Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to measure the changes in the intraaneurysmal fluid pressure and parent vessel flow characteristics resulting from packing the aneurysmal sac with hydrogel-coated coils.
METHODS: Platinum coils coated with an expansible hydrogel were used to embolize a silicone model of the basilar tip aneurysm. The intraaneurysmal fluid pressure was measured with a NeuroCare Camino Pressure Micro-Probe at various packing ratios. A programmable pulsatile pump was used to input a physiologically relevant pulsatile flow in the parent artery.
RESULTS: The intraaneurysmal fluid pressure did not increase when packing the aneurysm with hydrogel-coated platinum coils, even with a coil density up to 93%.
CONCLUSION: Packing the aneurysm with hydrogel-coated coils at a density up to 93% did not increase the intraaneurysmal fluid pressure.
Endovascular treatment for intracranial aneurysms has been developed over the past decade as an alternative to surgery. The Guglielmi detachable coil (GDC) system is extensively used. There are, however, potential problems associated with endovascular therapy using bare platinum coils, such the risk of aneurysmal recurrence or coil compaction. MicroVention has introduced the HydroCoil Embolic System (HES; MicroVention, Aliso Viejo, CA), which has been designed to fill the aneurysm cavity and potentially diminish the observed rates of aneurysmal recanalization by the means of an expansible hydrophilic polymer coating on platinum helical coils. It has been previously reported that placing noncoated coils (either a single coil [1], or multiple coils [2, 3]) with complete filling of the aneurysmal sac) does not change the mean intraaneurysmal fluid pressure. There is some uncertainty about the possibility that the expansible hydrogel coating the coils might increase the intraaneurysmal fluid pressure when fully expanded, extrude into the parent vessel, or exert pressure on the aneurysm wall.
The aim of our study was to evaluate and measure the effect of the fully hydrated gel on the intraaneurysmal fluid pressure for different degrees of aneurysmal packing. We also evaluated the possibility of parent vessel compromise in a particular aneurysm model. After the hydrocoils were placed, measurements of the velocity field were taken to characterize the effect of aneurysmal packing on the flow in the parent vessel and to ensure that no hydrogel has extruded into the vessel. We did not attempt to measure an exerted pressure on the aneurysm wall in the present study.
Methods
It has been shown that coiling is the preferred method of treatment for basilar aneurysms (4). Thus, we selected for our study a model of a basilar artery (4 mm in diameter) with a 10-mm saccular aneurysm located at its apex. The aneurysm model was made out of silicone, in an attempt to reproduce the compliance of the arterial wall (5).
The aneurysm model was perfused with 0.01 mol/L phosphate-buffered saline (PBS), a solution with the exact same pH as blood (normal blood pH is about 7.35), to ensure an expansion of the hydrogel similar to that occurring in vitro. The fluid is seeded with lycopodium powder (Carolina Biologic Supply Company, Burlington, NC) with a mean diameter ranging from 1 to 10 μm. To avoid distortion of the visualized flow by refraction, the model is placed in a transparent box filled with the same perfusion fluid.
A pulsatile flow corresponding to the flow through the carotid artery was supplied to the model by means of a pulsatile pump (UHDC flow system, Sidac Engineering, London, Ontario, Canada). The peak volume rate selected for this study was 360 mL/min. The period of the pulsatile flow was 0.83 seconds, corresponding to a cardiac rate of 72 beats per minute.
Coil Characteristics
Following the established protocol for placing coils, two MicroPlex 18 Complex coils (MicroVention)—Complex 1D 10 mm/26 cm and Complex 2D 9 mm/21 cm—were deployed first to create a framework for subsequent deposition of the hydrogel-coated coils. The HydroCoil Embolic System was used to place HydroCoils 14 of different sizes, ranging from 3 mm/7 cm to 8 mm/10 cm, until complete filling was achieved (see Table 1.)
