Abstract
Summary: Susceptibility artifact from platinum coil packs impairs the visibility of perianeurysmal soft tissues at conventional 3D time-of-flight MR angiography. These artifacts limit the evaluation for residual-recurrent aneurysm and parent vessel stenosis. Reducing the echo time can decrease the artifact and improve perianeurysmal visualization. The purpose of this study was to assess quantitatively the effect of decreasing the echo time on artifact production at different field strengths and coil pack densities.
Intracranial aneurysms are common lesions, with a prevalence estimated between 0.4–6.0% (1). Treatment options include open surgical clipping, endovascular occlusion, or both, with detachable coils (2–5). Despite the lack of long-term data, recent data from the International Subarachnoid Trial have shown favorable outcomes for endovascular coiling versus clipping at 1 year in a selected population and are sure to increase the number of coiling procedures performed (6).
Studies have shown that patients treated with platinum coils can have recurrence at the aneurysm neck, even in cases of initial total occlusion (5, 7, 8). It is therefore necessary to follow up these patients for recurrence. Because of its excellent temporal and spatial resolution, digital subtraction angiography (DSA) has been the criterion-standard examination to assess the completeness of the initial treatment and the presence or absence of recurrence. DSA, however, is an invasive procedure that poses a small but definite risk of neurologic complication (9). Although helical CT angiography (CTA) is an effective technique to detect aneurysms (10), beam-hardening artifact related to the platinum coils significantly limits its usefulness in the post-coil setting.
Multiple reports have suggested that 3D time-of-flight (3D-TOF) MR angiography (MRA) and contrast-enhanced (CE) MRA may be comparable to DSA to assess for recurrent-residual aneurysm and parent vessel stenosis after aneurysm coiling (11–17). Platinum coils do, however, create varying degrees of susceptibility-induced artifact with both MRA techniques. Lower echo-time techniques have been employed to reduce susceptibility-induced signal intensity loss from the coil packs, thereby improving perianeurysmal visualization (18, 19).
Methods
Aneurysm Models
Two aneurysm models were constructed by using Word Bartholin gland catheters (Rusch, Duluth, GA). Using an 18-gauge needle, 5 mL of saline were injected into each catheter. This volume was predetermined to allow for coil deployment and enable creation of an aneurysm model that was approximately 10 × 10 × 10 mm3. An introducer was inserted until the tip was visualized in the model to prepare for coil delivery.
Coil Pack Density
Assuming an aneurysm model of 10 × 10 × 10 mm3, the aneurysm volume was calculated by using the following formula:
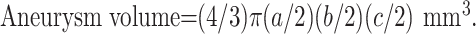 |
Using this aneurysm volume and the desired coil pack densities (CPDs) of 20% and 40%, the length of coils required could then be calculated by using a coil-specific formula provided by the vendor:
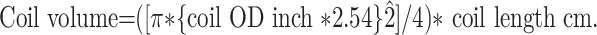 |
Given a 10 × 10 × 10 mm3 aneurysm model, the length of coil required was 145 cm for a 20% occlusion and 290 cm for a 40% occlusion.
Coil Preparation and Deployment
TruFill DCS detachable platinum coils (Cordis Endovascular Systems, Miami, FL) were cut at the site of detachment, leaving the entire coil length within the original microcatheter. These coils were loaded into the introducer and deployed into the models. Any air was aspirated from the models.
Once all the coils were in the distal end of the balloon, the balloon was twisted and tied off with a suture. Every attempt was made to maintain an aneurysm size of 10 × 10 × 10 mm3, but some fluid leaked, introducing some variability. The size of both models was manually measured in three orthogonal planes with an electrocardiogram caliper. By using these measurements and the known coil lengths deployed, we again used the formula to calculate our achieved CPD. Below are the actual coil lengths, aneurysm sizes, and achieved CPDs:
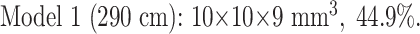 |
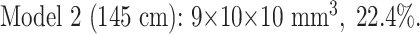 |
Both models were suspended in a gelatin mold to mimic intracranial placement.
