration is due to a factor of 4.5 in volume coverage and a factor of 7 reduction in voxel volume.
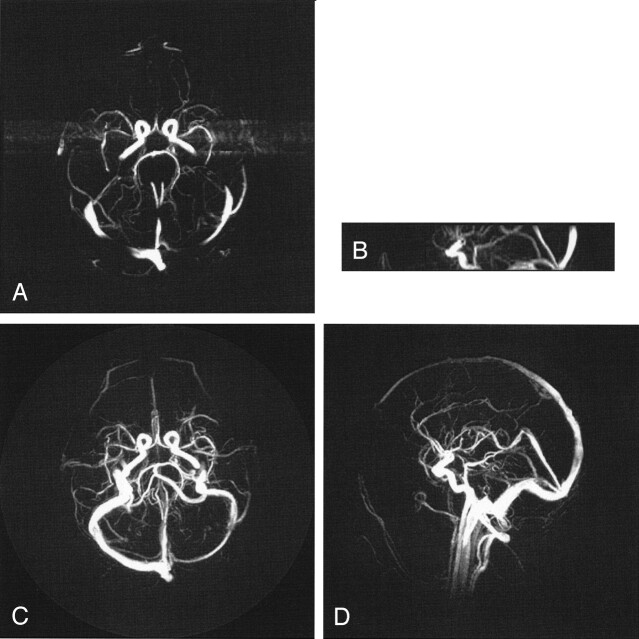
Fig 3.
Comparison of 3DPC (A, B) with a PCVIPR (C, D) acquisition having an acceleration factor of 61. The acceleration is due to a factor of 4.5 in volume coverage, a factor of 7 reduction in voxel volume, and a factor of 2 reduction in imaging time. Visualization of some small vessels is decreased in the PCVIPR examination, which indicates insufficient SNR to support this acceleration factor.
The advantages of the small isotropic voxels in PCVIPR are evident in the sagittal projection, where the PCVIPR pixel dimensions are 0.63 × 0.63 mm compared with 0.94 × 2 mm for 3DPC.
In Figure 2, the acceleration factor was increased to 30 by increasing the PCVIPR excitation section from 10 cm to 18 cm. The greater potential for signal intensity saturation in this larger volume was offset by the use of contrast material. The advantages of increased coverage, spatial resolution, and decreased artifacts are again evident.
In Figure 3 the acceleration factor was increased to 61 by reducing the number of acquired projections from 6400 to 5000 and increasing the receiver bandwidth to 32 kHz, resulting in an imaging time of 3 minutes, 50 seconds, compared with 7 minutes, 22 seconds, for 3DPC. In this case, the axial 3DPC examination shows more small vessels, which suggests that there is no longer sufficient SNR in the PCVIPR acquisition to support the small voxels at this acceleration factor.
Quantitative In Vitro and In Vivo Flow Measurements
In Vitro Quantitative Flow Measurements
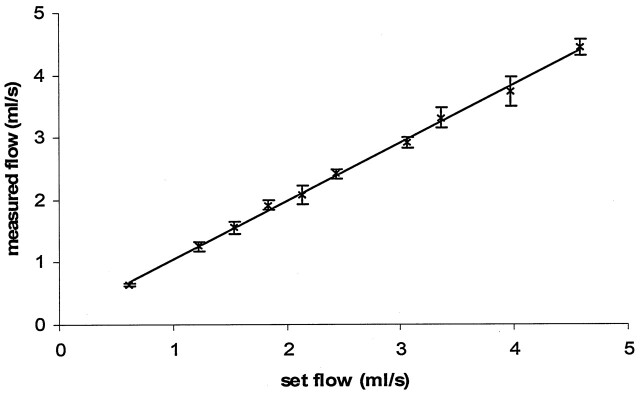
Flow rates calculated from the PCVIPR images varied between 0.75 and 5 mL/s at the 10 selected flow rates and correlated very closely with the flow rate prescribed in the phantom (Fig 4). One standard deviation was used as the error bar on each data point, which was less than 7% for any data point. The measured flow rates had a linear relationship to the actual flow rates with a slope of the fitted line equal to 0.94. The R2 was 0.99. The 0.94 slope (lower than the theoretical 1.0) may be due to the use of the 30% threshold applied to the complex difference image to define the region of interest for flow measurement.
Fig 4.
