Abstract
BACKGROUND AND PURPOSE: Lumbar zypapophyseal joints are innervated by the medial branch of the posterior lumbar ramus. The aim of this work was to describe the precise course of the medial ramus on axial CT scans and to define a precise location for its selective infiltration.
METHODS: Lumbar spines of two cadavers were first dissected to assess the route of the L1–L5 posterior ramus. Thirty lumbar spinal nerves of three cadavers were injected in the epineural space with a mixture of iodine contrast and stain to perform a correlation between anatomic gross sections and CT sections in the axial plane. A histologic study was also performed to ensure the neurologic nature of the structure identified.
RESULTS: The fibroosseous canal located between the mamillary and the accessory processes was a constant pathway for the medial branch of the L1–L4 posterior ramus. This former was always located closer to the accessory process. The L5 posterior ramus and its divisions could also be identified into a groove bounded laterally by the ala of the sacrum and medially by the base of the superior articular process of S1.
CONCLUSION: The accessory process and the groove bounded laterally by the ala of the sacrum and medially by the base of the superior articular process of S1 can be easily depicted on CT images and may allow a precise and selective infiltration of the medial branch of the posterior lumbar ramus.
The lumbrosacral spine is a major source of pain and disability. Three main pathologic mechanisms incriminated in the pathogenesis of these symptoms are intervertebral disk disease, vertebral body disease, and facet syndrome. The term “facet syndrome,” introduced by Ghormley in 1933 (1), corresponds to abnormalities of zygapophyseal joints related to segmental instability, synovitis, or degenerative arthritis (2–4). The clinical signs are local paralumbar tenderness, pain on hyperextension, absence of neurologic deficits, absence of root tension signs, and hip, buttock, or back pain when a straight leg is raised.
The usual treatment of facet syndrome is intraarticular corticoids injections; however, because the lumbar zypapophyseal joints are innervated by the medial branch of the posterior lumbar ramus, which arises from the spinal nerve just after they exit from the intervertebral foramen (5–9), steroid or ethanol infiltrations of the medial branch under fluoroscopic or CT guidance, as well as radio-frequency neurotomy, have been suggested as alternative therapies (7, 10–15). So far these injections have been performed at the junction of the base of the superior articular facet and the base of the transverse process (13). To the best of our knowledge, the precise course of the medial branch of the posterior lumbar ramus has not been described with axial imaging. This may have potential implications in the way the infiltrations are performed. The aim of this work was to describe the precise course of the medial branch of the posterior lumbar ramus on axial CT scans and to define precise bone landmarks that may allow selective infiltration of this nerve branch.
Methods
The lumbar columns of two cadavers were first dissected from L1 to S1 on their right side to assess the route of the posterior and medial ramus and their relationship with surrounding bony and soft tissues. The lumbar columns of three cadavers (two males, one female; age range, 70–87 years; mean age, 81 years) were then frozen and sawed in the medial sagittal plane to inject from the inside of the canal the cauda equina roots entering an intervertebral foramen. These cauda equina nerve roots were injected under their epineurium to realize a neurography, as described in the 1970s by others (16, 17). Thirty lumbar nerves (six L1, six L2, six L3, six L4, and six L5) were injected by this way with a mixture of iodine contrast (Iohexol 240 mg; Nycomed Amersham, Paris, France) and universal green stain. After injection, all specimens were studied by a helical CT scan. Scanning parameters were 120 KV, 120 mas, 1-mm section collimation, and 2.5-mm advance/rotation. Images were obtained in craniocaudal direction and were reconstructed every 0.8 mm in axial, sagittal, and frontal plane with a bone filter. Thereafter, the specimens were frozen and sawed into 3-mm-thick contiguous axial sections with a band saw.
