Abstract
BACKGROUND AND PURPOSE: The association of high-grade oligodendrogliomas with tumor contrast material enhancement on MR images has been reported. Some authors have even used contrast enhancement as a criterion for their oligodendroglioma grading system. The purpose of our study was to evaluate if tumor contrast enhancement is a specific finding for anaplastic oligodendroglioma.
METHODS: Pretreatment MR images of 24 oligodendrogliomas were reviewed retrospectively, and findings were compared with the histologic grade. The presence or absence and the pattern of tumor contrast enhancement were evaluated qualitatively. A contrast enhancement ratio (CER), a quantitative criterion, was calculated to assess the difference in degree of enhancement between the low-grade and anaplastic tumors. Tumor grade was diagnosed at pathologic examination according to the World Health Organization classification system.
RESULTS: Contrast enhancement was noted in nine (56%) of 16 low-grade tumors and in five (62%) of eight anaplastic tumors. A characteristic enhancement pattern, nodular-like enhancement, was found in eight tumors. The CERs were 2.12–40.88 (mean, 20.08) in low-grade tumors and were 3.20–62.52 (mean, 28.73) in anaplastic tumors (P > .05).
CONCLUSION: Tumor contrast enhancement was not statistically significantly different between the tumor groups. We believe that the presence or absence of tumor contrast enhancement is not a specific finding for simply discriminating low-grade from anaplastic oligodendrogliomas. Histologic confirmation is necessary even in tumors without contrast enhancement.
Oligodendrogliomas are uncommon primary intracranial tumors, but may be the second most common glioma in adults after glioblastoma multiforme (1). According to the World Health Organization (WHO) classification, they are divided into two groups: low grade (WHO II) and high-grade or anaplastic (WHO III) (1, 2). Some reports have demonstrated the association of anaplastic oligodendrogliomas with tumor contrast material enhancement on CT or MR images (3–6). Furthermore, some authors even use contrast enhancement as a grading system criterion that designates two grades: absence of endothelial hyperplasia and absence of contrast enhancement indicates low grade, and presence of endothelial hyperplasia and/or presence of contrast enhancement indicates high grade (7). In our practice, however, MR imaging frequently shows nonenhancing anaplastic oligodendrogliomas (WHO III). We therefore hypothesized that the presence of tumor contrast enhancement is not a specific finding for anaplastic oligodendrogliomas. To verify this hypothesis, we retrospectively reviewed the MR images in patients with oligodendrogliomas and compared the tumor contrast enhancement on the preoperative MR images with tumor histologic grade according to the WHO classification.
Methods
The study population consisted of 23 consecutive patients (12 male and 11 female patients; age range, 15–72 years; mean age, 38.8 years) with 24 oligodendrogliomas who underwent pretreatment MR imaging at the University of Iowa from 1993 to 2002.
MR imaging was performed at 1.5 or 1.0 T. T1-weighted spin-echo (SE) images were obtained with TR of 466–600 ms, TE of 14–20 ms, and imaging matrix of 192 × 256. T2-weighted fast SE images were obtained with TR of 2000–5266 ms, TE of 80–105 ms, and imaging matrix of 256–512 × 160–308. Approximately 10 minutes after injection of gadopentetate dimeglumine (0.1 mmol/kg of body weight, Magnevist; Princeton, NJ), postcontrast T1-weighted SE images were obtained in the same plane as used for the nonenhanced images. All sequences had the following parameters: section thickness, 5.0 mm; section gap, 2 or 2.5 mm; and field of view, 180 × 240 mm.
All 24 tumors were confirmed at pathologic examination by either resection (n=19) or biopsy (n=5). For histologic observations, formalin-fixed paraffin-embedded sections were stained with hematoxylin-eosin. These sections were reviewed by a board-certified neuropathologist (P.K.) without knowledge of the MR findings. Sixteen oligodendrogliomas were low-grade and eight were anaplastic according to the WHO classification system (Table 1). Low-grade oligodendrogliomas (WHO II) have even cellularity and little nuclear pleomorphism. There is no necrosis, endothelial proliferation, or mitotic activity. They may show microcystic areas. Anaplastic oligodendrogliomas (WHO III) are defined by increased cellular density and pleomorphism with endothelial proliferation, vascular proliferation, mitotic activity, and necrosis (1, 2).