Basic PCVIPR in vitro average flow measurement validation on a pulsatile flow on the flow phantom. Ten flow rates were measured. The PCVIPR results are plotted against the computer-set flow rates. One standard deviation is plotted as the error bar on each data point. A linear relationship was reached, with a linear fit slope of 0.94 and an R2 = 0.99.
In Vivo Quantitative Flow Measurements
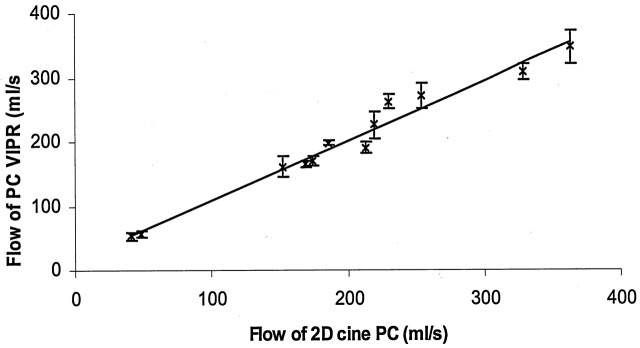
The mean flow rates measured with PCVIPR in the right internal carotid artery and the basilar artery matched very closely with those obtained from 2D cine PC. The correlation for the 12 values acquired by using these two methods is shown in Figure 5, with an R2 = 0.97. R2 is 0.87 for the right internal carotid artery and 0.99 for the basilar artery data if analyzed separately.
Fig 5.
In vivo flow measurement validation by using 2D cine PC MR on the right ICA and the basilar artery of six volunteers. One standard deviation is used to plot the error bar on each data point. The correlation of the two methods is shown here with R2 = 0.97.
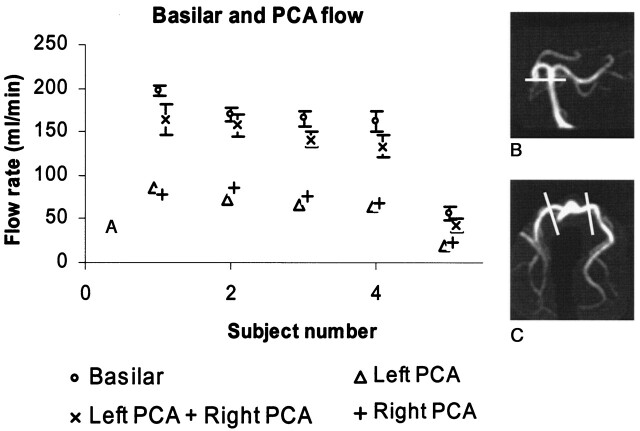
As shown in Figure 6, the flow in the left and right posterior communication arteries (PCAs) corresponded closely with the flow in the basilar artery except in one patient with a large PCA, whose data are not shown here. This study shows one of the advantages of the 3D PCVIPR data acquisition. Without additional images, flow information can be acquired in any vessel in the image volume. No independent flow measurements by using 2D PC MR were carried out to validate this study.
Fig 6.
When no posterior communicating arteries exist in the volunteer, the basilar artery is the only blood supplier to the two PCAs (B). The flow rates from the two PCAs were measured as shown in C, whereas the flow rate of the basilar artery was measured at the location as shown in B. As shown in A, the sum of the two PCA flow rates were very close to that of the basilar artery, where 1 SD was used as the error bar.
Discussion
PCVIPR offers a significant increase in the ability to acquire isotropic, large-volume high-resolution images in a short time. PCVIPR can produce arbitrarily large acceleration factors by increasing the readout resolution. This process ultimately can be limited by the available SNR. Therefore larger acceleration factors (higher resolution, coverage, imaging speed) can be accomplished when contrast material is present.
At very high acceleration factors, the voxels in PCVIPR may become so small or the acquisition time may become so short that inadequate SNR can limit the detection of vessels. Therefore a compromise between spatial resolution and SNR needs to be chosen. When contrast material is present, PCVIPR with a matrix of 3843 produces an SNR that appears to be adequate to support acceleration factors of about 30 relative to 3DPC. The resolution advantages of PCVIPR are particularly noticeable in imaging planes for which the 3DPC requires phase encoding.