Image Acquisition
MR angiographic studies were performed with a Siemens Sonata 1.5T systems (Erlangen, Germany) by using the standard receive-only head coil and a Siemens Trio 3T system by using the CP-head transmit-receive coil. Seven different 3D-TOF sequences and our standard True FISP CE MRA technique were performed at both field strengths (Table 1). Variables were incrementally changed to investigate coil pack artifact reduction. Variables included the echo time (TE), bandwidth (BW), acquired voxel dimension, and flow compensation. For all 3D-TOF experiments, the following parameters were kept constant: repetition time (TR), FOV, rectangular FOV aspect ratio, flip angle, section thickness, and phase-encoding direction.
TABLE 1:
| Constant 3D TOF Parameters | 1.5T |
3.0T |
|---|---|---|
| TR, 24; FOV, 172 × 230; % phase FOV, 75; FA, 25; ST, 1 | TR, 27 (except for sequence 1 which had a TR of 45); FOV, 172 × 230; % phase FOV, 75; FA, 25; ST, 1 | |
| Sequence 1 | TE, 7.20; BW, 105; acq mtx, 384 × 187; % samp, 65 | TE, 7.15; BW, 105; acq mtx, 384 × 216; % samp, 75 |
| Sequence 2 | TE, 7.20; BW, 105; acq mtx, 384 × 288; % samp, 100 | TE, 7.15; BW, 105; acq mtx, 384 × 288; % samp, 100 |
| Sequence 3 | TE, 3.52; BW, 105; acq mtx, 384 × 187; % samp, 65 | TE, 3.52; BW, 105; acq mtx, 384 × 216; % samp, 75 |
| Sequence 4 | TE, 2.75; BW, 250; acq mtx, 384 × 187; % samp, 65 | TE, 2.74; BW, 250; acq mtx, 384 × 216; % samp, 75 |
| Sequence 5 | TE, 2.72; BW, 450; acq mtx, 384 × 187; % samp, 65 | TE, 2.72; BW, 450; acq mtx, 384 × 216; % samp, 75 |
| Sequence 6 | TE, 2.72; BW, 450; acq mtx, 384 × 288; % samp, 100 | TE, 2.72; BW, 450; acq mtx, 384 × 288; % samp, 100 |
| Sequence 7 | TE, 2.51; BW, 450; acq mtx, 384 × 288; % samp, 100 | TE, 2.51; BW, 450; acq mtx, 384 × 288; % samp, 100 |
| No flow compensation | No flow compensation | |
| Sequence 8 CE MRA | TR, 3.17; TE, 1.24; BW, 490; acq mtx, 512 × 182; FOV, 172 × 230; % phase FOV, 62.5; FA, 5; % samp, 56.9; ST, 0.9; ETL, 1; PED, Row | TR, 3.77; TE, 1.49; BW, 490; acq mtx, 512 × 246; FOV, 172 × 230; % phase FOV, 68.8; FA, 5; % samp, 69.9; ST, 0.9; ETL, 1; PED, Row |
Note.—TR, repetition time; TE, echo time, BW, bandwidth; FOV, field of view; acq mtx, acquisition matrix; FA, flip angle; samp, sampling; ST, section thickness; ETL, echo train length; PED, phase encoding direction.
Data Analysis
The data were analyzed by using the Vitrea 2 3D Workstation (Vital Images, Plymouth, MN). The measurement tool was accurately calibrated by measuring a known standard (the catheter tubing) at a window and level (W/L) of 472/202 that would display the artifact optimally. Using the Vitrea 2 brain MR protocol and keeping the W/L setting constant, manual regions of interest were drawn to encompass the coil-induced signal intensity loss. The areas were summed, providing a volume in cubic millimeters. Also, the greatest diameter of signal intensity void produced by each coil pack was measured in three orthogonal planes. By using the measured diameters, the coil pack volumes were then calculated by using the following formula:
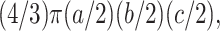 |
where a, b, and c represent the three orthogonal measurements.
