Abstract
Summary: A 36-year-old man presented with trouble speaking and bilateral progressive hearing loss. MR imaging and histopathologic results revealed a posterior fossa melanotic ependymoma. Pial surfaces appeared hyperintense on T1-weighted images and hypointense on T2-weighted images. Histopathologic examination revealed that tumor cells and interstitial spaces had abundant melanin accumulation. There was no evidence of hemosiderin in tumor cells and in interstitial spaces. Pial melanin accumulation secondary to a posterior fossa melanotic ependymoma explained our MR findings.
Intracytoplasmic melanin pigment has been described infrequently in primary intracranial tumors such as astrocytoma, ganglioglioma, choroid plexus papilloma and carcinoma, medulloblastoma, central neurocytoma, and schwannoma ( 1). Pigmented ependymomas are rare, with only six cases having been previously reported in the literature ( 1–5). We represent a case of pial melanosis, with widespread pial melanin accumulation secondary to a posterior fossa melanotic ependymoma, and we describe MR findings.
Case Report
A 36-year-old man presented with headache and difficulty swallowing. He had been complaining of trouble in speaking, bilateral progressive hearing loss, and dizziness for 2 years. On neurologic examination, there was total sensorineural hearing loss on the left and subtotal sensorineural hearing loss on the right. Left-sided central facial paralysis, hypesthesia on the left side of the body, wide-based gait, cerebellar ataxia, and bilateral clonus were present. His deep tendon reflexes were brisk and symmetrical with bilateral Babinski and Hoffman signs.
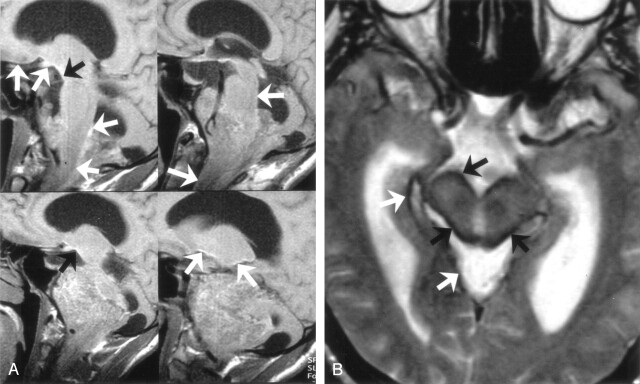
MR examination revealed a posterior fossa mass that extends cranially through 4th ventricle and left cerebellopontine cistern and caudally through foramen magnum (Fig 1A–D). T2-weighted images showed striking signal intensity voids, and noncontrast T1-weighted images revealed a linear band of slightly high intensity on the pial surfaces of the cerebrum, cerebellum, brain stem, and spinal cord (Fig 2A and B).
Fig 1.
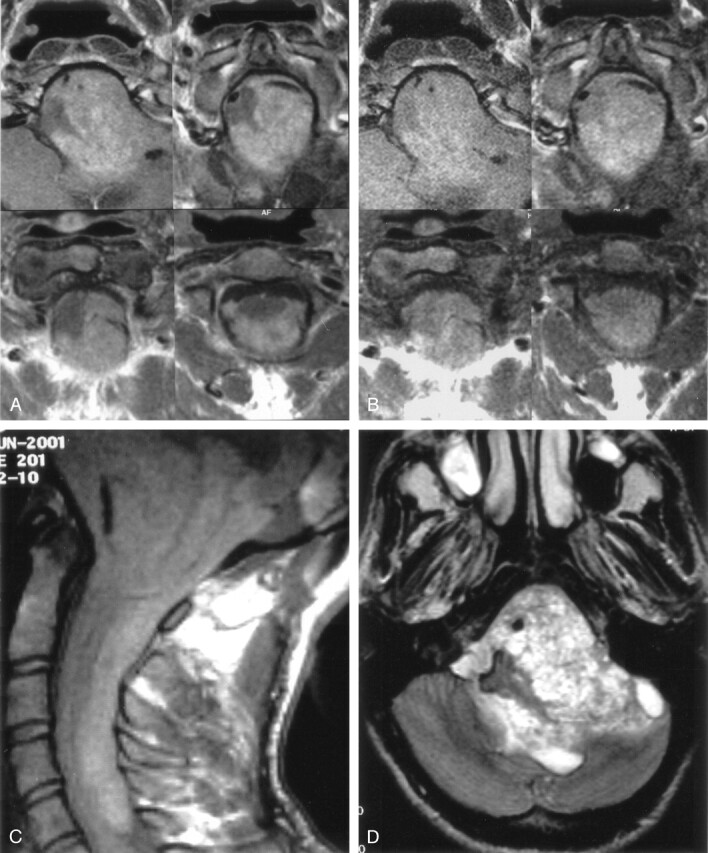
Contrast-enhanced (A) and nonenhanced (B) T1-weighted axial MR images reveal a posterior fossa mass that extends cranially through 4th ventricle and left cerebellopontine cistern and caudally through foramen magnum. Nonenhanced T1-weighted sagittal MR image (C) shows that a hyperintense melanin-containing mass displaces the medulla oblongata and spinal cord and extends down to the level of the 6th cervical vertebra. T2-weighted axial MR image (D) shows a heterogeneous posterior fossa mass.