The present article is limited to a comparison with 3D PC. For clinical studies, PCVIPR has provided significant advantages over conventional 3D PC methods. It might be possible that ultimately the PCVIPR examination could provide both anatomy and flow in a single examination. Although not reported here, ultimately we intend to compare PCVIPR with 3D TOF. Fifty patients have been imaged with both techniques and will be the subject of a future report. Acceleration factors relative to 3D TOF are typically 4–10. Voxel volumes are typically four times smaller than for 3D TOF. Qualitatively, preliminary results show a large number of images in which some structures were much more clearly seen with PC VIPR (10). Because of the longer echo times used in PCVIPR, however, there is a greater potential for intravoxel signal intensity loss. Although this should be at least partially offset by the small voxels in PCVIPR, a detailed comparison of image quality for various detection tasks must be carried out. If PCVIPR were found to be adequate to replace 3D TOF, the single PCVIPR examination could potentially be used to provide all anatomic and flow information.
In this article, our flow quantification was performed by using a 2563 matrix without contrast material. Good agreement with 2D cine PC MR has been achieved both in vitro and in vivo. Accurate average flow measurements can be acquired in any vessel in the 3DPCVIPR acquisition retrospectively. Time-resolved flow quantification can also be achieved by incorporating ECG gating into the PCVIPR acquisition.
The limitations in this study warrant some discussion. Flow in the phantom was underestimated by about 6%. The underestimation may be due to the use of the 30% threshold applied to the complex difference image to define the region of interest for flow measurement. The choice of this threshold is somewhat arbitary, and better agreement with the flow rates provided by the pump would be better with a somewhat lower threshold.
Conclusion
The PCVIPR sequence can be used to acquire angiographic images over a large imaging volume with high and isotropic spatial resolution in a short imaging time. It is not sensitive to pulsatile flow artifacts and provides useable acceleration factors up to about 30 relative to 3DPC when residual contrast material is present. Retrospective measurements of average flow rates in the 3D volume agree well with flow pump values and with in vivo 2D cine PC MR results.
Acknowledgments
This work was supported by National Institutes of Health grant 1RO1-HL62465.
References
- 1.Dumoulin CL, Souza SP, Walker MF, Wagle W. Three-dimensional phase contrast angiography. Magn Reson Med 1989;9:139–149 [DOI] [PubMed] [Google Scholar]
- 2.Block WF, Barger AV, Mistretta CA. Vastly undersampled isotropic projection imaging. In: Proceedings of the Eighth International Society for Magnetic Resonance in Medicine, Denver, CO. April 1–7, 2000. :161
- 3.Barger AV, Block WF, Toropov Y, Grist TM, Mistretta CA. Time-resolved contrast-enhanced imaging with isotropic resolution and broad coverage using a undersampling 3D projection trajectory. Magn Reson Med 2002;48:297–305 [DOI] [PubMed] [Google Scholar]
- 4.Wong STS, Roos MS. Strategy for sampling on a sphere with applications to 3D selective pulse design. In: Proceedings of 12th meeting of the Society of Magnetic Resonance in Medicine, New York, NY. August 14–20,1993. :1178
- 5.Peters DC, Korosec FR, Grist TM, et al. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med 2000;43:91–101 [DOI] [PubMed] [Google Scholar]
- 6.Dumoulin CL, Souza SP, Darrow RD, et al. Simultaneous acquisition of phase-contrast angiograms and stationary tissue images with Hadamard encoding of flow-induced phase shifts. J Magn Reson Imaging 1991;1:399–404 [DOI] [PubMed] [Google Scholar]
- 7.Pelc NJ, Bernstein MA, Shimakawa A, Glover GH. Encoding strategies for three-dimension phase-contrast MR imaging of flow. J Magn Reson Imaging 1991;1:405–413 [DOI] [PubMed] [Google Scholar]
- 8.Pelc LR, Pelc NJ, Rayhill SC, et al. Arterial and venous blood flow: noninvasive quantitation with MR imaging. Radiology 1992;185:809–812 [DOI] [PubMed] [Google Scholar]
- 9.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med 1992;28:275–289 [DOI] [PubMed] [Google Scholar]
- 10.Turk AS, Gu T, Mistretta CA, et al. Vascular imaging with phase contrast vastly undersampled isotropic projection imaging: an ultrafast phase contrast MR angiographic technique. In: Proceedings of the 42nd meeting of the American Society of Neuroradiology, Seattle, WA. June 7–11, 2004:123