Artifact overestimation factors (OEFs) for volume and diameter measurements were calculated on the basis of the measured artifact volume and diameters compared with actual coil pack volume and diameter measurements. The OEFs were calculated in the following manner:
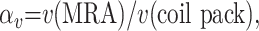 |
(1) |
with v(MRA) equal to the measured volume of the coil induced artifact and v(coil pack) the calculated volume of the coil pack,
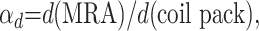 |
(2) |
where d(MRA) is a measured diameter of the artifact and d(coil pack) is the corresponding diameter of the actual coil pack.
An average of the diameter OEF, αd, was obtained from the three orthogonal measurements for all sequences. This average OEF was multiplied by the average actual diameter of each coil pack to obtain the average overestimation in millimeters.
Statistical Analysis
The signal intensity loss volume data and the overestimation data were analyzed with an analysis of variance (ANOVA) and protected least squares difference post hoc tests. The diameter data in the three planes (anterior-posterior [AP], cranial-caudal [CC], left-right [LR]) were also analyzed with repeated-measures ANOVA tests. The effect of parameter changes, CPDs and different field strengths were analyzed. The null hypothesis was rejected at P ≤ .05.
Results
Volume Measurement Data
Significantly more (P = .0038) volume artifact was produced at 3T than at 1.5T. At both field strengths, the more densely packed aneurysm (model 1) produced more artifact than the less densely packed aneurysm (model 2); however, this was statistically significant only at 3T (Table 2). There was a significant (P < .0001) effect on volume artifact as a function of sequence (TE) for both the 1.5T and 3T. At both field strengths, the longer TE sequences (1 and 2) produced the most artifact, with both of these sequences differing significantly from all the others.
TABLE 2:
Signal loss volumes (mm3) due to coil-induced susceptibility artifact
| 1.5T |
3T |
|||
|---|---|---|---|---|
| Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | |
| Sequence 1 | 2234 | 1385 | 3067 | 1827 |
| Sequence 2 | 1955 | 1073 | 2972 | 1963 |
| Sequence 3 | 1372 | 872 | 2055 | 1221 |
| Sequence 4 | 1160 | 745 | 1713 | 964 |
| Sequence 5 | 1112 | 688 | 1719 | 959 |
| Sequence 6 | 942 | 657 | 1491 | 933 |
| Sequence 7 | 875 | 622 | 1522 | 819 |
| Sequence 8 | 984 | 688 | 985 | 701 |
| Actual Volume of Coil Masses (mm3) | |
|---|---|
| Model 1: 44.9% occlusion |
Model 2: 22.4% occlusion |
| 471 | 471 |
Diameter Measurement Data
The 3T groups produced artifacts with significantly greater diameters than the 1.5T groups (P = .0004). The diameters were greatest for the CC plane (P < .0001). Model 1 produced the greatest artifact diameters compared with model 2 for both 1.5T and 3T groups for each plane (P = .0004), with CC yielding the highest diameters (Table 3). Sequence parameter changes yielded a statistically significant effect (P < .0001) on artifact diameter measurements, with CC yielding the highest diameters (P < .0001). For all three planes, the longer TE sequences (1 and 2) yielded significantly higher artifact diameters than any other sequence.
TABLE 3:
Greatest diameter (mm) in 3 orthogonal planes of signal loss due to coil-induced susceptibility artifact
| 1.5T (AP/CC/LR) |
3T (AP/CC/LR) |
|||
|---|---|---|---|---|
| Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | |
| Sequence 1 | 17.2/19.4/13.2 | 12.0/16.0/12.0 | 18.9/22.5/17.1 | 12.4/20.8/14.4 |
| Sequence 2 | 16.5/19.4/13.2 | 11.0/16.2/10.9 | 17.8/23.0/16.2 | 14.1/20.7/14.3 |
| Sequence 3 | 14.1/17.1/11.1 | 9.2/13.4/9.6 | 16.8/20.0/13.4 | 10.2/18.9/12.5 |
| Sequence 4 | 11.5/16.5/10.1 | 9.5/13.9/9.7 | 14.8/18.4/12.3 | 10.0/17.4/11.8 |
| Sequence 5 | 11.6/15.6/11.1 | 9.3/14.3/9.3 | 13.4/18.3/10.8 | 9.9/17.0/11.4 |
| Sequence 6 | 11.7/15.7/10.8 | 9.5/14.1/8.9 | 13.7/18.8/11.8 | 10.5/16.5/11.4 |
| Sequence 7 | 11.0/15.4/10.6 | 9.2/14.0/9.6 | 13.7/18.8/10.6 | 9.8/16.0/10.6 |
| Sequence 8 | 11.7/14.2/10.1 | 9.6/12.6/10.5 | 12.9/13.9/10.0 | 9.4/12.7/10.1 |
| Actual Diameters of Coil-Filled Aneurysm Models (mm) (AP/CC/LR) | |
|---|---|
| Model 1: 44.9% occlusion |
Model 2: 22.4% occlusion |
| 10/10/9 | 9/10/10 |
Note.—AP, anterior-posterior; CC, cranial-caudal; LR, left-right.