TABLE 1:
Histologic classification of oligodendrogliomas
| Type of Specimen | Tumor Grade |
Total | |
|---|---|---|---|
| Low-grade | Anaplastic | ||
| Biopsy | 4 | 1 | 5 |
| Resection | 12 | 7 | 19 |
| Total | 16 | 8 | 24 |
The MR images of these patients were evaluated in conference by two radiologists (M.L.W. and Y.Z.) with knowledge of the diagnosis of oligodendrogliomas but without knowledge of the histologic grade. The radiologists reached a consensus regarding the presence or absence and the pattern of tumor contrast enhancement. For the pattern of tumor enhancement, homogeneous or inhomogeneous enhancement, complete or partial enhancement, and the presence or absence of nodular-like enhancement were evaluated. To minimize the visual misjudgment in evaluating tumor enhancement, the precontrast and postcontrast images were windowed and leveled identically. Also, we tried varying windows and levels in reviewing the images to obtain the best qualitative evaluation.
In 12 oligodendrogliomas (six low-grade and six anaplastic) for which MR images were available digitally, contrast enhancement ratios (CERs) were calculated to evaluate the difference in the degree of enhancement between the low-grade and anaplastic tumors. The calculation formula used was (S -S0) × 100/S0, where S is the average signal intensity of the region of interest that shows the greatest enhancement visually on the postcontrast T1-weighted SE image. In tumors without visible contrast enhancement, a region of interest was placed on a solid part of the tumor. S0 is the average signal intensity of the region of interest obtained on the precontrast T1-weighted SE image. Three tumors with digital images were not included in calculating the CER, because they were confirmed to be low-grade oligodendrogliomas only by biopsy. Without a complete histologic evaluation in these three tumors, we could not exclude the possible presence of a high-grade component.
The paired t test was used to asses associations between the CERs and histologic grades. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated by using MR contrast enhancement for discriminating anaplastic oligodendrogliomas from low-grade oligodendrogliomas.
The MR images were also analyzed with regard to tumor size (the largest diameter), signal intensity heterogeneity (inhomogeneous signal intensity on T1- and/or T2-weighted images) of tumor, and definition of tumor margin (sharp or fuzzy). The tumor margin was defined as the outermost boundary of the tumoral area demonstrating high signal intensity on T2-weighted fast SE images.
All the features of MR imaging mentioned above were compared with the histologic grade of the tumors. Presence or absence of increased neovascularity was evaluated at pathologic examination and compared with tumor enhancement. Given the possibility of sampling error in biopsies, the four low-grade oligodendrogliomas proved only with biopsy were not included in the comparison between tumor enhancement and histologic grade. Because resected specimens were not cut in the exact same plane as those of MR images, comparison of pathologic descriptions by the pathologist with image findings on a site-to-site basis could not be obtained.
Results
MR contrast enhancement of tumor was noted in 14 (58%) of 24 tumors. Of 12 low-grade tumors proved by resection, six (50%) showed contrast enhancement. Of eight anaplastic tumors, five (62%) showed contrast enhancement (Table 2). Another four low-grade tumors, in which three showed contrast enhancement, were proved only by biopsy and were not included in the comparison between the enhancement and the tumor grade. MR contrast enhancement for descrimating anaplastic oligodendrogliomas from low-grade oligodendrogliomas resulted in a sensitivity of 63%, specificity of 50%, positive predictive value of 45%, and a negative predictive value of 67%.
TABLE 2:
Contrast enhancement in oligodendrogliomas
| Tumor Grade | Enhancement* | No Enhancement |
|---|---|---|
| Anaplastic (n = 8) | 5† (3) | 3 |
| Resected low-grade (n = 12) | 6 (1) | 6‡ |
Numbers in parentheses are the number of tumors with increased neovascularity.
Sensitivity is 63%.
Specificity is 50%.
The contrast enhancement pattern of the tumors on postcontrast T1-weighted SE images revealed inhomogeneous and total tumor enhancement in five, and partial tumor enhancement in nine. In six resected low-grade tumors that were enhanced, one showed inhomogeneous and total tumor enhancement and five demonstrated partial enhancement. In five enhanced anaplastic tumors, two showed inhomogeneous and total tumor enhancement and three demonstrated partial enhancement.