The gross anatomic sections and the corresponding CT scans were evaluated in consensus by two observers (X.D., C.V.) to perform a radioanatomic correlation. The observers had to identify green-stained structures on gross anatomic sections and round or linear structures filled by contrast medium on CT scans of the theoretical course (5–9) of the posterior and medial ramus. A histologic study was performed each time a correlation was possible between anatomic and CT sections to confirm the nervous nature of the structure identified. The tissue for light examination was fixed in 10% buffered formoline and embedded in parafin. Four-micrometer-thick sections were stained with hematoxylin, eosin, and saffron. An L2 and a lumbosacral junction from a skeleton were employed to take photographs to show and illustrate the bone landmarks demonstrated in this study.
Results
The posterior ramus and its divisions into a lateral and medial branch could be identified in all anatomic sections. The correlation between anatomic and CT sections was conclusive in 18 cases for the posterior ramus (three L1, five L2, four L3, three L4, and three L5) and in 14 cases for the medial branch (three L1, four L2, three L3, two L4, and two L5). The histologic study confirmed in all these cases the nervous nature of the structure depicted both on CT sections and on the anatomic sections.
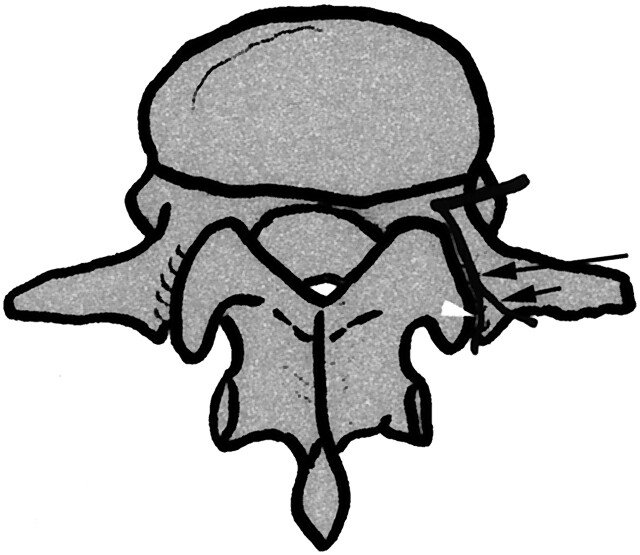
The results of the radioanatomic correlations and dissections were as follows: the L1–L4 posterior ramus was seen arising at a right angle from the spinal nerve just after its exit from the intervertebral foramen in each case. It then run dorsally and caudally along the anterolateral edge of the superior articular process down to the angle formed by this articular process and the transverse process where it divided into a medial branch and a lateral branch (Figs 1 and 2). The lateral branch then runs laterally and slightly dorsally and caudally behind the transverse process, which is of no further interest to this study. The medial branch continued its route dorsally, caudally, and medially against the lateral surface of the caudal edge of the superior articular process, separated from cortical bone by connective tissue, and entered a fibroosseous canal bounded anteriorly by the dorsal surface of the transverse process, medially by the mamillary process, laterally by the accessory process, and dorsally by a ligament connecting the accessory and mamillary processes (Figs 1 and 3).
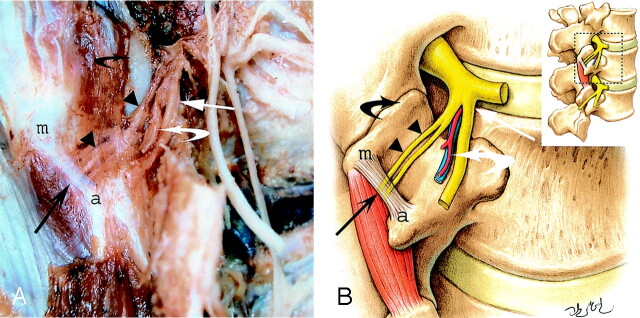
Fig 1.