Volume Overestimation Data
The 3T group produced significantly more (P = .0029) overestimation than did the 1.5T group (Table 4). Also, the more densely packed aneurysm produced more artifact than did the more loosely packed aneurysm at both field strengths. There was a significant (P < .0001) effect on overestimation as a function of sequence and field strength. For both the 1.5T and 3T groups, the longest TE sequences (1 and 2) produced the greatest overestimation, with both of these sequences differing significantly from all the others.
TABLE 4:
Volume overestimation factor for given coil pack density and sequence
| 1.5T |
3.0T |
|||
|---|---|---|---|---|
| Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | |
| Sequence 1 | 4.74 | 2.94 | 6.51 | 3.88 |
| Sequence 2 | 4.15 | 2.28 | 6.31 | 4.17 |
| Sequence 3 | 2.91 | 1.85 | 4.36 | 2.59 |
| Sequence 4 | 2.46 | 1.58 | 3.64 | 2.05 |
| Sequence 5 | 2.36 | 1.46 | 3.65 | 2.04 |
| Sequence 6 | 2.00 | 1.39 | 3.17 | 1.98 |
| Sequence 7 | 1.86 | 1.32 | 3.23 | 1.74 |
| Sequence 8 | 2.09 | 1.46 | 2.09 | 1.49 |
Diameter Overestimation Data
The data in the three planes (AP, CC, LR) were analyzed with repeated-measures ANOVA tests (Table 5). The 3T groups produced more overestimation than did the 1.5T groups (P = .0005). The overestimation was greatest for the CC plane, followed by AP and LR (P < .0001). Model 1 produced greater diameter overestimation than did model 2 at 1.5T and 3T for each plane (P = .0002), with CC yielding the highest overestimation (P < .0001). Sequence parameter alterations yielded a statistically significant effect relative to plane (P = .0001), with CC yielding the greatest diameters (P < .0001). For all three planes, the longest TE sequences (1 and 2) yielded significantly higher artifact diameters than did any of the other sequences.
TABLE 5:
Average diameter overestimation factor for each coil pack density
| 1.5T |
3.0T |
|||
|---|---|---|---|---|
| Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | Model 1, 44.9%Occlusion | Model 2, 22.4% Occlusion | |
| Sequence 1 | 1.71 | 1.38 | 2.01 | 1.63 |
| Sequence 2 | 1.69 | 1.31 | 1.96 | 1.69 |
| Sequence 3 | 1.45 | 1.11 | 1.72 | 1.42 |
| Sequence 4 | 1.31 | 1.14 | 1.56 | 1.34 |
| Sequence 5 | 1.32 | 1.13 | 1.47 | 1.31 |
| Sequence 6 | 1.31 | 1.12 | 1.52 | 1.32 |
| Sequence 7 | 1.27 | 1.13 | 1.48 | 1.25 |
| Sequence 8 | 1.24 | 1.13 | 1.26 | 1.11 |
Discussion
In this in vitro study, we have defined some of the major characteristics related to MRA imaging of coil packs. The main conclusions to be drawn from this study are 1) reducing the TE is the main factor in improving perianeurysmal visualization on 3D TOF MRA images; 2) susceptibility artifact from platinum coil packs significantly increases with increasing field strength; 3) susceptibility artifact increases with increased CPDs; and 4) asymmetric artifact occurs in the frequency-encoding direction on 3D TOF MRA images.
