Abstract
BACKGROUND AND PURPOSE: Relative cerebral blood volume (rCBV) measurements derived from perfusion-weighted imaging (PWI) may be useful to evaluate angiogenesis and preoperatively estimate the grade of a glioma. We hypothesized that rCBV is correlated with vascular endothelial growth factor (VEGF) expression as marker of the angiogenic stimulus in presumed supratentorial low-grade gliomas (LGGs).
METHODS: From February 2001 to February 2004, we examined 20 adults (16 men, four women; mean age 36 years; range, 23–60 years) with suspected (nonenhancing) supratentorial LGG on conventional MR imaging. Preoperative MR imaging used a dynamic first-pass gadolinium-enhanced, spin-echo echo-planar PWI. In heterogeneous tumors, we performed stereotactic biopsy in the high-perfusion areas before surgical resection. Semiquantitative grading of VEGF immunoreactivity was applied.
RESULTS: Nine patients had diffuse astrocytomas (World Health Organization grade II), and 11 had other LGG and anaplastic gliomas. In patients with heterogeneous tumors on PWI, the high-rCBV focus had areas of oligodendroglioma or anaplastic astrocytoma on stereotactic biopsy, whereas the surgical specimens were predominantly astrocytomas. Anaplastic gliomas had high rCBV ratios and positive VEGF immunoreactivity. Diffuse astrocytomas had negative VEGF expression and mean rCBV values significantly lower than those of the other two groups. Three diffuse astrocytomas had positive VEGF immunoreactivity and high rCBV values.
CONCLUSION: Our results confirmed the correlation among rCBV measurements, VEGF expression, and histopathologic grade in nonenhancing gliomas. PWI may add useful data to the preoperative assessment of nonenhancing gliomas. Its contribution in predicting tumor behavior and patient prognosis remains to be determined.
Microvascular proliferation is one of the most important criteria for determining the malignancy of gliomas (1). Several authors have demonstrated the relevance of assessing tumoral angiogenesis in diagnosing gliomas and in treatment planning and follow-up (2–7). Although tumor enhancement on conventional gadolinium-based MR imaging has been an important criterion in preoperatively predicting the malignancy of gliomas, contrast enhancement itself reflects only disruption of the blood-brain barrier and not tumor angiogenesis. According to the literature, 20% of low-grade gliomas (LGG) enhance after the administration of Gd-based contrast agent, but approximately one-third of nonenhancing gliomas are malignant (8, 9). For this reason, tumor enhancement is not accurately predictive of the grade of the glioma, and the diagnosis of LGG cannot be based on conventional MR imaging alone, particularly in the case of nonenhancing tumors.
Some authors have suggested that relative cerebral blood volume (rCBV) measurements derived from perfusion-weighted imaging (PWI) can be useful tools to evaluate tumor angiogenesis and to preoperatively estimate the grade of tumor, as they add diagnostic information not available with conventional MR studies (10–13). In addition, rCBV measurements can potentially reduce the sampling error in the histopathologic diagnosis of gliomas, improving the selection of targets for stereotactic biopsy (10–17).
Several growth factors are responsible for the angiogenic process, particularly vascular endothelial growth factor (VEGF) (18). Expression of VEGF has been demonstrated in a wide variety of malignant tumors, including gliomas (19–21). High-grade gliomas have marked microvascular proliferation, and VEGF expression is thought to be prognostic in astrocytic tumors (19).
The correlation between PWI and VEGF has been described. Many authors reported the application of this technique to monitor treatment with anti-VEGF compounds (22–27). Our aim was to evaluate the correlation between rCBV and VEGF to obtain quantitative measurements that could improve preoperative examination and offer potential parameters for use in the follow-up assessment of nonenhancing gliomas.
Methods
Our institutional review board approved the study protocol. From February 2001 to February 2004, we enrolled 20 adults (16 men, four women; mean age, 36 years; range, 23–60 years) with suspected supratentorial nonenhancing LGG, as shown on conventional MR imaging. All provided informed consent.