Eight of the 14 enhanced tumors had nodular-like enhancement. In comparison with tumor histologic grade, the nodular-like enhancement was found in three of six enhanced low-grade tumors proved by resection and four of five enhanced anaplastic tumors. The remaining tumor with the nodular-like enhancement was a low-grade tumor proved only by biopsy. In three of four anaplastic tumors with nodular-like enhancement, histologic examination revealed that there were similar nodular-like areas showing higher cell density, neovascularity, and necrosis (Fig 1). In another anaplastic tumor with nodular-like enhancement, histologic study showed an uncertain nodular-like area with variable cellularity. However, all these histologic findings were not compared with nodular-like enhancement on a site-to-site basis. In the three low-grade tumors with nodular-like enhancement, no definite nodular-like areas with higher cell density, neovascularity, and/or necrosis were found at histologic examination.
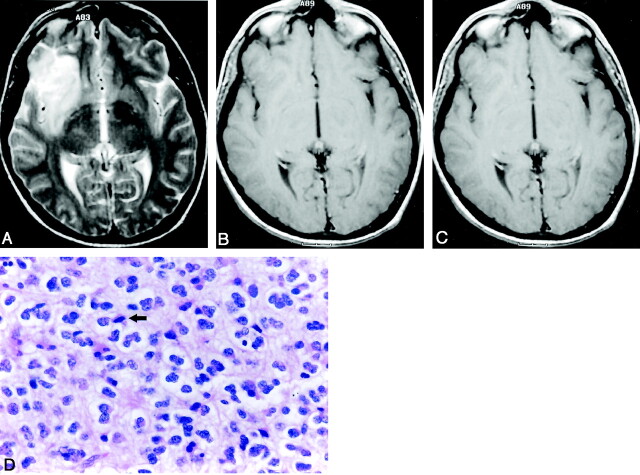
Fig 1.
Anaplastic oligodendroglioma obtained in a 28-year-old man.
A, Axial T2-weighted (3588/99 TR/TE) fast SE image shows a high-signal-intensity mass in the left frontal lobe.
B, Axial T1-weighted (540/14) image shows that the mass has low signal intensity.
C, Axial contrast-enhanced T1-weighted (540/14) image shows nodular-like enhancement within the mass.
D, Photomicrograph of the specimen (hematoxylin-eosin stain; original magnification, x25) shows a nodular area that has the high-grade features consisting of high cellularity and nuclear hyperchromasia; however, this is not a specifically sampled area of the nodular-like enhancement on the T1-weighted image. Note the area of necrosis (arrows).
E, Photomicrograph of the specimen (hematoxylin-eosin stain; original magnification, x25) shows the area that has the relatively low-grade features, such as even cellularity and little nuclear pleomorphism. No necrosis is present.
As to degree of contrast enhancement, the mean CER was 20.08 in six resected low-grade tumors and 28.73 in six anaplastic tumors (P > .05) (Table 3). The CER of one tumor was only 2.12. Histologic examination revealed it to have even cellularity and little nuclear pleomorphism. No mitosis was found. All these were consistent with histologic findings of low-grade oligodendroglioma. However, in the other two tumors with similar low CERs (3.20 and 5.82, respectively), histologic examination showed findings of anaplastic oligodendroglioma, such as increased cellular density, pleomorphism, mitosis, and so forth (Fig 2). However, in one tumor with a higher CER of 40.88, no malignant finding was noted at histologic examination (Fig 3).
TABLE 3:
CERs for 12 oligodendrogliomas
| Low-Grade (n = 6) | Anaplastic (n=6) |
|---|---|
| 2.12 | 3.20 |
| 17.24 | 5.82 |
| 17.77 | 15.86 |
| 17.87 | 38.35* |
| 24.58 | 46.63† |
| 40.88 | 62.52* |
| 20.08‡ | 28.73‡ |
Tumors with increased vascularity.
This tumor was proved only by biopsy; all others were proved by resection.
Mean CER of each tumor group.
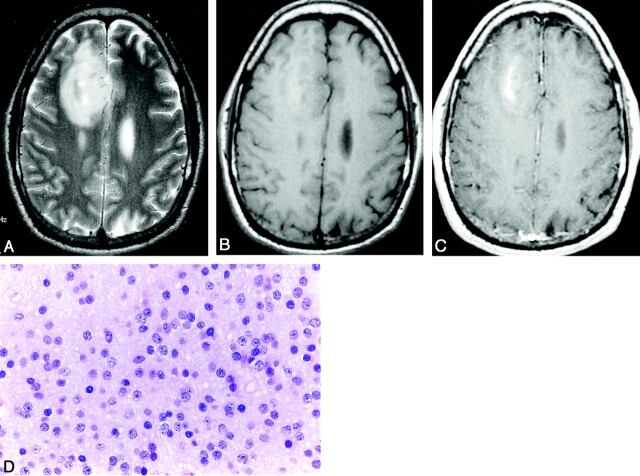
Fig 2.