Fig 2.
Four sequential T1-weighted nonenhanced sagittal MR images (A) show linear high signal intensity on the pial surfaces of the cerebrum, cerebellum, brain stem, and spinal cord secondary to pial melanin accumulation (arrows). T2-weighted axial MR image (B) shows striking signal intensity voids on the pial surfaces of brain stem (arrows).
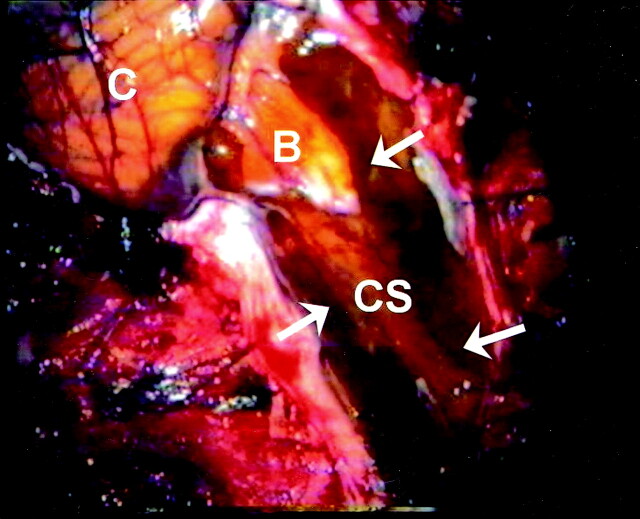
The patient underwent subtotal resection of the posterior fossa mass. During the operation, a black and dark brown mass and diffuse black pigmentation of the leptomeninges were identified (Fig 3).
Fig 3.
Photograph of the surgery shows diffuse black and dark brown pigmentation of the pial surfaces of medulla oblongata and medulla spinalis (arrows). C indicates cerebellum; B, bulbus; CS, cervical spinal cord.
Histopathologic examination revealed typical appearance of ependymoma with true rosettes, pseudorosettes, and perivascular orientation (Fig 4A). The same tumor cells had abundant brown pigment. The brown pigment did not stain with Prussian blue and showed prominent positivity when stained with Masson-Fontana, which is a characteristic of melanin that was scattered throughout the ependymoma (Fig 4B). The immunohistochemical pattern of this tumor included positivity for glial fibrillary acidic protein, which is important in the exclusion of other melanotic diseases such as meningeal melanocytomas.
Fig 4.
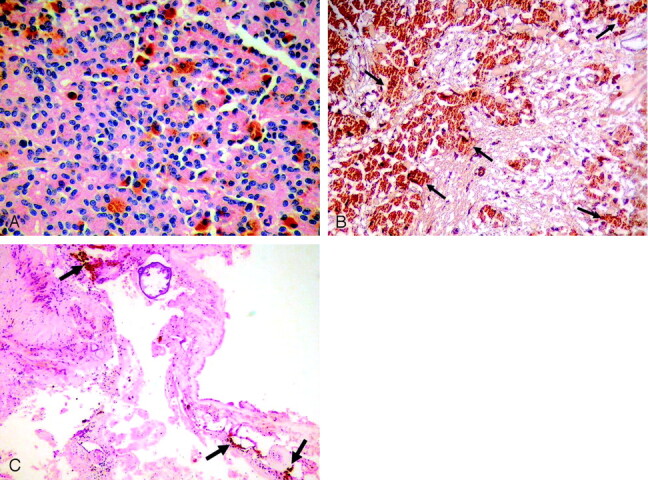
A, Microscopy shows characteristic histopathologic features of ependymoma with true rosettes and pseudorosettes, perivascular orientation (hematoxylin-eosin; original magnification × 200). B, Tumor cells have abundant brown melanin pigment (arrows) in the cytoplasm that shows positivity for Masson-Fontana (original magnification ×100). C, Brown melanin pigment is also present in the interstitial space of the tumor (arrows; hematoxylin-eosin; original magnification ×40).