The patients were examined by using 1.5-T equipment with echo-planar capability. Only a single bolus of contrast material was administered for each examination. Eleven axial section levels (5 mm thick, 2-mm gap) were chosen for echo-planar imaging; this selection was based on the extent of the lesion estimated on conventional MR images. Axial T2-weighted, spin-echo echo-planar images were acquired by using the following parameters: TR/TE=1700/80, frequency R/L, 128 × 128 matrix, 28-cm FOV. Intravenous administration of 0.3 mmol/kg of a Gd-based contrast agent at a rate of 5-mL/second followed by a 20-mL saline flush was accomplished by using an MR-compatible power injector.
All dynamic MR images were transferred via Ethernet to a workstation (Sun Ultra 10; Sun Microsystems, Santa Clara, CA) and evaluated with ADW 4.0 software (GE Medical Systems, Milwaukee, WI). Knopp et al (12) previously describe the details of data processing. At least six rCBV measurements were obtained in different regions of interest within the lesion.
In homogeneous lesions, mean and maximum rCBV values were recorded to analyze the overall degree of tumor vascularity. The maximum obtained value was the method that provided the best interobserver and intraobserver reproducibility, according to previous studies (16, 28). Cerebral blood volume must be expressed relative to an internal reference, such as the normal contralateral white matter. We referred to these values as rCBV. Tumors with homogeneous rCBV maps (low or high ratios compared with those of normal white matter) were surgically removed. In tumors with heterogeneous rCBV maps and focal areas of high perfusion, mean and maximum rCBV measurements were recorded separately, and the higher values of both were considered for statistical analysis. We performed stereotactic biopsy in the focus with highest perfusion before surgical resection, by using a Micromar Stereotactic Planning System software (29). This was done to better evaluate the possible correlation between rCBV and VEGF expression. This method of multimodality image fusion and treatment planning in stereotactic conditions was classified as semiautomatic, with three dimensions, and uses rigid-body transformation with referential markers defined in both modalities to accomplish CT and MR image fusion. The registration errors were smaller than the voxel size on the primary images (29). Despite the potential limitations of the stereotactic biopsy (30, 31) and the possible geometric distortion of fused color maps with a low-resolution matrix (128 × 128), this method allowed us to obtain a sample of the high-perfusion focus to be compared with the residual tumor that has been removed by craniotomy after biopsy.
One neuropathologist (J.N.S.), who was blinded to the MR imaging results, analyzed all pathology slides. Surgical specimens were fixed in 10% buffered formaldehyde in preparation for light microscopy and stained with hematoxylin-eosin. The histopathologic diagnosis was based on the World Health Organization (WHO) classification (1).
A semiquantitative grading of VEGF immunoreactivity was applied, as Chaudhry et al (32) described. Deparaffinized sections were immunohistochemically stained by using the avidin-biotin-peroxidase complex and monoclonal antibody against VEGF (clone A20, 1:30; Santa Cruz Biotechnology, Santa Cruz, CA). Stains were graded from 0 to 3 according to extent and intensity of staining: Grade 0 indicated no staining; grade 1, focal weak staining; grade 2, focal strong staining; and grade 3, widespread strong staining. We further simplified the grading scale to distinguish strong immunostaining from equivocal weak or no staining: Grades 0 and 1 were classified as negative (VEGF absent or focal weak staining of uncertain significance), whereas grades 2 and 3 were grouped as positive, indicating that VEGF was present. This grading scale was adapted from that of Abdulrauf et al (19).
To analyze the rCBV values and VEGF expression observed in different gliomas, we classified the tumors into three groups: diffuse astrocytomas (WHO grade II); oligodendrogliomas and oligoastrocytomas, grouped as other LGG; and anaplastic gliomas (anaplastic astrocytomas or oligoastrocytomas). To assess the relationship among rCBV ratio, VEGF expression, and histopathologic diagnosis, we analyzed rCBV ratios in the three groups by using the Kruskal-Wallis test and evaluated VEGF expression by using the Fisher exact test. We compared rCBV ratios and VEGF expression by using the Mann-Whitney U test. P value <.05 indicated a statistically significant difference. Statistical analysis was performed by using a SPSS statistical software package (SPSS, Chicago, IL).
Results
Nine patients had diffuse astrocytomas (WHO grade II), and the remaining 11 had different diagnoses: four LGGs (one oligodendroglioma and three oligoastrocytomas) and seven anaplastic gliomas (one anaplastic oligoastrocytoma and six anaplastic astrocytomas).