Photograph (A) and schematic drawing (B) of a right posterolateral view of a dissection of a posterior lumbar ramus and its divisions at L2–L3. The medial branch of a L2 posterior ramus (arrowheads) runs against the lateral surface of the caudal edge of the superior articular process (black curved arrow) and then passes under a ligament (long black arrow) connecting the accessory process (a) and the mamillary process (m). Note the lateral branch of L2 posterior ramus (long white arrow) and vessels (white curved arrow) as well as the duplicity of the medial branch in the fibroosseous canal (twin medial branch).
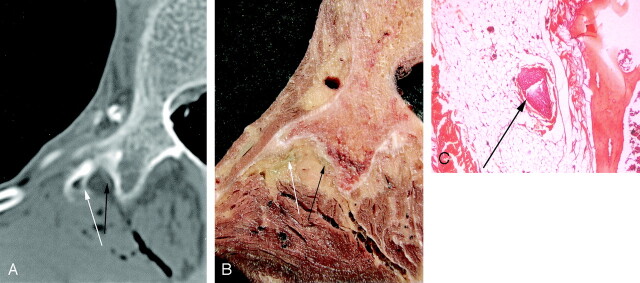
Fig 2.
Axial CT section (A) and corresponding gross anatomic section (B) show the L1 posterior ramus dividing into a medial branch (black arrow) and a lateral branch (white arrow) at the back of the transverse process. The medial branch is demonstrated (black arrow) on the corresponding histologic section (C).
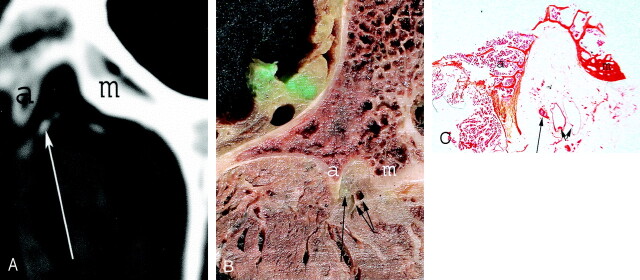
Fig 3.
Anatomic axial CT scan (A) after nerve injection and corresponding gross anatomic section (B) showing the L2 posterior ramus (long arrow) located into the groove bounded by the accessory process (a) and the mamillary process (m). Note the vascular pedicle (short arrows) located at the medial aspect of the medial branch. The histologic section (C) performed at this level demonstrates the medial branch (long arrow), the vascular pedicle (short arrows), the accessory process (a), and the mamillary process (m).
This branch does not run at the bottom of the bony gutter but constantly through its lateral part (i.e., against the medial face of the accessory process) (Fig 3). The accessory and mamillary processes were identified on all the anatomic and CT images. In this canal, blood vessels were also found consistently on the medial side of the medial branch in anatomic sections and in CT images on which correlations could be performed. Our dissections (Fig 4) showed that, emerging from this canal, the medial branch sent a branch to the adjacent zygapophyseal joint and just after another to the subajacent zygapophyseal joint. The medial branch then turned medially and caudally immediately below to the zygapophyseal joint. Note that, in one of our dissections we found a case of twin medial branch through the fibroosseous canal (Fig 1) as described by Auteroche (9). A schematic course of the posterior ramus and its divisions are demonstrated in Figure 5.
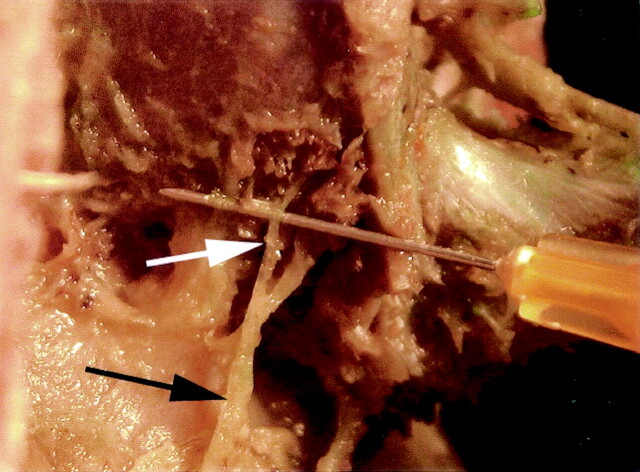
Fig 4.