The diagnosis was a melanotic ependymoma of the posterior fossa. Brown pigment was also found in the interstitial spaces and did not stain with Prussian blue but showed prominent positivity when stained with Masson-Fontana, which means releasing of melanin outside of the tumor (Fig 4C). Pial melanin accumulation secondary to posterior fossa melanotic ependymoma explained the reason why pial surfaces were hyperintense on T1-weighted images and hypointense on T2-weighted images. The patient’s condition progressively worsened, and he died 12 days after surgery.
Discussion
Primary intracranial tumors such as astrocytoma, ganglioglioma, choroid plexus papilloma and carcinoma, medulloblastoma, central neurocytoma, and schwannoma may rarely contain intracytoplasmic melanin pigment (1). Melanotic ependymoma is an uncommon tumor, with only six cases having been reported in the literature (1–5). In the brain, melanogenesis is normally found in the leptomeningeal melanocytes, in a few neurons situated in the roof of the 4th ventricle, and certain brain stem nuclei, but melanin has not been clearly recognized in ependymal cells. Because glial elements and melanotic pigmented layer of the retina are derived from the same ciliated epithelium of the embryonic neural tube, the presence of melanin in an ependymal tumor seems to be the expression of the neuroepithelial cells, which usually are lost during normal maturation (2, 3).
Melanin has a high affinity and binding capacity for metal ions. Natural melanin contains a wide variety of bound metals in vivo, which indicates that melanin may have cytoprotective function as an intracellular scavenger of free metals such as iron, copper, manganese, and zinc (6). Melanomas and some other melanin containing tumors appear hyperintense on T1-weighted images and hypointense on T2-weighted images. Some authors hypothesize that binding of paramagnetic metals is responsible for this characteristic MR imaging appearance (6). Enochs et al (6) stated that substantial increases in the signal intensity on T1-weighted images and substantial decreases in the signal intensity on T2-weighted images were obtained in their experimental study with increasing concentrations of melanin.
In previously reported cases, MR imaging features of melanotic ependymoma have not been discussed. In our case, the posterior fossa mass was heterogeneously isohyperintense on T1-weighted images and heterogeneously hypohyperintense on T2-weighted images. On MR examination, the signal intensity of the leptomeninges of the cerebrum, cerebellum, brain stem, and spinal cord were high on T1-weighted images and low on T2-weighted images. The hypointense rim that is produced by hemosiderin accumulations coating the cerebellum, brain stem, cranial nerves, optic chiasm, cerebral hemispheres, and spinal cord on T2-weighted images is also a characteristic MR feature of superficial siderosis (7). On T1-weighted images, however, pial structures do not appear hyerintense in superficial siderosis. The best clue for the differential diagnosis of superficial siderosis and pial melanosis is based on hyperintensity of melanin accumulated on the pial surfaces on T1-weighted images.
CSF findings may also help differentiate siderosis from melanosis by demonstrating the presence of hemorrhage, erythrophages, or siderophages with raised iron and ferritin levels in superficial siderosis and by showing Masson-Fontana staining pigmented cells in pial melanosis. We could not investigate CSF for melanin because of the large tumoral mass in the posterior fossa. In our case, a black and dark brown mass and diffuse black pigmentation of the leptomeninges were identified intraoperatively. The macroscopic appearance of the tumor was a reminiscent of metastatic malignant melanoma. During surgery, no hemorrhage was evident in the tumor or in CSF. Histopathologic examination revealed the typical appearance of ependymoma with true rosettes, pseudorosettes, and perivascular orientation (Fig 3A). These tumor cells had abundant brown pigment. The brown pigment did not stain with Prussian blue, and thus the possibility of hemosiderin deposition was excluded. The brown pigment showed prominent positivity when stained with Masson-Fontana, which is a characteristic of melanin that was scattered throughout the ependymoma and in the interstitial spaces of the tumor.