Table 1 summarizes our results. All anaplastic gliomas had positive VEGF immunoreactivity and high rCBV ratios compared with those of normal contralateral white matter (rCBV > 1.0) (Fig 1). Both rCBV (P=.0006, Kruskal-Wallis test) and VEGF expression (P=.02, Fisher exact test) were significantly higher in anaplastic gliomas than in diffuse astrocytomas. rCBV values were significantly correlated VEGF immunoreactivity (P = .0007, Mann-Whitney U test).
rCBV ratios, histologic results, and VEGF expression
| Patient/Sex/Age (y) | Histology | rCBV* | VEGF |
|---|---|---|---|
| WHO grade II | |||
| 1/M/30 | Diffuse astrocytoma | 0.65/0.77 | − |
| 2/M/35 | Diffuse astrocytoma | 0.55/0.72 | − |
| 3/F/33 | Diffuse astrocytoma | 0.57/0.7 | − |
| 4/M/27 | Diffuse astrocytoma | 0.69/0.81 | − |
| 5/M/41 | Diffuse astrocytoma | 0.62/0.65 | − |
| 6/M/39 | Diffuse astrocytoma | 1.73/2.1 | + |
| 7/M/38 | Diffuse astrocytoma | 1.07/1.2 | + |
| 8/F/22 | Diffuse astrocytoma | 1.61/1.9 | + |
| 9/M/32 | Diffuse astrocytoma | 0.68/0.82 | − |
| 10/M/39 | Oligodendroglioma | 1.27/1.62 | − |
| 11/M/26 | Oligoastrocytoma | 1.72/1.88 | − |
| 12/F/37 | Oligoastrocytoma | 1.59/1.97 | + |
| 13/M/27 | Oligoastrocytoma | 2.64/2.99 | + |
| WHO grade III | |||
| 14/M/42 | Anaplastic astrocytoma | 2.78/3.11 | + |
| 15/M/30 | Anaplastic astrocytoma | 2.89/3.08 | + |
| 16/M/58 | Anaplastic astrocytoma | 3.45/3.6 | + |
| 17/F/55 | Anaplastic astrocytoma | 3.79/3.9 | + |
| 18/M/25 | Anaplastic astrocytoma | 3.22/3.7 | + |
| 19/M/56 | Anaplastic astrocytoma | 3.30/3.8 | + |
| 20/M/25 | Anaplastic oligoastrocytoma | 2.99/3.19 | + |
Mean/maximum.
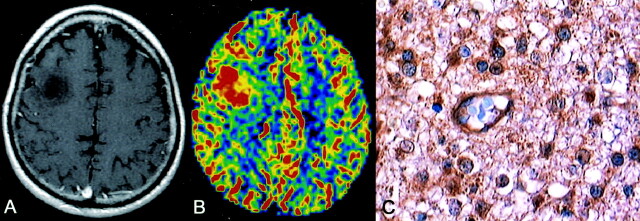
Fig 1.
Anaplastic astrocytoma. Axial enhanced T1–weighted image demonstrates a right frontal, nonenhancing lesion (A). rCBV map shows a markedly elevated rCBV of 3.6, which is consistent with biopsy-proved anaplastic astrocytoma (B). Immunohistochemical staining (400×) with monoclonal antibody against VEGF reveals strong cellular and endothelial positivity (C).
Although tumors with low rCBV ratios (rCBV < 1.0) and negative VEGF expression were invariably diffuse astrocytomas (Fig 2), three diffuse astrocytomas (in patients 7–9) showed positive VEGF immunoreactivity. All of these also had rCBV values higher than those of normal contralateral white matter (rCBV > 1.0) (Fig 3).
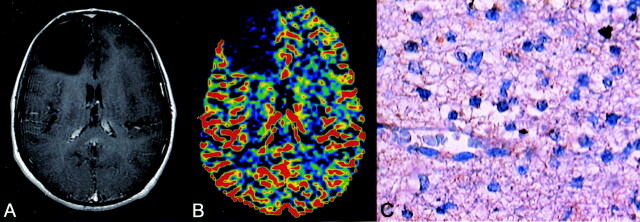
Fig 2.