Volumetric aneurysmal occlusion achieved with the coils used to pack a basilar tip aneurysm model
| Placement Order | Type of Coils* | Secondary Wind Diameter (mm) | Length (cm) | Volumetric Aneurysmal Occlusion (%) |
|---|---|---|---|---|
| 1 | MCS 18 | 10 | 26 | 6 |
| 2 | MCS 18 | 9 | 21 | 10 |
| 3 | HES 14 | 8 | 10 | 17 |
| 4 | HES 14 | 6 | 10 | 24 |
| 5 | HES 14 | 6 | 10 | 31 |
| 6 | HES 14 | 6 | 10 | 38 |
| 7 | HES 14 | 6 | 10 | 46 |
| 8 | HES 14 | 6 | 10 | 53 |
| 9 | HES 14 | 4 | 10 | 60 |
| 10 | HES 14 | 4 | 10 | 67 |
| 11 | HES 14 | 4 | 10 | 74 |
| 12 | HES 14 | 4 | 10 | 81 |
| 13 | HES 14 | 4 | 10 | 88 |
| 14 | HES 14 | 3 | 7 | 93 |
Note.—MCS indicates MicroPlex Coil System; HES, HydroCoil Embolic System.
Pressure Measurements
Pressure measurements were made by using a Camino Parenchymal Intracranial Pressure Catheter (Integra Neurosciences, Neurosurgery Division of Integra LifeSciences, Plainsboro, NJ). This device is widely used to directly monitor intracranial pressure by inserting a transducer-tipped catheter in the parenchymal or subarachnoid space. The accuracy of the measurements is ±2 mmHg. The analog signal intensity was acquired with an A/D converter and processed by using LabVIEW (National Instruments, Austin, TX) software.
The pressure was measured by placing the catheter tip at the fundus of the sac. Measurements were taken for the control and subsequently after placing each coil, up to a total of 14 coils.
Velocity Measurements
To ensure that the flow in the parent artery was reestablished after placing the hydrocoils, measurements of the velocity field inside the parent vessel were simultaneously taken by using the digital particle image velocimetry technique. The system is composed of two Nd:YAG lasers, a synchronizer, a charge-coupled device (CCD) camera and a personal computer. The pulsed lasers provide a laser sheet that illuminates the small-size tracer particles added to the flowing fluid. The firing of the two pulsed lasers is synchronized with the image acquisition. Two consecutive images, corresponding to the firing of each laser, 0.4 milliseconds apart, are captured when the CCD camera is triggered by the synchronizer, thus recording the light scattered by the tracer particles. The velocity field is then computed by the appropriate software (Insight, TSI Inc., Saint Paul, MN) cross-correlating the two images and measuring the displacements of the particles. The cross-correlation function is computed by performing 2D fast Fourier transforms.
Results
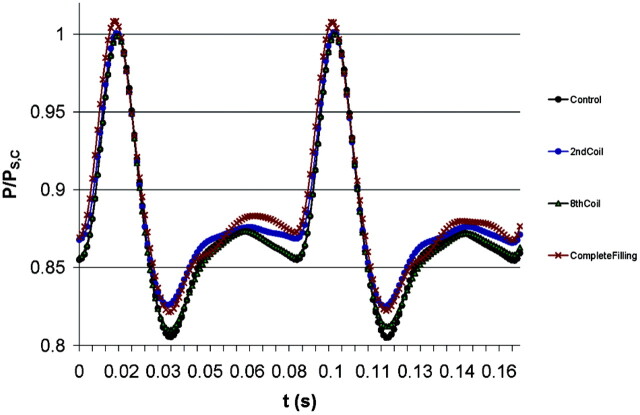
To determine possible changes in the intraaneurysmal pressure due to the expansion of the polymer that coats the HydroCoils, we first measured the pressure at the aneurysmal fundus before placing the coils (control). Then, pressure measurements were taken after the deployment of each coil, including the noncoated platinum coils used as a support structure. The different degrees of packing achieved after placing each of the coils is shown in Table 1. A summary of these measurements is shown in Figure 1, where we have included the measurements for the control and after placing the two structural coils (denoted as the second coil in Fig 1). We have also included the results for two representative cases: after placing six hydrogel-coated coils and after complete filling (12 HydroCoils). Our results indicate that there is not a significant change in the intraaneurysmal fluid pressure with the expansion of the polymer coating the platinum coils (the slight difference of the results was within the measurement error), not even after placing twelve HydroCoils and achieving a filling capacity to 93%. We did not measure wall tension specifically.
