Abstract
Summary: Benign triton tumors (neuromuscular hamartomas) are rare neoplasms composed of well-differentiated striated muscle fibers admixed with peripheral nerve fibers. To the best of our knowledge, this is the third case reported in the world literature of a benign triton tumor involving cranial nerve V (trigeminal nerve) and the first in the radiology literature. The previous reports of this lesion have focused on its unusual pathology and not on the imaging findings. In light of the imaging appearance of this lesion, we consider that the imaging findings may suggest this uncommon diagnosis.
Primary tumors of the trigeminal nerve are uncommon and are usually schwannomas or meningiomas. This case report documents a rare triton tumor originating from the trigeminal nerve. Our review of the literature revealed very few reports of this rare lesion, which has relatively unusual imaging and histopathologic findings.
Case Report
After a motor vehicle accident, a 15-year-old male patient underwent noncontrast CT of the head that showed an incidental right middle cranial fossa mass. The patient had asymptomatic facial asymmetry due to decreased bulk of the right masticator muscles and weakness of the right masseter muscle, noted on careful examination following the CT and MR imaging examination. The unenhanced CT demonstrated a well-circumscribed hyperattenuated right parasellar mass centered in Meckel’s cave (Fig 1). The mass caused scalloping of the anterior surface of the right petrous apex, which suggests a long-standing process (Fig 1). The mass extended inferiorly and smoothly expanded the foramen ovale (Fig 2). No other lesions were noted. Head MR imaging without and that with contrast revealed a well-circumscribed mass centered in Meckel’s cave that measured 18.8 × 14.9 × 13.9 mm in anteroposterior, transverse, and craniocaudal dimensions, respectively. Intracranially, the mass appeared to extend to the root entry zone of the right trigeminal nerve at the pons, with a cystic component along the right anterolateral aspect of the pons. The mass was centered in Meckel’s cave and extended into the foramen ovale along the V3 division. Denervation atrophy of the muscles of mastication was noted. The lesion was homogeneously hypointense relative to the brain on the precontrast T1 images and profoundly hypointense on T2-weighted images. The solid component in Meckel’s cave demonstrated mild fairly homogeneous enhancement after the administration of gadolinium, whereas the portion of the lesion in the prepontine cistern and the cystic portion did not enhance (Figs 3–7). A diagnosis of atypical schwanomma of the right V3 nerve was suggested.
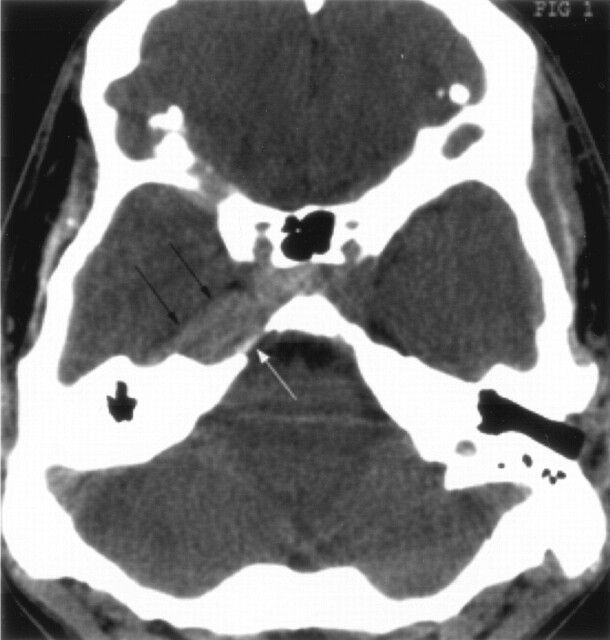
Fig 1.
Unenhanced axial CT scan shows a hyperattenuated mass in the right parasellar region and Meckel’s cave (double arrows). Remodeling of the petrous apex is also noted (single white arrow).