Anaplastic oligodendroglioma in a 47-year-old woman.
A, Axial T2-weighted (3200/96) fast SE image shows a high-signal-intensity mass that mainly involves the right frontal lobe and insula.
B, Axial T1-weighted (583/20) image shows that the mass is isointense to slightly hypointense.
C, Axial contrast-enhanced T1-weighted (583/20) image does not show obvious tumor contrast enhancement. The CER is 5.82.
D, Photomicrograph of the specimen (hematoxylin-eosin stain; original magnification x40) shows high cellularity, nuclear pleomorphism and hyperchromasia, and mitosis (arrow). There is no increased neovascularity.
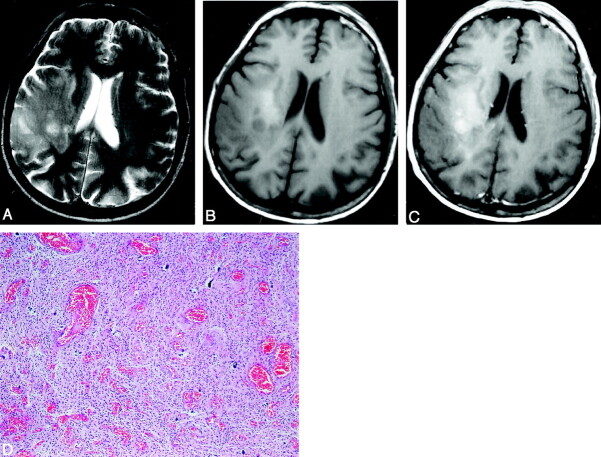
Fig 3.
Low-grade oligodendroglioma in a 42-year-old man.
A, Axial T2-weighted (4750/105) fast SE image shows a high-signal-intensity mass in the right frontal lobe.
B, Axial T1-weighted (466/14) image shows that the mass is isointense to slightly hyperintense.
C, Axial contrast-enhanced T1-weighted (466/14) image shows partial tumor contrast enhancement. The contrast enhancement ratio is 40.88.
D, Photomicrograph of the specimen (hematoxylin-eosin stain; original magnification, x40) shows even cellularity and little nuclear pleomorphism and hyperchromasia. No mitosis or necrosis is present. There is no increased neovascularity.
In 12 low-grade tumors proved by resection, only one tumor that partially enhanced on MR images had increased neovascularity at histologic examination. In this tumor, the CER was not calculated since a digital MR study was not available. In five anaplastic tumors with contrast enhancement on MR images, three tumors had increased neovascularity (Table 2; Fig 4). The other three anaplastic tumors that did not enhance on MR images showed no increased neovascularity. When comparing the neovascularity with the measured CERs, we found that two anaplastic tumors with increased neovascularity had the higher CERs (38.35 and 62.52, respectively). In another anaplastic tumor with increased neovascularity, digital images were not available. An anaplastic tumor in which the CER was 46.63 showed no increased neovascularity, but it was proved only by biopsy (Table 3).
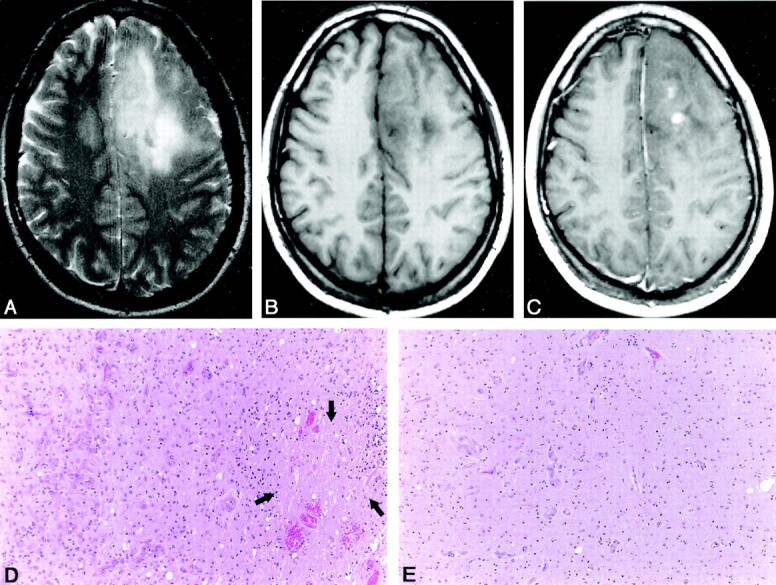
Fig 4.