Diffuse astrocytoma. Axial enhanced T2-weighted image shows a left frontal nonenhancing lesion (A). rCBV map shows an obviously reduced rCBV of 0.72, which is consistent with biopsy-proved diffuse astrocytoma (WHO grade II). Immunohistochemical staining (400×) with the monoclonal antibody against VEGF is negative (C).
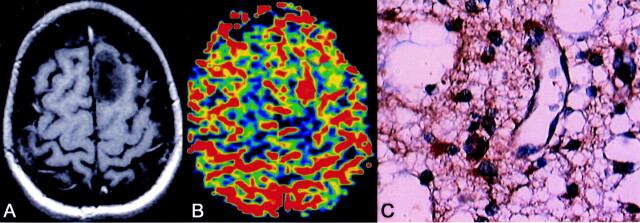
Fig 3.
Diffuse astrocytoma. Axial enhanced T1-weighted image shows a left frontal nonenhancing lesion (A). rCBV map shows an elevated rCBV of 1.9 (B). Histologic section (400×) reveals a diffuse astrocytoma (WHO grade II), but VEGF staining shows strong cellular and endothelial positivity (C).
Six patients had heterogeneous rCBV maps. The samples obtained during stereotactic biopsy of the highest rCBV focus allowed for the histologic diagnosis of two oligoastrocytomas and four anaplastic gliomas, whereas their surgical resection samples proved to be predominantly diffuse astrocytomas at histopathologic analysis (Fig 4). In these patients, expression patterns were also correlated with malignancy, as all anaplastic gliomas had positive immunoreactivity for VEGF.
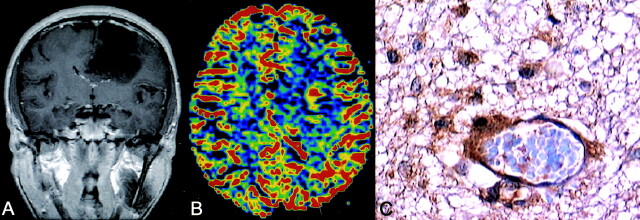
Fig 4.
Anaplastic astrocytoma. Coronal enhanced T1-weighted image shows a heterogeneous left frontal lesion (A). rCBV map demonstrates a focal area with a high rCBV of 3.11. Histologic section (400X) of this focus is consistent with biopsy-proved anaplastic astrocytoma, and VEGF staining shows strong cellular and endothelial positivity (C).
Oligodendroglioma and oligoastrocytoma had rCBV ratios higher than those of diffuse astrocytomas, but significantly lower ratios than those of anaplastic tumors. Two oligoastrocytomas had positive immunoreactivity for VEGF.
Discussion
Gliomas constitute a wide range of heterogeneous tumors, which differ greatly in terms of their potential for growth, invasiveness, and tendency to progression (1). LGG account for 10–20% of the adult primary brain neoplasms and their clinical management remains controversial, particularly in young, asymptomatic patients with controlled epilepsy and tumors involving functionally eloquent brain areas (33–38). Accurate histopathologic diagnosis is crucial to define the best management and prognosis of these tumors. Despite the lack of evidence-based definitive recommendations to support the benefit of gross total resection in gliomas (39, 40), most neuro-oncologists believe that safe and feasible resection improves the outcomes for these patients (36, 37). In addition, gliomas are known to be heterogeneous tumors, and one of the main advantages of surgery is to avoid potential diagnostic error with biopsy samples (40). However, many gliomas are not amenable to gross total resection because of their infiltrative nature and their involvement of eloquent brain structures. In this case, stereotactic biopsy may be an option to confirm the diagnosis. In this context, nonenhancing gliomas remain a major diagnostic challenge. Despite recent advances in sequences and protocols, conventional MR imaging is unreliable in predicting the diagnosis and grade of brain gliomas (16), and it does not provide consistent morphologic criteria to select the target for stereotactic biopsy in patients with nonenhancing tumors.
Marked neovascularization is characteristic of high-grade gliomas (1). The degree of tumor vascularity and the extent of peritumoral vasogenic edema are correlated with much of the morbidity and mortality observed with malignant and certain benign CNS neoplasms. In the last 2 decades, some reports have focused on mechanisms of glioma vascularization, including growth factors, receptors, and possible mechanisms involved in tumor-associated angiogenesis and capillary permeability (18, 19, 32, 41, 42).