Fig 1.
Intraaneurysmal pressure measurements of the nontreated aneurysm model (control), after placing two structural coils (2nd coil), after placing six hydrogel-coated coils (8th coil), and for the complete filling (14th coil). Measurements were normalized with the measured pressure at peak systole for the control case, PS,C. The slight difference between the curves remains within the measurement error (±2 mmHg).
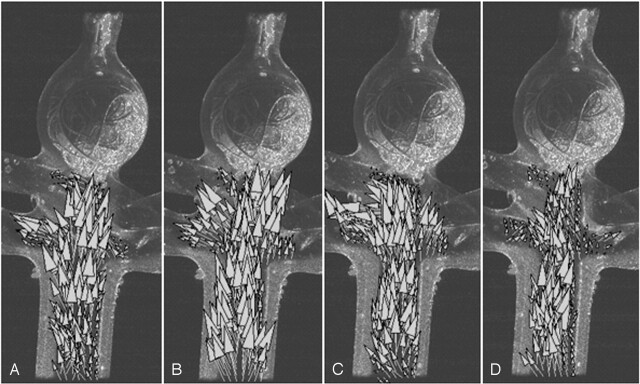
Measurements of the velocity field in the parent artery performed at four representative stages of the cardiac cycle (at the acceleration and deceleration phases of the systole, A and B; at peak diastole, C; and at the resting stage, D) are shown in Figure 2. These measurements show that the placement of the hydrocoils does not perturb the flow in the parent vessel and the flow is redirected from the basilar artery toward the two posterior cerebral arteries.
Fig 2.
Resulting velocity field after complete filling at the four representative points of the cardiac cycle: at the acceleration stage of the systole, A; deceleration stage of the systole, B; peak diastole, C; and resting period, D. The flow in the parent artery is shown to be reestablished.
Discussion
The use of detachable embolic coils in the treatment of aneurysms often presents a lower incidence of complications and may have a comparable patient outcome to surgical methods (6, 7). This is even the case when treating both ruptured and unruptured aneurysms. The use, safety, efficacy, and complications of the use of GDCs have been extensively reported in recent years (8–12). There are potential risks associated with this treatment, such as the possible recanalization of the aneurysm with risk of rupture or the risk of coil protrusion into the parent vessel.
To overcome the limitations of the GDCs, modifications on the coil design have been made, such as 3D coils, or bioabsorbable polymeric-coated coils (13–17). In this study we have considered the HES to treat in vitro a basilar aneurysm model. The HES consists of a platinum helical coil coated with a layer of a hydrophilic, acrylic polymer (hydrogel) that provides additional filling of a vascular space. This polymeric material is capable of swelling in the presence of blood (blood diffuses through the polymer, causing disentanglement of polymer chains and swelling), expanding up to three times its initial volume.
The goal of HES is to fill the sac entirely, theoretically reducing the risk of aneurysm recurrence. The mechanisms behind aneurysm regrowth remain unknown but likely relate to thrombolysis and recanalization of thrombus that are initially induced by the coils. It has been shown that the HES significantly improves aneurysm packing, from a 20–30% volumetric aneurysmal occlusion with bare platinum coils to about 70% volumetric occlusion with HES coils (14, 18).
Our in vitro measurements show that the intraaneurysmal fluid pressure remains virtually unchanged, even after achieving a 93% coil filling after full hydration. We have not, however, studied the effect of hydrocoils on the aneurysm wall tension. Overpacking (inserting more than 100% of the calculated volume of the aneurysm) could potentially lead to an increased pressure, which, depending on the wall’s modulus of elasticity, will result either in a volumetric expansion of the sac or in some of the polymeric hydrogel extruding into the parent vessel.
It is interesting to note that our measurements are consistent with previous results where GDCs have been used, which have shown that coiling aneurysms does not decrease the pressure transmitted from the vascular system (1–3). Therefore, the presence of the expansible hydrogel does not modify the effect of the coils on the intraaneurysmal fluid pressure even with up to 93% filling. The protective effect may be due to thrombus formation promoted by coils, reduction in shear forces against the wall, or other contributing factors (19).