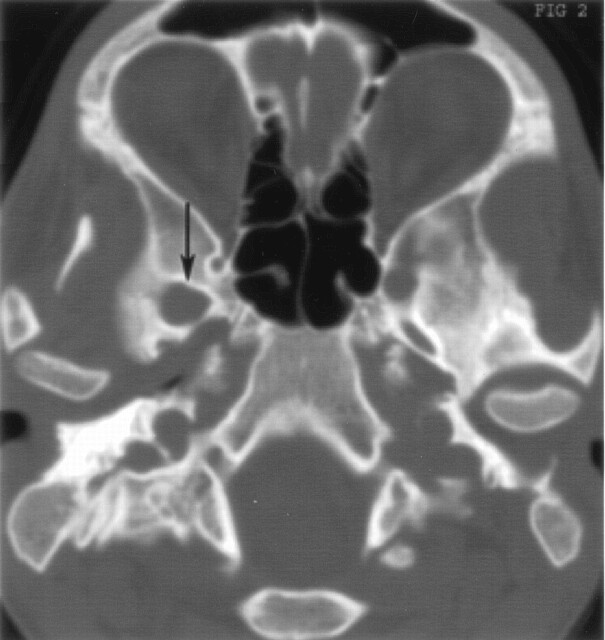
Fig 2.
Bone windows on axial CT scan show a smoothly enlarged right foramen ovale (arrow).
Fig 3.
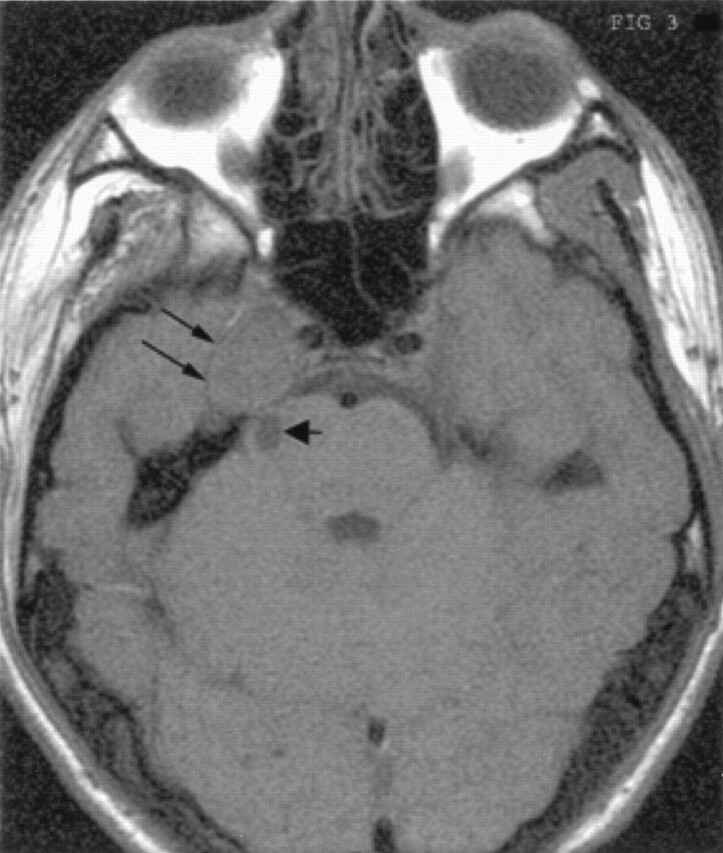
Unenhanced T1-weighted axial MR image shows the mass in the right parasellar and right Meckels cave region (double arrows). Also seen is the medially located cystic component (short broad arrow).
Fig 7.
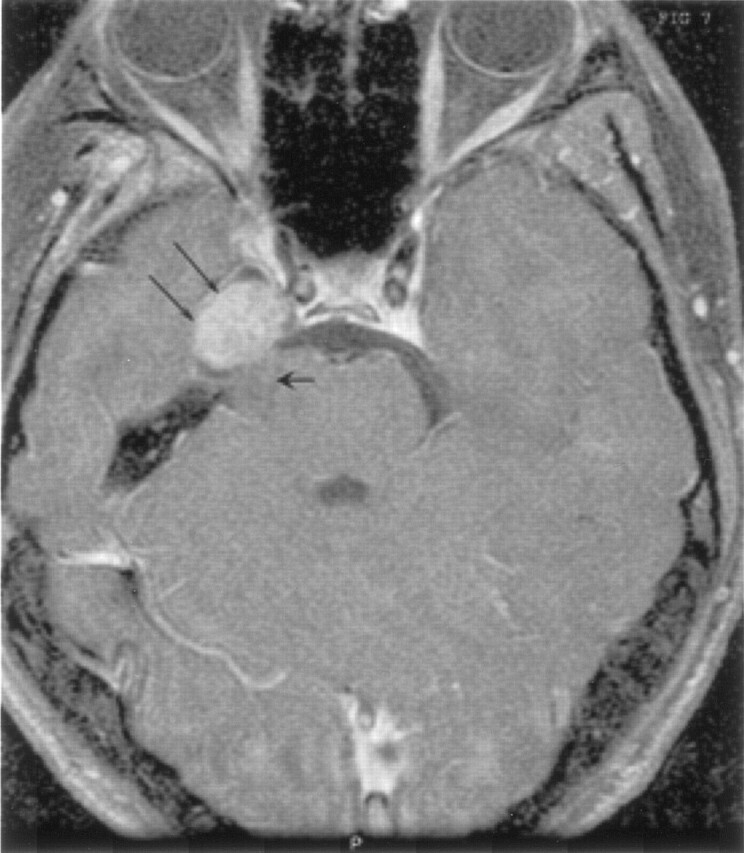
Axial postcontrast T1-weighted image shows intense enhancement of the right parasellar mass (double arrows), with the nonenhancing posterior fossa solid component (single broad arrow) adjacent to the trigeminal root entry zone.