Anaplastic oligodendroglioma in a 59-year-old man.
A, Axial T2-weighted (3986/99) fast SE image shows a high-signal-intensity mass in the right temporal and frontal lobes.
B, Axial T1-weighted (600/14) image shows that the mass is isointense to slightly hyperintense.
C, Axial contrast-enhanced T1-weighted (600/14) image shows the inhomogeneous and partial tumor contrast enhancement with a CER up to 62.52. There are nodular-like enhancement areas within the tumor.
D, Photomicrograph of the specimen (hematoxylin-eosin stain; original magnification, x100) shows an area with high cellularity and increased neovascularity.
The 12 resected low-grade tumors ranged from 0.7 to 7.5 cm (mean, 5.1 cm) and the eight anaplastic tumors from 2.0 to 9.0 cm (mean, 6.28 cm) in maximum diameter. With regard to signal intensity heterogeneity of tumor, six of the 12 resected low-grade tumors were inhomogeneous on either T1-weighted SE images or T2-weighted fast SE images. Among the eight anaplastic tumors, five and seven were inhomogeneous on T1-weighted SE images and T2-weighted fast SE images, respectively.
On T2-weighted fast SE images, the tumor margins were relatively sharp in 11 of 12 low-grade and five of eight anaplastic tumors, and the margins were fuzzy in one low-grade and three anaplastic tumors.
Discussion
Treatment of oligodendrogliomas typically has been surgical resection followed by radiation therapy (8, 9), but recent studies have found that anaplastic oligodendrogliomas are chemosensitive tumors. Alkylating agent chemotherapy has been found to be an adjuvant treatment of choice for anaplastic oligodendrogliomas (5, 10). Therefore, grading these tumors correctly is critical for guiding therapy.
The MR contrast enhancement of oligodendrogliomas has been thought to correlate with anaplastic oligodendrogliomas (3–6). A pathoradiologic study using the WHO grading system revealed that all anaplastic oligodendrogliomas showed contrast enhancement on images (3). Furthermore, in a new grading system based on morphology and imaging, the presence of contrast enhancement is even used as a criterion for discriminating anaplastic oligodendrogliomas from low-grade oligodendrogliomas (7). These results could convince us that low-grade oligodendrogliomas tend not to enhance and anaplastic oligodendrogliomas should have obvious enhancement. In our study, however, six of 12 low-grade tumors showed contrast enhancement and three of eight anaplastic tumors had no visible contrast enhancement. These findings result in a positive predictive value of only 45% for MR contrast enhancement indicating that a tumor is an anaplastic oligodendroglioma. Moreover, the mean CERs in both tumor groups were not statistically significantly different. The low CER values of less than 10.0 were found not only in low-grade tumors but also in anaplastic tumors. One low-grade tumor also showed a CER value of more than 40.
All four tumors that had increased neovascularity showed contrast enhancement on MR images. This is not unexpected because gadolinium enhancement is directly related to tumor neovascularity (11–13). Lack of obvious contrast enhancement in the anaplastic oligodendrogliomas could be attributed to a low degree of neovascularity. However, five low-grade tumors with contrast enhancement had no increased neovascularity at histologic study. Apart from the tumor neovascularity, therefore, it is presumed that another histologic feature like the degree of disruption of the blood-brain barrier might be related to tumor contrast enhancement. One anaplastic tumor with a high CER (46.63) showed no neovascularity; however, it was proved only by biopsy and a complete histologic evaluation could not be performed.
With regard to the enhancement rate of anaplastic oligodendrogliomas, our result is different from that in the study by Reiche et al (3). The latter study showed that all eight anaplastic oligodendrogliomas enhanced on MR images. However, this finding of a 100% enhancement rate of the anaplastic oligodendrogliomas might only be a contingency that occurred because of the small number of cases.