Angiogenesis is regulated by a number of both stimulating and inhibiting angiogenic factors (43). Some authors have identified many growth factors with chemotactic and mitogenic activity for endothelial cells (44). Among them, VEGF is thought to be a major regulator in the development of tumor angiogenesis (42, 45, 46). Some reports describe a positive correlation of VEGF levels and vascularity in gliomas (47–49).
MR imaging is widely accessible and is the best option for diagnosis and follow-up assessment of brain tumors (12, 33, 36, 37, 50). Furthermore, in the past few years, technical advances have allowed the use of MR imaging for noninvasive evaluation of angiogenesis.
Several previous studies described the rCBV ratios of gliomas (10–12, 51, 52), and although some authors reported a wide range of rCBV ratios that overlap among neoplasms of different grades, they observed notable differences between LGGs and high-grade gliomas, as we did. However, previous series included patients with heterogeneous tumors—both enhancing and nonenhancing—as well as important information regarding perienhancement edema, distant tumor foci, hemorrhage, necrosis, mass effect, which are helpful data for characterizing tumor aggressiveness and hence tumor grade on conventional MR imaging (16). Our patients had relatively homogeneous and nonenhancing tumors; therefore, high-grade features could not be accurately predicted on the basis of conventional MR imaging results.
We chose spin-echo echo-planar images because of their presumed increased sensitivity to microvascular changes (eg, capillary-level blood volume) (11, 53, 54). In fact, this is a somewhat contentious issue. Donahue et al (55) argue that spin-echo PWI is less accurate in predicting glioma grade than gradient-echo PWI. Sugahara et al (10) found that tumor rCBV obtained with the gradient-echo echo-planar technique was significantly higher than that obtained with the spin-echo echo-planar technique in the high-grade gliomas but not in LGGs. These findings have been related to the increased sensitivity of the gradient-echo echo-planar technique to large vessels, which can be clearly observed in high-grade gliomas. On the other hand, Lev et al (52) reported that spin-echo rCBV maps show fewer falsely elevated values related to larger cerebral vessels, draining veins, and shunt vascularity than do gradient-echo MR-PWI maps. Because the gradient-echo echo-planar technique is sensitive to susceptibility effects from total vascular beds, normal arteries and veins on the surface of the brain tissue and ventricles might be misinterpreted as tumor vascularity.
Correction for the leakage of contrast material was not performed because our patients had only nonenhancing lesions. Although contrast enhancement might not be visible, an element of leakage may be present in nonenhancing regions, and the T1 shortening effect from the extravascular space accumulated during the first pass could not be completely excluded. Finally, substantial methodologic variability may explain the lack of a consistent relationship between angiogenesis markers and prognosis in our study compared with previous series.
Despite the limitation of the sample size, we confirmed a positive correlation between increased tumoral perfusion (rCBV ratios) and VEGF expression in our patients. Diffuse astrocytomas usually show negative VEGF expression, as we observed. Furthermore, on PWI, all patients had rCBV values lower than those of normal contralateral white matter. One hypothesis to explain the absence of vascular proliferation in VEGF-expressing WHO grade II astrocytomas is that, though VEGF is upregulated, specific receptors on endothelium are not expressed (18). This would prevent tumor neovascularization induced by VEGF. Another possibility is that additional signals are required to trigger angiogenesis in these neoplasms (18). However, three of our patients with low-grade astrocytomas had positive VEGF expression. These patients also had rCBV values higher than those of normal contralateral white matter. Although the regulatory mechanisms involved in the upregulation of VEGF in LGG have not been elucidated, this finding may be of biologic relevance because VEGF expression may be correlated with the prognosis in diffuse, low-grade fibrillary astrocytomas. Abdulrauf et al (19) reported that VEGF positivity in low-grade astrocytomas is associated with increased progression to high-grade tumors and with a poor prognosis. Our relatively small sample size was essentially based on our intention of including only nonenhancing supratentorial tumor in adults. In addition, the limited follow-up precluded further statistical analysis of the association between VEGF expression and tumor progression or prognosis.