Because of the MR appearance, surgical observations, and histopathologic examinations of tumor and the interstitial spaces, we decided that melanin was accumulated throughout the leptomeninges secondary to melanin release from the melanotic ependymoma of the posterior fossa to CSF and the pial surfaces.
In their study, Baumgarten et al (8) injected a labeled synthetic melanin into the left lateral ventricle of adult rats that were followed up by autoradiography and transmission electron microscopy. Shortly after injection, melanin particles were seen ingested by supraependymal and epiplexus cells, cells residing in the pia-arachnoid, and subependymally located microglia-like cells ( 8). This experimental study shows that melanin existing in CSF can accumulate on the pial surfaces. Lawrence et al ( 9) have previously reported leptomeningeal melanin accumulation in humans in the pia of the ventral surface of the pons, medulla, cerebellum, upper cervical spinal cord, and lumbosacral cord. On MR examination, pial melanin accumulations were recognized with high signal intensity on T1-weighted images and low signal intensity on T2-weighted images, which reflects the paramagnetic properties of the mature melanin (9). From this point of view, in our case, melanotic ependymoma seems to be the underlying cause of superficial melanosis. We concluded that melanin was released from the melanotic ependymoma of the posterior fossa to the interstitial area and to CSF and, over time, accumulated throughout the pial surfaces.
Conclusion
Melanotic ependymoma is a rare tumor, with only a few cases reported in the literature. In this study, we saw for the first time that these uncommon tumors can release melanin into subarachnoid spaces where melanin accumulates throughout the leptomeninges. Superficial pial melanosis should be considered in the differential diagnosis of superficial siderosis, in cases of pigmented intracranial primary neoplasms other than meningeal melanoma. MR imaging has an undeniable role in the diagnosis of pial melanosis and is very useful in differentiating from other diseases such as superficial siderosis by demonstrating prominent high signal intensity of melanin pigment on T1-weighted images.
References
- 1.Chan AC, Ho LC, Yip WW, Cheung FC. Pigmented ependymoma with lipofuscin and neuromelanin production. Arch Pathol Lab Med 2003;127:872–875 [DOI] [PubMed] [Google Scholar]
- 2.Kakkar N, Vasishta RK, Banerjee AK. Pathology teach and tell: melanotic ependymoma. Pediatr Pathol Mol Med 2003;22:171–174 [DOI] [PubMed] [Google Scholar]
- 3.McCloskey JJ, Parker JC Jr, Brooks WH, Blacker HM. Melanin as a component of cerebral gliomas: the melanotic cerebral ependymoma. Cancer 1976;37:2373–2379 [DOI] [PubMed] [Google Scholar]
- 4.Rosenblum MK, Erlandson RA, Aleksic SN, Budzilovich GN. Melanotic ependymoma and subependymoma. Am J Surg Pathol 1990;14:729–736 [DOI] [PubMed] [Google Scholar]
- 5.Panyathanya R, Chantarakul N. Melanotic ependymoma with distant metastases. J Med Assoc Thai 1982;65:454–458 [PubMed] [Google Scholar]
- 6.Enochs WS, Petherick P, Bogdanova A, et al. Paramagnetic metal scavenging by melanin: MR imaging. Radiology 1997;204:417–423 [DOI] [PubMed] [Google Scholar]
- 7.Salem A, Krainik A, Helias A, et al. MRI findings in a case of a superficial siderosis associated with an ependymoma. J Neuroradiol 2002;29:136–138 [PubMed] [Google Scholar]
- 8.Baumgarten HG, Moritz F, Schlossberger HG. Accumulation of 14c-5,6-dihydroxytryptamine-melanin in intrathecal and subependymal phagocytes of the rat CNS and possible routes of their elimination from brain. Prog Clin Biol Res 1981;59A:187–196 [PubMed] [Google Scholar]
- 9.Poe LB, Roitberg D, Galyon DD. Neurocutaneous melanosis presenting as an intradural mass of the cervical canal: magnetic resonance features and the presence of melanin as a clue to diagnosis: case report. Neurosurgery 1994;35:741–774 [DOI] [PubMed] [Google Scholar]