One characteristic finding seen in the pattern of oligodendroglioma enhancement was nodular-like enhancement. Seven of 11 enhanced oligodendrogliomas showed this finding in our study. Based on the small number of patients in this study, it was difficult to form any statistical conclusion about the difference in showing nodular-like enhancement between the low-grade and anaplastic tumors. However, in contrast with three of six enhanced low-grade tumors having nodular-like enhancement, four of five enhanced anaplastic tumors revealed this finding. Nodular enhancement has been thought to be the most important predictor of malignancy in astrocytomas (13–15). Lee et al (16) also found seven cases of nodular enhancement in 15 mixed oligodendrogliomas.
At pathologic examination, nodular-like enhancement might correlate with the presence of nodular foci with distinct higher cell density, neovascularity, and endothelial hyperplasia (11). Comparison of pathologic evaluation with areas of nodular-like enhancement was not performed on a site-to-site basis in the current study, and the underlying pathologic processes in nodular-like enhancement could not be elucidated accurately. However, histologic evaluation in three of four anaplastic tumors with nodular-like enhancement did show that there were similar nodular-like areas with higher cell density, vascularity, and necrosis. Interestingly, in the three low-grade tumors with nodular-like enhancement, nodular regions were not identified at pathologic examination. This is probably because cellularity and vascularity had not increased enough to give the pathologic nodular pattern in the low-grade tumors. Sampling error could be another reason, but these three low-grade tumors were totally resected, not only biopsied, which would allow for a good pathologic sampling.
Considering the close correlation between neovascularity and malignancy, the areas of nodular-like enhancement should be included in histologic analysis if possible. The result in the series by Daumas-Duport et al (7, 11) suggested that neovascularity is a crucial event in the progression of oligodendrogliomas toward more aggressive behavior. Therefore, areas of nodular-like enhancement within low-grade oligodendrogliomas could be the early presage of anaplastic change.
No significant difference was noted in tumor size between low-grade and anaplastic oligodendrogliomas. Tumors of varying sizes were found in both groups. The presence of tumor heterogeneity usually supports the diagnosis of high-grade astrocytoma (15). In this study, we did find that seven of eight anaplastic oligodendrogliomas showed inhomogeneous signal intensity, but half (six of 12) of low-grade oligodendrogliomas also had inhomogeneous signal intensity. Owing to the overlap in the two groups, we do not think that signal intensity heterogeneity could be used as a strong factor in determining the malignancy grade of oligodendrogliomas.
In our study, we defined the tumor margin as the outermost boundary of the tumoral area demonstrating high signal intensity on T2-weighted fast SE images, since there is infrequently peritumoral edema in oligodendrogliomas (14). Also, supporting this approach is that microscopic tumor infiltration generally extends at least as far as the signal intensity change on T2-weighted SE images (17). Therefore, we believe that it is clinically important to define the outermost boundary of abnormal high signal intensity as the radiologic tumor margin.
Extensive edema is thought to be an important predictor of high-grade brain tumors (4, 15). However, oligodendrogliomas are not typically associated with vasogenic edema. Edema may not be a sensitive marker in grading oligodendrogliomas.
In our comparison of definition of tumor margin between low grade and anaplastic oligodendrogliomas, a fuzzy margin was found in only one of 11 low-grade tumors but in three of eight anaplastic tumors. Because of the presence of overlap, definition of tumor margin may not be used as a predictor of malignancy, but could indirectly reflect the degree of tumor infiltration and should be evaluated in diagnosing and treating oligodendroglioma.
In this study, no information about therapy, clinical follow-up, and survival rate was traced because of the inclusion of recent cases. We therefore could not compare the presence or absence of contrast enhancement with prognosis in oligodendrogliomas, as was done by Daumas-Duport et al (7, 11). This is one limitation of the current study. To conclusively clarify the significance of tumor enhancement in grading oligodendrogliomas, a further study about the relation between contrast enhancement on MR images and survival rate is necessary. Another limitation is that subtle enhancement in some cases could be visually missed in the qualitative study.
Perfusion MR imaging with the creation of relative cerebral blood volume (rCBV) maps results in the qualitative and quantitative assessment of glial tumor vascularity. Elevated rCBV could be a more sensitive marker for high-grade histopathologic finding (18). Further study of rCBV analysis of oligodendrogliomas is needed.