In our study, oligoastrocytomas and the only case of oligodendroglioma had rCBV ratios higher than those of diffuse astrocytomas; this was probably related to their increased microvascular density (56). Lev et al (52) reported different perfusion patterns in diffuse astrocytomas and oligodendrogliomas, probably in association with the fine capillary network typically observed in oligodendrogliomas. Some reports show conflicting results regarding VEGF expression in oligodendrogliomas. Although some authors (20, 57) did not find immunoreactivity for VEGF in oligodendrogliomas, Christov et al (21) showed immunohistochemical expression of VEGF in 31 of 34 oligodendrogliomas. In their study, as in ours, VEGF expression patterns were also correlated with malignancy, as our anaplastic oligoastrocytoma exhibited strong immunoreactivity for VEGF.
These technical differences include differences in markers to highlight vessels, counting methods (manual vs imaging analysis), quantification methods (average microvessel count per square millimeter vs highest microvessel distribution count per square millimeter), and microvascular distribution in various regions of the tumor. Our intention in using the semiquantitative method was to detect areas of highest vascularity, not as a prognostic marker but as a diagnostic tool for a possible high-grade tumor.
Conclusion
Our results confirmed the correlation of rCBV measurements, VEGF expression, and histopathologic grade in nonenhancing gliomas. PWI is a feasible supplementary sequence to conventional MR imaging that may add important and useful data for the preoperative assessment of nonenhancing gliomas. In this study, we were able to improve MR imaging analysis by adding noteworthy information, such as the VEGF finding as an immunohistochemical marker of angiogenesis. PWI may provide new approaches for functional studies and become a valuable method for noninvasive, in vivo monitoring of tumor growth and angiogenesis. Its contribution in predicting tumor behavior and patient prognosis remains to be determined.
References
- 1.Kleihues P, Cayenee W. Pathology and Genetics of Tumors of the Nervous System. Lyon: International Agency for Research on Cancer;2000
- 2.Wenz F, Rempp K, Hess T, et al. Effect of radiation on blood volume in low-grade astrocytomas and normal brain tissue: quantification with dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol 1996;166:187–193 [DOI] [PubMed] [Google Scholar]
- 3.Tomoi M, Maeda M, Yoshida M, et al. Assessment of radiotherapeutic effect on brain tumors by dynamic susceptibility contrast MR imaging: a preliminary report. Radiat Med 1999;17:195–199 [PubMed] [Google Scholar]
- 4.Pardo FS, Aronen HJ, Kennedy D, et al. Functional cerebral imaging in the evaluation and radiotherapeutic treatment planning of patients with malignant glioma. Int J Radiat Oncol Biol Phys 1994;30:663–669 [DOI] [PubMed] [Google Scholar]
- 5.Sugahara T, Korogi Y, Tomiguchi S, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 2000;21:901–909 [PMC free article] [PubMed] [Google Scholar]
- 6.Henry RG, Vigneron DB, Fischbein NJ, et al. Comparison of relative cerebral blood volume and proton spectroscopy in patients with treated gliomas. AJNR Am J Neuroradiol 2000;21:357–366 [PMC free article] [PubMed] [Google Scholar]
- 7.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 1996;77:362–372 [DOI] [PubMed] [Google Scholar]
- 8.Barker FG II, Chang SM, Huhn SL, et al. Age and the risk of anaplasia in magnetic resonance-nonenhancing supratentorial cerebral tumors. Cancer 1997;80:936–941 [PubMed] [Google Scholar]
- 9.Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002;59:947–949 [DOI] [PubMed] [Google Scholar]
- 10.Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 1998;171:1479–1486 [DOI] [PubMed] [Google Scholar]
- 11.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 1994;191:41–51 [DOI] [PubMed] [Google Scholar]