Echo Time
Image degradation from metal in 3D TOF MRA is the result of magnetic field heterogeneity and resultant susceptibility-induced signal intensity loss. Local distortion of the magnetic field is secondary to differences in the static magnetic susceptibility of the metal and surrounding tissue and the presence of dynamic eddy currents as from radio-frequency pulses and gradient magnetic fields (20). Reducing the TE has been shown to reduce heterogeneity of the magnetic field that occurs with metal (18). Many investigators have reported some quantitative but primarily qualitative data demonstrating this principle (19).
In our study, the only parameter change that resulted in a statistically significant reduction in susceptibility-induced artifact was reducing the TE. This held true at both field strengths. Reducing the TE from 7.2 ms to 3.5 ms (∼51% reduction) was effective at minimizing artifact while limiting overall image degradation. Smaller reductions below 3.5 ms established a trend of decreasing artifact, but there was no significant incremental gain.
Our data support previous assertions that reducing the TE can result in improved visualization of perianeurysmal soft tissues and also establish that there is less incremental benefit to be gained once the TE is below 3.5 ms. Optimizing a 3D TOF sequence for postcoiling MRA can be focused on manipulating the TE. On our systems, the target TE is 3.5 ms.
Field Strength
As expected, susceptibility-induced signal intensity loss increased from 1.5T to 3T for all sequences and at both CPDs. The effect of reducing the TE was similar at both field strengths. The True FISP sequence created the least amount of artifact overall and was not significantly increased at 3T, which suggests that there may be room for incremental gain at 3T for CE MRA of coiled aneurysms. The potential of increased signal-to-noise ratio (SNR), improved spatial resolution, improved temporal resolution, and similar artifact magnitude at 3T favors further development of CE MRA at 3T. The same cannot be said for the 3D TOF sequences, which showed significant increases in artifact at 3T for both CPDs and all TEs compared with 1.5T. These data suggest that the best chance at examining a coiled aneurysm at 3T with 3D TOF techniques will be a low-density packed aneurysm with a short TE. All things being equal, however, 1.5T is superior to 3T when 3D TOF techniques are used.
CPD
A more densely packed aneurysm will create more susceptibility-induced signal intensity loss than will a less densely packed aneurysm at 1.5T and 3T. Although a definite trend, there were no statistical differences at 1.5T relative to CPD across 3D TOF sequences. At 3T, there were significant differences between models 1 and 2: what was a trend at 1.5T became a statistical difference at 3T because of the inherent differences in field strength (i.e., SNR) and incremental effect on susceptibility-induced artifacts. There were no statistical differences relative to CPD and field strength for CE MRA sequence, which again supports our assertion that the gains at 3T may outweigh the increase in artifacts relative to imaging of coiled aneurysms by using CE MRA methods.
Frequency-Encoding Direction
The frequency-encoding direction yielded the highest artifact across all CPDs and at both field strengths. The data were collected in the coronal plane such that the frequency-encoding gradient was oriented along the CC direction. With this prescription, the frequency gradient was significantly smaller in magnitude than the other two directions (4mT/m vs. 23 and 20mT/m). Thus, the relative effect of magnetic susceptibility was greatest in the frequency-encoding direction. In typical clinical applications, the frequency-encoding gradient for 3D TOF MRA is oriented in the AP direction. These data suggest that the orientation of the frequency-encoding direction matters and will asymmetrically degrade image quality in that direction. This is not an easily controlled factor, because vessels, aneurysms, and aneurysm necks are a heterogeneous group with variable orientations. On a case-by-case basis, this effect should be considered and the MRA prescribed in such a manner as to direct the frequency-encoding direction away from the area in question.
TOF versus True FISP
True FISP is a fast gradient-echo technique used for CE MRA. The TE is short (TE, 1.24 ms, sequence 8), and susceptibility-induced signal intensity loss is minimal. The True FISP sequence produced the least amount of artifact overall and differed significantly from the longer TE sequences. Because there was not a statistically significant increase in artifact at 3T compared with 1.5T, incremental gain may occur at 3T for CE MRA.
