Abstract
BACKGROUND AND PURPOSE: The index of mean blood flow velocity (V) in the middle cerebral artery (MCA) divided by respective velocity in the ipsilateral internal carotid artery (ICA), or VMCA/VICA index, is commonly used as a marker of vasospasm, although reference values are not established. We sought to provide reference data for these velocities and index.
METHODS: Transcranial color-coded duplex and carotid duplex sonography was performed in 335 healthy volunteers (211 women, 124 men; mean age ± SD, 42 ± 18 years; range, 18–86 years). Age analyses were based on three groups: I, <40; II, 40–60; and III, >60 years. The VMCA/VICA index was calculated based on angle-corrected blood flow velocities determined in the MCA and extracranial ICA.
RESULTS: Mean flow velocities in the MCA and ICA diminished with increasing age, most pronounced in those subjects >40 years. The VMCA/VICA index increased significantly (1.67 + 0.005 [age]; P < .05) with age in women, but not in men. In women, reference values and ranges for the VMCA/VICA index were as follows, by group: I, 1.82 (range, 0.88–2.68); II, 1.91 (range, 0.94–2.88); and III, 2.06 (range, 0.59–3.53). Respective values for men were as follows, by group: I, 2.10 (range, 0.96–3.24); II, 2.04 (range, 0.71–3.37); and III, 1.78 (range, 0.81–2.75). In subjects <40 years, the VMCA/VICA index was significantly higher in men than in women.
CONCLUSION: The VMCA/VICA index significantly varies with age and sex. Sonographic diagnosis of cerebral vasospasm should be based on age- and sex-adjusted reference values of the VMCA/VICA index.
The ability of transcranial Doppler sonography (TCD) to detect elevated blood flow velocities associated with narrowing of an intracranial artery has led to extensive application of TCD for the bedside detection and serial evaluation of cerebral vasospasm (1,2). Transcranial color-coded duplex sonography (TCCS) has enabled more accurate estimates of intracranial blood flow velocities (3,4). Considerable efforts have been devoted to establishing a threshold of blood flow velocities that reliably differentiate cerebral vasospasm from hyperemia or physiologic blood flow (5–7). However, the diagnostic reliability of any isolated velocity threshold is limited because of the multifactorial determinants of blood flow velocity in a particular vascular segment (8–10).
TCD diagnosis of cerebral vasospasm is limited by false-negative results associated with increased cerebrovascular impedance and false-positive results caused by hyperemia and/or hyperperfusion (11,12) Aaslid et al (13) and Lindegaard et al (14) originally proposed the use of a ratio to relate blood flow velocity (V) in the middle cerebral artery (MCA) to that in the ipsilateral extracranial internal carotid artery (ICA), and they used this VMCA/VICA index to help distinguish vasospasm from normal blood flow. Despite relatively widespread acceptance of this ratio, reference values for the VMCA/VICA index have not been established. Because anatomic characteristics and elastic properties of the intracranial and extracranial vessels differ by age and sex, velocities and corresponding indices are also expected to vary (15–18). The purpose of this study was to determine reference values for mean blood flow velocities and the VMCA/VICA index based on age and sex in a large group of healthy subjects.
Methods
The local ethics committee approved the research protocol, and informed consent was obtained. The study population consisted of 335 healthy volunteers recruited from the individuals from the medical school, the hospital staff, and their relatives. They included 211 women (mean age ± standard deviation [SD], 40 ± 18.8 years; range, 18–86 years) and 124 men (mean age ± SD, 48 ± 16.7; range, 19–84 years). Selection criteria excluded individuals with a significant medical history, including those with psychiatric, cardiovascular, endocrine, or neurologic disorders. Pregnant women were also excluded. Individuals were also excluded if they were receiving medication or hormonal therapy. Screening procedures also excluded those with body temperature above 37°C (98.6°F), systolic blood pressure greater than 160 mm Hg, diastolic blood pressure greater than 100 mm Hg, or moderate or severe carotid atherosclerosis.