Right posterior view of a dissection at L2–L3 intervertebral level showing the division of the medial branch into a branch (white arrow) innervating the adjacent zypapophyseal joint and into another branch (black arrow) innervating the subadjacent zygapophyseal joint.
Fig 5.
Drawing showing on a left posterolateral view the course of a posterior lumbar ramus (long arrow) and its division into a lateral branch (short arrow) running at the back of the transverse process and into a medial branch (arrowhead) running dorsally and caudally against the anterolateral edge of the superior articular process and then passing between the accessory and mamillary process.
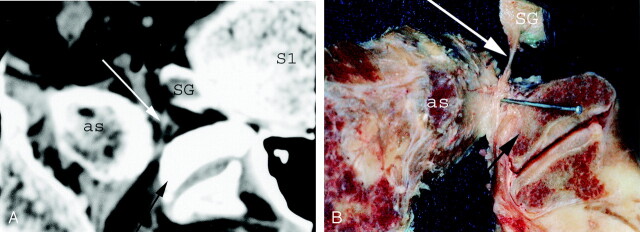
The L5 posterior ramus was identified on the dissections as well as on the anatomic and CT sections. It runs dorsally at the bottom of a groove bounded medially by the superior articular process of S1, and caudally and laterally by the ala of the sacrum (Fig 6). Along its course in this groove the posterior ramus divided into two branches: a medial branch that curved medially around the lateral aspect of the lumbosacral zygapophyseal joint and a lateral branch that runs downward to join the S1 posterior ramus (Fig 7). The posterior ramus was accompanied at that point by vessels located just laterally and cranially.
Fig 6.
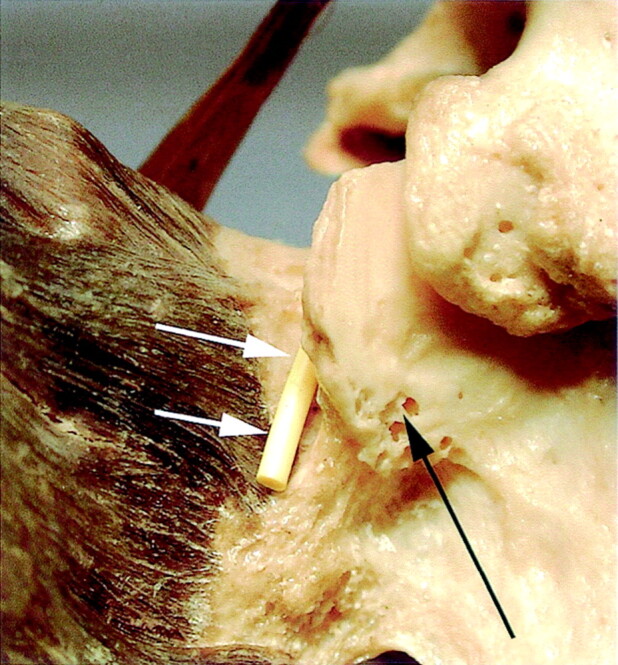
Posterior view of the left part of the first sacral vertebrae (S1) showing the groove bounded by the base of the superior articular process of S1 (black arrow) and the ala of the sacrum. The course of the medial branch of the posterior ramus is materialized by a yellow plastic wire (white arrows).
Fig 7.
Axial CT scan (A) and corresponding gross anatomic section (B) showing the L5 posterior ramus (white arrow) located into a groove bounded medially by the superior articular process of S1 (black arrow) and laterally by the ala of the sacrum (as). The gross anatomic section was dissected to better show the whole course of the posterior ramus into the bony groove. S1, first sacral vertebrae; SG, spinal ganglia.