Conclusion
In vitro measurements of the pressure inside a saccular aneurysm filled with hydrocoils and perfused with a physiologically representative pulsatile flow have shown that the expansion of the hydrated polymer does not contribute to any appreciable changes in the intraaneurysmal fluid pressure. This is shown to be the case when the fully hydrated coil volume is as high as 93% of the aneurysmal sac volume. It should be emphasized here that our study has been limited to cases in which the volume of the fully hydrated coils does not exceed the initial volume of the aneurysmal sac.
References
- 1.Novak P, Glikstein R, Mohr G. Pulsation-pressure relationship in experimental aneurysms: observation of aneurysmal hysteresis. Neurol Res 1996;18:377–382 [DOI] [PubMed] [Google Scholar]
- 2.Boecher-Schwarz HG, Ringel K, Kopacz L, et al. Ex vivo study of the physical effect of coils on pressure and flow dynamics in experimental aneurysms. AJNR Am J Neuroradiol 2000;21:1532–1536 [PMC free article] [PubMed] [Google Scholar]
- 3.Sorteberg A, Sorteberg W, Turk AS, et al. Effect of Guglielmi detachable coil placement on intraaneurysmal pressure: experimental study in canines. AJNR Am J Neuroradiol 2001;22:1750–1756 [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber DP, Zimmerman GA, Tomsick TA, et al. A comparison between endovascular and surgical management of basilar artery apex aneurysms. J Neurosurg 1999;90:868–874 [DOI] [PubMed] [Google Scholar]
- 5.Kerber CW, Heilman CB, Zanetti PH. Transparent elastic arterial models I: a brief technical note. Biorheology 1989;26:1041–1049 [DOI] [PubMed] [Google Scholar]
- 6.International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 7.Johnston SC, Zhao S, Dudley RA, et al. Treatment of unruptured cerebral aneurysms in California. Stroke 2001;32:597–605 [DOI] [PubMed] [Google Scholar]
- 8.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284–293 [DOI] [PubMed] [Google Scholar]
- 9.Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Circulation 2000;102:2300–2308 [DOI] [PubMed] [Google Scholar]
- 10.Brilstra EH, Rinkel GJE, van der Graaf Y, et al. Treatment of intracranial aneurysms by embolization with coils. Stroke 1999;30:470–476 [DOI] [PubMed] [Google Scholar]
- 11.Dovey A, Misra M, Thornton J, et al. Guglielmi detachable coiling for intracranial aneurysms: the story so far. Arch Neurol 2001;58:559–564 [DOI] [PubMed] [Google Scholar]
- 12.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998–2004 [DOI] [PubMed] [Google Scholar]
- 13.Cloft HJ, Joseph GJ, Tong FC, et al. Use of three-dimensional Guglielmi detachable coils in the treatment of wide-necked cerebral aneurysms. AJNR Am J Neuroradiol 2000;21:1312–1314 [PMC free article] [PubMed] [Google Scholar]
- 14.Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR Am J Neuroradiol 2002;23:1580–1588 [PMC free article] [PubMed] [Google Scholar]
- 15.Murayama Y, Suzuki Y, Viñuela F, et al. A new surface modification technique of platinum coils by ion implantation and protein coating: use in intravascular treatment of brain aneurysms. Nucl Instrum Meth B 1997;127/128:1015–1018 [Google Scholar]
- 16.Murayama Y, Viñuela F, Tateshima S. Matrix: new bio-absorbable polymeric coils for the treatment of intracranial aneurysms. Int Cong Ser 2002;1247:119–126 [Google Scholar]
- 17.Murayama Y, Viñuela F, Tateshima S, et al. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg 2001;94:454–463 [DOI] [PubMed] [Google Scholar]
- 18.Cloft HJ, Kallmes DF. Aneurysms packing with HydroCoil embolic system versus platinum coils: initial clinical experience. AJNR Am J Neuroradiol 2004;25:60–62 [PMC free article] [PubMed] [Google Scholar]
- 19.Malek AM, Alper SL, Izumu S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999;282:2035–2042 [DOI] [PubMed] [Google Scholar]