During surgery a strictly intradural hard pink soft tissue mass was found to be firmly attached to the trigeminal nerve, mainly to the V3 branch. The nerve had to be resected with the tumor. The postoperative course was uneventful. The facial asymmetry and mild masseter weakness was unchanged. Postoperatively, the patient developed paresthesias in the right V1 and V2 distribution and the corneal reflex was lost in the right eye. Follow-up MR imaging examination at 6 months and again at 1 year showed thin postoperative dural enhancement in Meckel’s cave and no evidence of recurrent or residual tumor. The cystic component seen by use of preoperative MR imaging was not seen and was considered to have spontaneously resolved. The patient continues to be followed up.
Histopathologic analysis revealed a lesion composed of disorderly proliferation of well-differentiated skeletal muscle. The skeletal muscle fibers varied in size and were haphazardly distributed within connective tissue. The muscle fibers were strap shaped, with multiple subplasmalemmal nuclei and cross striations, and resembled normal skeletal muscle cells. No cytologic atypia was present, and mitoses were absent. Although nerve fibers were not readily apparent on light microscopic examination of hematoxylin-eosin–stained sections, Bielschowsky silver impregnation stains for axons highlighted nerve fibers lying among skeletal muscle fibers. S-100 protein reactivity was seen in accompanying Schwann cells. Trichrome stain showed connective tissue outlining neuromuscular bundles. These microscopic findings, with consideration of location of the lesion, were diagnostic of neuromuscular hamartoma or benign triton tumor.
Discussion
The trigeminal nerve is the largest cranial nerve and has sensory and motor functions. Tumors originating from the trigeminal nerve are uncommon and are usually schwannomas or meningiomas rarely associated with neurofibromatosis type II. Neuromuscular hamartomas are rare tumors of the peripheral nerve characterized by the presence of mature neural tissue and well-differentiated striated muscular elements. Involvement of the cranial nerves by this lesion is exceptional. To the best of our knowledge, this is the third case of benign triton tumor in the trigeminal nerve reported in the world literature. This tumor has also been reported earlier in the 7th cranial nerve. Similar tumors arising from the nerve containing a mature smooth muscle component are referred to as choristomas and have been reported as a separate entity.
Neuromuscular hamartomas can present in different age groups. However, in all previously published case reports, cranial nerves have been involved in younger patients (1–6). All cases described in the literature presented with progressive cranial nerve dysfunction and mass effect, depending upon the site of involvement. Our patient was asymptomatic, but clinical examination revealed facial asymmetry and weakness in the right masticator muscles.
Radiologically, the tumor was reported to be hyperattenuated on unenhanced CT (1). A previous case report (2) did not describe the CT findings. The MR imaging characteristics of the tumor reported by Zwick et al (2) were not mentioned, but the MR images included in the article demonstrated a hypointense lesion on T1-weighted images. Another previous case report (3) did not contain a detailed description of imaging findings, although the images included with the report demonstrated an avidly enhancing mass in the left parasellar region with an enlarged foramen rotundum. A subsequent case report (4) described a tumor with intracranial and extracranial components with extensive bony destruction and low T1 and intermediate T2 characteristics with patchy internal enhancement. This lesion was described as a neuromuscular hamartoma with fibromatosis and was more destructive in nature. The case report by Tiffee et al (5) again did not describe the imaging characteristics, but the description of the radiologic extent and surgical pathology suggested a more extensive and aggressive lesion.