Discussion
We have depicted the medial branch of the posterior lumbar nerve using a neurography technique, which was described in the 1970s (16, 17), because it appeared to us as an interesting way to demonstrate the posterior ramus and its divisions by a radioanatomic correlation. We chose to perform CT instead of MR imaging after neurography because infiltrations of the posterior lumbar ramus or zygapophyseal joint are frequently performed under CT guidance. The treatment of the facet syndrome remains controversial (12). Some authors have described intraarticular steroid injection into zygapophyseal joints in which temporary pain relief is obtained in most cases. Facet joint denervation has been considered and several ways of performing this procedure have been suggested. Schmid et al (14) described a CT-guided injection of alcohol dorsal to the zygapophyseal joint capsule. Schmid et al checked the extraarticular placement of the needle by injecting a small amount of contrast agent; however, this technique, which lacks precise landmarks for injection, is not selective of the medial branch course. Bogduk and Long (13) recommended performing under fluoroscopy the infiltration of the medial branch of L1–L4 nerve roots at the junction between the superior articular process and the base of the transverse process, immediately below the most medial end of its superior edge. Indeed, they demonstrated by anatomic dissections that the medial branch was within 5 mm of this point. Gangi et al (10) also suggested the same landmarks under CT guidance. We stress that using this landmark includes a risk of alcohol diffusion through the intervertebral foramen, which can be toxic for the anterior ramus. Moreover, injection in this area may not be selective for the medial branch because of the proximity of the division of the posterior ramus.
Our study confirmed that the fibroosseous canal located between the mamillary and the accessory process is a constant pathway for the medial branch of L1–L4 nerve roots (5–9). More precisely, both injected CT scans and anatomic sections revealed a constant positioning of the nerve within this canal (Fig 3). Indeed, the medial branch was always located in the lateroposterior part of the canal, closer to the accessory process than to the mamillary process. Therefore the accessory process, which is easily recognized on the axial CT images, appears to be an important bony landmark for CT-guided injections, which should then be done just medially to it. Using this landmark may allow a precise injection targeting the medial branch of the posterior ramus with decreased risk of ethanol diffusion through the intervertebral foramen.
The posterior ramus of the L5 nerve root and its division into a medial and lateral branches could also be identified into a groove bounded laterally by the ala of the sacrum and medially by the root of the superior articular process of S1 as yet described (7, 8). Here too, this bony groove is easily identified on CT images and represents an important bony landmark for infiltration of the posterior ramus under CT guidance. This bony groove has been described as a landmark for infiltrations by Bogduk and Long (7). We agree with these authors that infiltration into this groove would not be selective of the medial branch. Indeed, it is the L5 posterior ramus that runs in this groove to divide distally at its posterior part into the medial and lateral branches. Our study also confirmed the dual innervation of the zygapophyseal joints, which implies that denervation of one joint requires injection of both the branch of the corresponding level and the branch of the supra-adjacent level. Finally, our study confirms the presence of blood vessels coursing along the posterior ramus and its medial branch, which implies that the radiologist should consider injecting some iodine contrast agent before steroids or alcohol administration, to make sure that the needle is not placed in these vessels.
Our study had some limitations. Opacification of the posterior and medial ramus was not obtained at all vertebral levels on CT images. This is first due to the fact that, unlike veins and arteries, nerves are not hollow structures and thus the contrast media cannot fill the whole nerve but has to diffuse around the fibers. Moreover performing nerve injection under the epineurium is a delicate undertaking because of the nerve fragility and the small size of the nerve roots.
Conclusion
Our study reports a constant relationship of the L1–L4 medial branch of the posterior ramus with the accessory process and of the L5 medial branch of the posterior ramus with the groove bounded laterally by the ala of the sacrum and medially by the root of the superior articular process of S1. These bone structures can be easily depicted on CT images, which may allow a selective infiltration of these structures. In our experience the use of these landmarks allows an easy infiltration of the medial branch.