Overestimation Factors
Overestimation factors were calculated to compare volume and diameter artifact measurements to actual volume and diameter measurements. As expected, less overestimation occurred at lower TEs, field strengths, and CPDs. Extrapolating the diameter OEF to the measured model provides an assessment in millimeters of potential overestimation (Table 6). For example, at 3T with a CPD of 44.9%, sequence 1 will produce 9.8 mm (range, 8.1–12.5 mm) of artifact and sequence 7 will produce 2.5 mm (range, 1.6–8.8 mm). These absolute increases in artifact production should be taken into account when interpreting MRA images.
TABLE 6:
Average millimeters of actual overestimation in any one direction
| 1.5T |
3.0T |
|||
|---|---|---|---|---|
| Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | Model 1, 44.9% Occlusion | Model 2, 22.4% Occlusion | |
| Sequence 1 | 6.9 | 3.7 | 9.8 | 6.1 |
| Sequence 2 | 6.7 | 3.0 | 9.3 | 6.7 |
| Sequence 3 | 4.4 | 1.1 | 7.0 | 4.1 |
| Sequence 4 | 3.0 | 1.4 | 5.4 | 3.3 |
| Sequence 5 | 3.1 | 1.3 | 4.6 | 3.0 |
| Sequence 6 | 3.0 | 1.2 | 5.0 | 3.1 |
| Sequence 7 | 2.6 | 1.3 | 4.6 | 2.4 |
| Sequence 8 | 2.3 | 1.3 | 2.5 | 1.1 |
Clinical Relevance
Coiled aneurysms can be assessed by MRA, but there are many factors to consider that affect imaging strategy. All things being equal, 3D TOF imaging of coiled aneurysms will be more successful at 1.5T than at 3T. Significant reductions in susceptibility-induced signal intensity loss can be achieved by optimizing or reducing the TE to the 3.5-ms range. Aneurysms of lower CPD and aneurysms that are considered partially or incompletely treated will be more easily studied particularly at 1.5T because of the relative decrease in artifact production. A heterogeneous coil pack may favorably affect visualization if the dome, for instance, is more densely coiled than the neck, which is often the case. Finally, imaging of the aneurysm neck will be more favorable if the neck is not oriented in the frequency-encoding direction.
CE techniques produce the least amount of artifact compared with all TOF sequences and do not considerably change with increase in field strength. As such, the benefits of 3T imaging, which include improved SNR, spatial resolution, and temporal resolution, may outweigh the slight but statistically insignificant increase in susceptibility-induced signal intensity loss and provide an opportunity for CE MRA applications in patients with coiled aneurysms.
Study Limitations
There are several limitations to this study, including a static model (no flow), hand measurement, heterogeneity of the coil packs or artifact, and the timing of imaging. Our aneurysm model was a closed system with no flow: no estimation on the effects of flowing blood, particularly within the aneurysm, can be made. Also, without flow information, the effect of reducing the TE on the overall quality of the MRA image cannot be determined. The balloon models were hand measured, which can introduce error. Furthermore, the volume measurements were obtained by hand measuring each section only once. No assurance can be given regarding coil pack homogeneity, although clinically, coil packing is typically heterogeneous. Artifact from metal is heterogeneous and is represented as signal intensity loss (hypointense) and signal intensity displacement (hyperintense). We made no effort to measure the displaced signal intensity, because it was the minority of artifact and had borders that would be impossible to identify confidently. The results described were obtained by using specific imaging sequences as implemented on Siemens Sonata and Trio equipment. Different absolute results might be obtained with other equipment or vendors because of different gradient amplitude combinations employed in the sequence structure; however, the conclusions should remain the same. Finally, approximately 16 hours elapsed between imaging at 1.5T and 3T. Although unlikely, it is conceivable that the aneurysm sizes could have reduced by leaking fluid into the adjacent mold.
Conclusion
Improved visualization of perianeurysmal soft tissues is best accomplished by reducing the TE on 3D TOF sequences to approximately 3.5 ms. Imaging at higher field strengths does not provide incremental gain for 3D TOF sequences because of significant increases in susceptibility-induced artifacts. Aneurysms with higher CPDs produce more artifact under all conditions, and more artifact is produced in the frequency-encoding direction. CE MRA techniques produce the least amount of artifact at 1.5T and 3T and will show incremental gain at 3T.