In our patient, the lesion was hypointense on T1-weighted images and profoundly hypointense on the T2-weighted images and demonstrated mild, fairly homogeneous enhancement after the administration of gadolinium. A cystic component was identified in the anterior lateral right pons near the trigeminal root entry zone. This cystic component was assumed to represent a tumor associated cyst, which was shown to have resolved on follow-up examination.
An earlier case report (1) described an arachnoid cyst associated with the posterior fossa component of the trigeminal triton tumor. Although initially diagnosed as an atypical schwanomma, in retrospect it did not appear as such on the basis of imaging findings. On nonenhanced CT scans, schwannomas tend to be iso- or slightly hypoattenuated relative to adjacent brain. On MR images, most schwannomas are slightly T1 hypointense relative to brain parenchyma. Thirty percent are T1 isointense. Most schwannomas are hyperintense relative to brain on proton density–and T2-weighted images. Foci of cystic degeneration are common in larger lesions. Nearly all schwannomas demonstrate intense enhancement after the administration of contrast medium. Apart from the trigeminal nerve, neuromuscular hamartoma has also been described in the intracranial facial nerve (6).
Apart from schwannomas, neurofibromas and neuromuscular hamartomas, other uncommon tumors that have been described in the trigeminal nerve include lipomas (7), hemangiopericytomas (8), gangliogliomas (9), and ganglioneuromas (10).
Conclusion
To our knowledge, this is the third case report of a benign triton tumor or neuromuscular hamartoma of the trigeminal nerve described in the literature and the first report in the radiology literature. MR imaging may help to distinguish this lesion from other primary tumors involving the trigeminal nerve. In our case, the neuroimaging appearance of this tumor differs significantly from that expected of a schwanomma or a meningioma of the trigeminal nerve. Although this lesion is very rare, benign triton tumor should be considered in the differential diagnosis of tumors with atypical characteristics affecting the 5th cranial nerve particularly in young patients.
Fig 4.
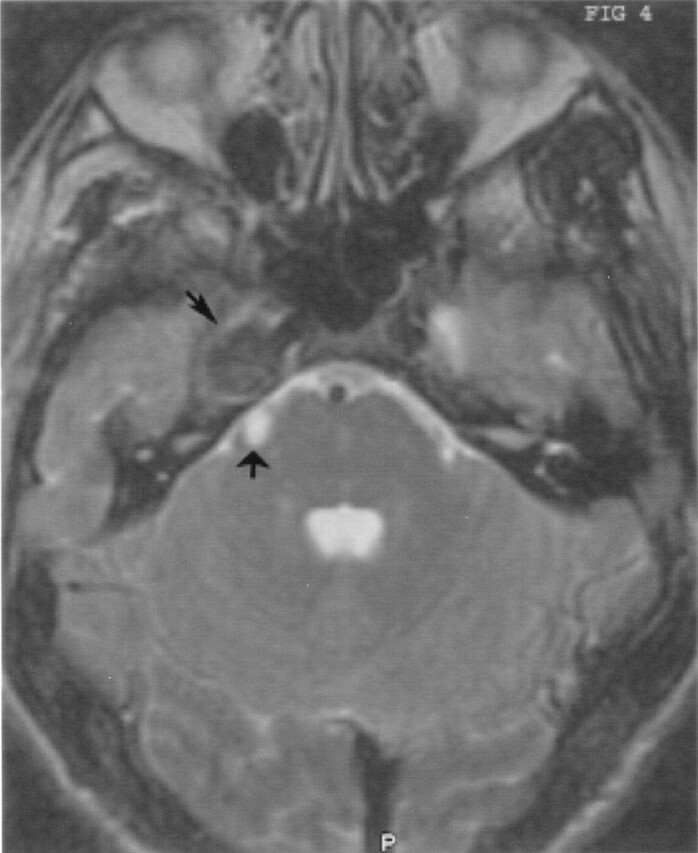
Axial T2-weighted image demonstrates the markedly hypointense parasellar mass (small arrow) and the very hyperintense cystic component in the prepontine cistern (broad arrow).