Acknowledgments
We thank Professor He-Jin Kim, and the illustrator, Kwan-Hyun Youn, from the Dental College, Yonsei University of Seoul, Korea, for their assistance with the drawings. We also acknowledge Doctor Hervé Cotten from the Pathology Laboratory, Boulevard de la Liberté, Lille, France, for his assistance with preparation and interpretation of histologic sections.
References
- 1.Ghormley R. Low back pain with special reference to the articular facet, with presentation of an operative procedure. JAMA 1933;101:1773–1777 [Google Scholar]
- 2.Schellinger D, Werner L, Ragsdale B, Patronas NJ. Facet joint disorder and their role in production of back pain and sciatica. Radiographics 1987;7:923–944 [DOI] [PubMed] [Google Scholar]
- 3.Helbig T, Casey K. Lee. The lumbar facet syndrome. Spine 1988;13:61–64 [DOI] [PubMed] [Google Scholar]
- 4.Fukui S, Ohseto K, Shiotani M, et al. Distribution of referred pain from the lumbar zygapophyseal joints and dorsal rami. Clin J Pain 1997;13:303–307 [DOI] [PubMed] [Google Scholar]
- 5.Lazorthes G. La branche postérieure des nerfs rachidiens: l’innervation des articulations interapophysaires vertébrales [in French]. In: Compte-rendu de l’Association des Anatomistes. 43ème reunion. Lisbonne:000;1966. :488–494
- 6.Lazorthes G, Juskiewenski S. Etude comparative des branches postérieures des nerfs dorsaux et lombaires et de leurs rapports avec les articulations interapophysaires vertébrales [in French]. In: Beau A, ed. Bulletin de l’Association des Anatomistes. 49ème reunion. Nancy, France: Imprimerie Georges Thomas;1964. :1025–1033
- 7.Bogduk N, Long DM. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg 1979;51:172–177 [DOI] [PubMed] [Google Scholar]
- 8.Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat 1982;134:383–397 [PMC free article] [PubMed] [Google Scholar]
- 9.Auteroche P. Innervation of the zygapophyseal joints of the lumbar spine. Anat Clin 1983;5:17–28.14 [Google Scholar]
- 10.Gangi A, Dietemann JL, Mortazavi R, et al. CT-guided interventional procedures for pain management in the lumbosacral spine. Radiographics 1998;18:621–633 [DOI] [PubMed] [Google Scholar]
- 11.Goupille P, Cotty P, Fouquet B, et al. Denervation of the posterior lumbar vertebral apophyses by thermocoagulation in chronic low back pain: results of the treatment of 103 patients. Rev Rhum Ed Fr 1993;60:791–796 [PubMed] [Google Scholar]
- 12.el-Khoury GY, Renfrew DL. Percutaneous procedures for the diagnosis and treatment of lower back pain: diskography, facet-joint injection, and epidural injection. AJR Am J Roentgenol 1991;157:685–691 [DOI] [PubMed] [Google Scholar]
- 13.Bogduk N, Long DM. Percutaneous lumbar medial branch neurotomy: a modification of facet denervation. Spine 1980;5:193–200 [DOI] [PubMed] [Google Scholar]
- 14.Schmid G, Vetter S, Göttmann D, Strecker EP. CT-guided epidural perineural injections in painful disorders of the lumbar spine: short- and extended-term results. Cardiovasc Intervent Radiol 1999;22:493–498 [DOI] [PubMed] [Google Scholar]
- 15.Silbergleit R, Mehta BA, Sanders WP, Talati SJ. Imaging-guided injection techniques with fluoroscopy and CT for spinal pain management. Radiographics 2001;21:927–939 [DOI] [PubMed] [Google Scholar]
- 16.Ingianni G. Neurography: experimental positive visualization of peripheral nerves by x-rays. Unfallchirurgie 1982;8:128–130 [DOI] [PubMed] [Google Scholar]
- 17.Bourrel P. Neurography: technique and value. Rev Chir Orthop Reparatrice Appar Mot 1974;60:89–96 [PubMed] [Google Scholar]