- 12.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 1999;211:791–798 [DOI] [PubMed] [Google Scholar]
- 13.Provenzale JM, Wang GR, Brenner T, Petrella JR, Sorensen AG. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol 2002;178:711–716 [DOI] [PubMed] [Google Scholar]
- 14.Cha S, Lu S, Johnson G, Knopp EA. Dynamic susceptibility contrast MR imaging: correlation of signal intensity changes with cerebral blood volume measurements. J Magn Reson Imaging 2000;11:114–119 [DOI] [PubMed] [Google Scholar]
- 15.Lam WW, Chan KW, Wong WL, Poon WS, Metreweli C. Pre-operative grading of intracranial glioma. Acta Radiol 2001;42:548–554 [DOI] [PubMed] [Google Scholar]
- 16.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003;24:1989–1998 [PMC free article] [PubMed] [Google Scholar]
- 17.Uematsu H, Maeda M, Sadato N, et al. Blood volume of gliomas determined by double-echo dynamic perfusion-weighted MR imaging: a preliminary study. AJNR Am J Neuroradiol 2001;22:1915–1919 [PMC free article] [PubMed] [Google Scholar]
- 18.Machein MR, Plate KH. VEGF in brain tumors. J Neurooncol 2000;50(1–2):109–120 [DOI] [PubMed] [Google Scholar]
- 19.Abdulrauf SI, Edvardsen K, Ho KL, Yang XY, Rock JP, Rosenblum ML. Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytoma. J Neurosurg 1998;88:513–520 [DOI] [PubMed] [Google Scholar]
- 20.Pietsch T, Valter MM, Wolf HK, et al. Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol (Berl) 1997;93:109–117 [DOI] [PubMed] [Google Scholar]
- 21.Christov C, Adle-Biassette H, Le Guerinel C, Natchev S, Gherardi RK. Immunohistochemical detection of vascular endothelial growth factor (VEGF) in the vasculature of oligodendrogliomas. Neuropathol Appl Neurobiol 1998;24:29–35 [DOI] [PubMed] [Google Scholar]
- 22.Pradel C, Siauve N, Bruneteau G, et al. Reduced capillary perfusion and permeability in human tumour xenografts treated with the VEGF signalling inhibitor ZD4190: an in vivo assessment using dynamic MR imaging and macromolecular contrast media. Magn Reson Imaging 2003;21:845–851 [DOI] [PubMed] [Google Scholar]
- 23.Checkley D, Tessier JJ, Kendrew J, Waterton JC, Wedge SR. Use of dynamic contrast-enhanced MRI to evaluate acute treatment with ZD6474, a VEGF signalling inhibitor, in PC-3 prostate tumours. Br J Cancer 2003;89:1889–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Checkley D, Tessier JJ, Wedge SR, et al. Dynamic contrast-enhanced MRI of vascular changes induced by the VEGF-signaling inhibitor ZD4190 in human tumour xenografts. Magn Reson Imaging 2003;21:475–482 [DOI] [PubMed] [Google Scholar]
- 25.Jayson GC, Zweit J, Jackson A, et al. Molecular imaging and biological evaluation of HuMV833 anti-VEGF antibody: implications for trial design of antiangiogenic antibodies. J Natl Cancer Inst 2002;94:1484–1493 [DOI] [PubMed] [Google Scholar]
- 26.Pham CD, Roberts TP, van Bruggen N, et al. Magnetic resonance imaging detects suppression of tumor vascular permeability after administration of antibody to vascular endothelial growth factor. Cancer Invest 1998;16:225–230 [DOI] [PubMed] [Google Scholar]
- 27.Badruddoja MA, Krouwer HG, Rand SD, Rebro KJ, Pathak AP, Schmainda KM. Antiangiogenic effects of dexamethasone in 9L gliosarcoma assessed by MRI cerebral blood volume maps. Neuro-oncology 2003;5:235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 2002;224:797–803 [DOI] [PubMed] [Google Scholar]
- 29.Bouza A. Desenvolvimento de uma técnica para fusão de imagens para planejamento cirúrgico em condições estereotáxicas: Tese de Mestrado em Neurociências. São Paulo, Brazil: UNIFESP-EPM,1999
- 30.Gilles FH, Brown WD, Leviton A, et al. Limitations of the World Health Organization classification of childhood supratentorial astrocytic tumors: Children Brain Tumor Consortium. Cancer 2000;88:1477–1483 [PubMed] [Google Scholar]