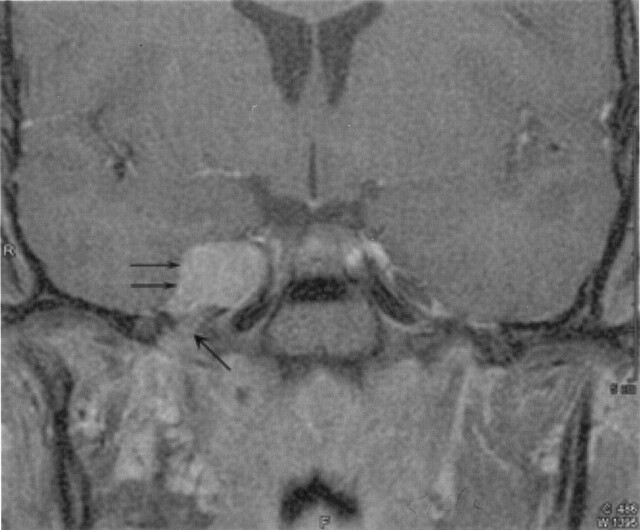
Fig 5.
Coronal postcontrast T1-weighted image shows intense enhancement of the right parasellar mass (double arrows), with the enhancement extending into the foramen ovale (single arrow).
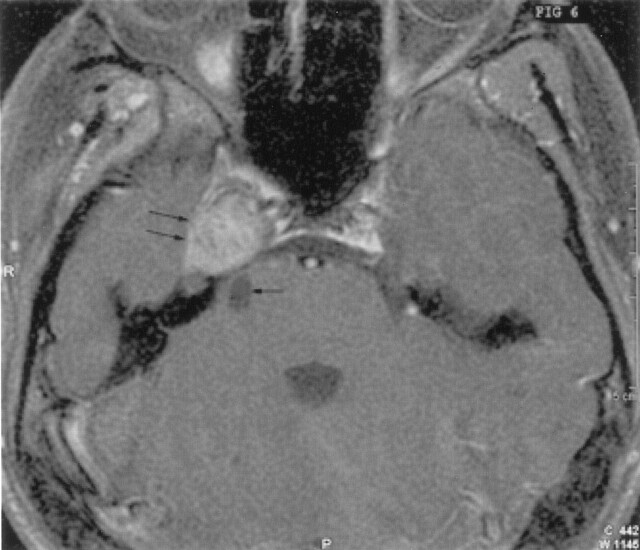
Fig 6.
Axial postcontrast T1-weighted image shows intense enhancement of the right parasellar mass (double arrows), with the nonenhancing posterior fossa cystic abnormality (single arrow).
References
- 1.Vajramani G, Devi I, Santosh V, et al. Benign triton tumor of the trigeminal nerve. Childs Nerv Syst 1999;15:140–144 [DOI] [PubMed] [Google Scholar]
- 2.Zwick DL, Livingston K, Clapp L, et al. Intracranial trigeminal nerve rhabdomyoma/choristoma in a child: a case report and discussion of possible histogenesis. Hum Pathol 1989;20:390–392 [DOI] [PubMed] [Google Scholar]
- 3.Leaa G, Dufour T, Gambrelli D, et al. Choristoma of the intracranial maxillary nerve in a child: case report. J Neurosurg 1994;81:788–791 [DOI] [PubMed] [Google Scholar]
- 4.Oeppen RS, Harden SP, Argent JD. Neuromuscular hamartoma: imaging features of a rare paediatric craniofacial tumour. Pediatr Radiol 2002;33:50–2 [DOI] [PubMed] [Google Scholar]
- 5.Tiffee JC, Barnes EL. Neuromuscular hamartomas of the head and neck. Arch Otolaryngol Head Neck Surg 1998;124:212–216 [DOI] [PubMed] [Google Scholar]
- 6.Vandewalle G, Brucher JM, Michotte A. Intracranial facial nerve rhabdomyoma: case report. J Neurosurg 1995;83:919–922. [DOI] [PubMed] [Google Scholar]
- 7.Yuh WT, Barloon TJ, Jacoby CG. Trigeminal nerve lipoma: MR findings. J Comput Assist Tomogr 1987;11:518–521 [DOI] [PubMed] [Google Scholar]
- 8.Tan I, Soo MY, Ng T. Haemangiopericytoma of the trigeminal nerve. Australas Radiol 2001;45:350–353 [DOI] [PubMed] [Google Scholar]
- 9.Athale S, Hallet KK, Jinkins JR. Ganglioglioma of the trigeminal nerve: MRI. Neuroradiology 1999;41:576–578 [DOI] [PubMed] [Google Scholar]
- 10.Abe T, Asano T, Manabe T, et al. Trigeminal ganglioneuroma. Brain Tumor Pathol 1999;16:49–53 [DOI] [PubMed] [Google Scholar]