Conclusion
We compared MR contrast enhancement with malignancy grade in oligodendrogliomas and found no significant difference in contrast enhancement between the low-grade and anaplastic tumors. Nodular-like enhancement within tumor may be a characteristic finding for oligodendrogliomas. Further study, which should focus on interpreting the histologic meaning of nodular-like enhancement and comparing this finding with malignancy grade, is necessary. Our results showed no specific finding from MR imaging for simply discriminating low-grade from anaplastic oligodendrogliomas, and pathologic sampling is necessary to grade oligodendrogliomas accurately by using the WHO grading system.
Footnotes
Presented as a scientific paper at the 42nd annual meeting of the American Society of Neuroradiology, Seattle, WA, June 5–11, 2004.
References
- 1.Giannini C, Scheithauer BW, Weaver AL, et al. Oligodendrogliomas: reproducibility and prognostic value of histologic diagnosis and grading. J Neuropathol Exp Neurol 2001;60:248–262 [DOI] [PubMed] [Google Scholar]
- 2.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 2002;61:215–225 [DOI] [PubMed] [Google Scholar]
- 3.Reiche W, Grunwald I, Hermann K, Deinzer M, Reith W. Oligodendrogliomas. Acta Radiol 2002;43:474–482 [PubMed] [Google Scholar]
- 4.Margain D, Peretti-Viton P, Perez-Castillo AM, Martini P, Salamon G. Oligodendrogliomas. J Neuroradiol 1991;18:153–160 [PubMed] [Google Scholar]
- 5.Lee YY, Van Tassel P. Intracranial oligodendrogliomas: imaging findings in 35 untreated cases. AJR Am J Roentgenol 1989;152:361–369 [DOI] [PubMed] [Google Scholar]
- 6.Fortin D, Cairncross GJ, Hammond RR. Oligodendroglioma: an appraisal of recent data pertaining to diagnosis and treatment. Neurosurgery 1999;45:1279–1291 [DOI] [PubMed] [Google Scholar]
- 7.Daumas-Duport C, Tucker ML, Kolles H, et al. Oligodendrogliomas, II: a new grading system based on morphological and imaging criteria. J Neurooncol 1997;34:61–78 [DOI] [PubMed] [Google Scholar]
- 8.Walker DG, Kaye AH. Diagnosis and management of astrocytomas, oligodendrogliomas and mixed gliomas: a review. Australas Radiol 2001;45:472–482 [DOI] [PubMed] [Google Scholar]
- 9.Leonardi MA, Lumenta CB. Oligodendrogliomas in the CT/MR-era. Acta Neurochir 2001;143:1195–1203 [DOI] [PubMed] [Google Scholar]
- 10.Cairncross JG, Macdonald DR, Ramsay DA. Aggressive oligodendroglioma: a chemosensitive tumor. Neurosurgery 1992;31:78–82 [DOI] [PubMed] [Google Scholar]
- 11.Daumas-Duport C, Varlet P, Tucker ML, Beuvon F, Cervera P, Chodkiewicz JP. Oligodendrogliomas, I: patterns of growth, histological diagnosis, clinical and imaging correlations—a study of 153 cases. J Neurooncol 1997;34:37–59 [DOI] [PubMed] [Google Scholar]
- 12.Zagzag D, Goldenberg M, Brem S. Angiogenesis and blood-brain barrier breakdown modulate CT contrast enhancement: an experimental study in a rabbit brain-tumor model. AJR Am J Roentgenol 1989;153:141–146 [DOI] [PubMed] [Google Scholar]
- 13.Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 1996;27:65–73 [DOI] [PubMed] [Google Scholar]
- 14.Osborn AG. Diagnostic Neuroradiology. St Louis: Mosby-Year Book;1994. :529–578
- 15.Castillo M. Contrast enhancement in primary tumors of the brain and spinal cord. Neuroimaging Clin N Am 1994;4:63–80 [PubMed] [Google Scholar]
- 16.Lee C, Duncan VW, Young AB. Magnetic resonance features of the enigmatic oligodendroglioma. Invest Radiol 1998;33:222–231 [DOI] [PubMed] [Google Scholar]
- 17.Essig M, Hawighorst H, Schoenberg SO, et al. Fast fluid-attenuated inversion-recovery (FLAIR) MRI in the assessment of intraaxial brain tumors. J Magn Reson Imaging 1998;8:789–798 [DOI] [PubMed] [Google Scholar]
- 18.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol 2004;25:214–221 [PMC free article] [PubMed] [Google Scholar]