References
- 1.Rinkel GJE, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998;29:251–256 [DOI] [PubMed] [Google Scholar]
- 2.Chang SD, Steinberg GK. Management of intracranial aneurysms. Vasc Med 1998;3:315–326 [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi G, Vinuela F, Sepetka I, Macellari F. Electrothrombosis of saccular aneurysms via endovascular approach. 1. Electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1–7 [DOI] [PubMed] [Google Scholar]
- 4.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. 2. Preliminary clinical experience. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 5.Vineula F, Duckwiller G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–82 [DOI] [PubMed] [Google Scholar]
- 6.Molyneux A, Kerr, R, Stratton I, et al. International Subarachnoid Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360:1267–1274 [DOI] [PubMed] [Google Scholar]
- 7.Hope AJK, Byrne JV, Molyneaux AJ. Factors influencing successful angiographic occlusion of aneurysms treated by coil embolization. AJNR Am J Neuroradiol 1999;20:391–399 [PMC free article] [PubMed] [Google Scholar]
- 8.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348–356 [DOI] [PubMed] [Google Scholar]
- 9.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 1994;15:1401–1407 [PMC free article] [PubMed] [Google Scholar]
- 10.Korogi Y, Takahashi M, Katada K, et al. Intracranial aneurysms: detection with three-dimensional CT angiography with volume rendering-comparison with conventional angiographic and surgical findings. Radiology 1999;211:497–506 [DOI] [PubMed] [Google Scholar]
- 11.Nome T, Bakke SJ, Nakstad PH. MR angiography in the follow-up of coiled cerebral aneurysms after treatment with Guglielmi detachable coils. Acta Radiol 2002;43:10–14 [DOI] [PubMed] [Google Scholar]
- 12.Anzalone N, Righi C, Simionato F, et al. Three-dimensional time-of-flight MR angiography in the evaluation of intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 2000;21:747–752 [PMC free article] [PubMed] [Google Scholar]
- 13.Kahara VJ, Seppanen SK, Ryymin PS, et al. MR angiography with three-dimensional time-of-flight and targeted maximum-intensity-projection reconstructions in the follow-up of intracranial aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1999;20:1470–1475 [PMC free article] [PubMed] [Google Scholar]
- 14.Derdeyn CP, Graves VB, Turski PA, et al. MR angiography of saccular aneurysms after treatment with Guglielmi coils: preliminary experience. AJNR Am J Neuroradiol 1997;18:279–286 [PMC free article] [PubMed] [Google Scholar]
- 15.Adams WM, Laitt RD, Jackson A. Time-of-flight 3D magnetic resonance angiography in the follow-up of coiled cerebral aneurysms. Intervent Neuroradiol 1999;5:127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leclerx X, Navez JF, Gauvrit JY, et al. Aneurysms of the anterior communicating artery treated with Guglielmi detachable coils: follow-up with contrast-enhanced MR angiography. AJNR Am J Neuroradiol 2002;23:1121–1127 [PMC free article] [PubMed] [Google Scholar]
- 17.Boulin A, Pierot L. Follow-up of intracranial aneurysms treated with detachable coils: comparison of gadolinium-enhanced 3D time-of-flight MR angiography and digital subtraction angiography. Radiology 2001;219:108–113 [DOI] [PubMed] [Google Scholar]
- 18.Schmalbrock P, Yaun C, Chakeres DW, et al. MR angiography: methods to achieve very short echo times. Radiology 1990;175:861–865 [DOI] [PubMed] [Google Scholar]
- 19.Gonner F, Heid O, Remonda L, et al. MR angiography with ultrashort echo time in cerebral aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1324–1328 [PMC free article] [PubMed] [Google Scholar]
- 20.Camacho CR, Plewes DB, Henkelman RM. Nonsusceptibility artifacts due to metallic objects in MR imaging. J Magn Reson Imaging 1995;5:75–88 [DOI] [PubMed] [Google Scholar]