- 31.Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro-oncology 2001;3:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhry IH, O’Donovan DG, Brenchley PE, Reid H, Roberts IS. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology 2001;39:409–415 [DOI] [PubMed] [Google Scholar]
- 33.Frapazz D, Chinot O, Bataillard A, et al. Summary version of standards, options and recomendations for the management of adult patients with intracranial glioma. Br J Cancer 2003;89:S73–S83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recht LD, Lew R, Smith TW. Suspected low-grade glioma: is deferring treatment safe? Ann Neurol 1992;31:431–436 [DOI] [PubMed] [Google Scholar]
- 35.Vecht CJ. Effect of age on treatment decisions in low-grade glioma. J Neurol Neurosurg Psychiatry 1993;56:1259–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeAngelis LM. Brain tumors. N Engl J Med 2001;344:114–123 [DOI] [PubMed] [Google Scholar]
- 37.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet 2003;361:323–331 [DOI] [PubMed] [Google Scholar]
- 38.Cairncross JG, Laperriere NJ. Low-grade glioma. To treat or not to treat? Arch Neurol 1989;46:1238–1239 [DOI] [PubMed] [Google Scholar]
- 39.Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg 2001;95:735–745 [DOI] [PubMed] [Google Scholar]
- 40.Sawaya R. Extent of resection in malignant gliomas: a critical summary. J Neurooncol 1999;42:303–305 [DOI] [PubMed] [Google Scholar]
- 41.Toi M, Matsumoto T, Bando H. Vascular endothelial growth factor: its prognostic, predictive, and therapeutic implications. Lancet Oncol 2001;2:667–673 [DOI] [PubMed] [Google Scholar]
- 42.Klagsbrun M, D’Amore PA. Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev 1996;7:259–270 [DOI] [PubMed] [Google Scholar]
- 43.Risau W. What, if anything, is an angiogenic factor? Cancer Metastasis Rev 1996;15:149–151 [DOI] [PubMed] [Google Scholar]
- 44.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353–364 [DOI] [PubMed] [Google Scholar]
- 45.Klagsbrun M, D’Amore PA. Regulators of angiogenesis. Ann Rev Physiol 1991;53:217–239 [DOI] [PubMed] [Google Scholar]
- 46.Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol 2000;50:121–137 [DOI] [PubMed] [Google Scholar]
- 47.Machein MR, Kullmer J, Fiebich BL, Plate KH, Warnke PC. Vascular endothelial growth factor expression, vascular volume, and, capillary permeability in human brain tumors. Neurosurgery 1999;44(4):732–740 [DOI] [PubMed] [Google Scholar]
- 48.Berkman RA, Merrill MJ, Reinhold WC, et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest 1993;91:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samoto K, Ikezaki K, Ono M, et al. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res 1995;55:1189–1193 [PubMed] [Google Scholar]
- 50.Bird CR, Drayer BP, Medina M, Rekate HL, Flom RA, Hodak JA. Gd-DTPA-enhanced MR imaging in pediatric patients after brain tumor resection. Radiology 1988;169:123–126 [DOI] [PubMed] [Google Scholar]
- 51.Lee SJ, Kim JH, Kim YM, et al. Perfusion MR imaging in gliomas: comparison with histologic tumor grade. Korean J Radiol 2001;2:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendroglimoas. AJNR Am J Neuroradiol 2004;25:214–221 [PMC free article] [PubMed] [Google Scholar]
- 53.Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 1995;34:555–566 [DOI] [PubMed] [Google Scholar]
- 54.Abu-Hajir M, Rand SD, Krouwer HG, Schmainda KM. Noninvasive assessment of neoplastic angiogenesis: the role of magnetic resonance imaging. Semin Thromb Hemost 2003;29:309–315 [DOI] [PubMed] [Google Scholar]
- 55.Donahue KM, Krouwer HG, Rand SD, et al. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med 2000;43:845–853 [DOI] [PubMed] [Google Scholar]
- 56.Chan AS, Leung SY, Wong MP, et al. Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol 1998;22:816–826 [DOI] [PubMed] [Google Scholar]
- 57.Nishikawa R, Cheng SY, Nagashima R, Huang HJ, Cavenee WK, Matsutani M. Expression of vascular endothelial growth factor in human brain tumors. Acta Neuropathol (Berl) 1998;96:453–462 [DOI] [PubMed] [Google Scholar]