Transcranial Color-Coded Duplex Sonography
After the subjects rested for 15 minutes in the supine position, the intracranial arteries were studied bilaterally by using a sonographic scanner (Toshiba, Toshiba Medical System, Tokyo, Japan) equipped with a 2.5-MHz, 90° phase-array probe for B-mode and Doppler imaging. Proximal segments of the basal cerebral arteries were insonated via a transtemporal approach on gray-scale and color imaging and examined on the basis of their anatomic relationship to identifiable intracranial structures (4). A 3-mm wide sample volume was placed on the color image of the proximal MCA (M1) about 10 mm distal to the terminal carotid. Under visual guidance, a linear marker was placed on the color image of the insonated vascular segment along the long axis of the vessel to determine the angle of insonation. The angle between this linear marker and the ultrasound beam, displayed automatically on the screen of the scanner, was considered a two-dimensional approximation of the angle of insonation. Angle-corrected peak-systolic (VPS), mean (VMEAN), and end-diastolic (VED) blood flow velocities were subsequently measured. Automatic determinations of flow velocities were used. In cases with weak Doppler signal intensity, the maximum frequency envelope of the Doppler waveform was manual traced to obtain these values.
Carotid Duplex Sonography
After the TCCS study was completed, bilateral ICA measurements were obtained with a broadband (7.5–11 MHz) linear-array transducer by using the same color scanner. We used a standard approach with gray-scale, pulsed Doppler, and color Doppler flow imaging (16). The sample volume, adjusted to the size of the insonated vessel, was placed within the ICA 15–20 mm distal to the common carotid bifurcation. Automatic determinations of angle-corrected VPS, VMEAN, and VED were used. In cases with weak Doppler signal intensity, the maximum frequency envelope of the Doppler waveform was manual traced.
The VMCA/VICA index was independently calculated on the basis of VPS, VMEAN, and VED in the MCA and ICA as the VMCA/VICA PS index, the VMCA/VICA mean index, and the VMCA/VICA ED index, respectively.
Statistical Analysis
Data were analyzed by using statistical software (Systat for Windows; Systat, Evanston, IL). Mean, range, median, and SD across subjects were calculated for each sonographic parameter. A normal distribution of measurements was verified by the normal probability plot method provided by Systat.
Mean VMCA, mean VICA, and the VMCA/VICA index were plotted for all subjects on the basis of age and sex. Trend curves obtained for distance-weighted least-squares smoothing of the mean VMCA and VICA values were computed to reveal the points of deflection of the age-flow dependence relation. The course of the age dependence of the curve for mean VMCA enabled us to define three groups according to age: younger than 40, 40–60, and older than 60 years (15). Values of the VMCA/VICA index from both hemispheres were compared by using a paired t test. Data between age and sex groups were compared with a one-way analysis of variance and the Tukey test for pairwise probability comparisons with probability adjustment and with a nonpaired t test, respectively. Relationships between the VMCA/VICA ratio and age were estimated by means of linear regression analysis.
We used traditional, normal theory to establish normal reference ranges because the data were normally distributed (19). Therefore, normal reference ranges were estimated by using a mean and 2 (actually 1.96) SDs of the dataset. This calculation yielded the 2.5% and 97.5% reference intervals.
Results
Poor transtemporal windows on at least one side excluded the data from 31 (9.3%) subjects from further analysis. The highest prevalence of insufficient windows was noted in subjects older than 60 years. As a result, VMCA/VICA indices were calculated on the basis of measurements of blood flow velocity in 304 subjects: 193 women and 111 men (Table 1).
TABLE 1:
Normal reference values for mean MCA and ICA blood flow velocities by age group
| Mean Velocity (cm/s) | Age Group |
Between-Group Differences | |||
|---|---|---|---|---|---|
| All | I (<40 y) | II (40–60 y) | III (>60 y) | ||
| All subjects (n = 304) | |||||
| MCA | 71 (35–107)** | 78 (44–112) | 70 (36–104) | 57 (32–82) | I vs II†, II vs III†, I vs III† |
| ICA | 39 (18–60)** | 43 (28–61) | 38 (16–60) | 33 (16–50) | I vs II†, II vs III†, I vs III† |
| Women (n = 193) | |||||
| MCA | 75 (39–111)** | 80 (45–115)‡ | 74 (41–107) | 58 (31–85) | II vs III†, I vs III† |
| ICA | 42 (20–64)* | 45 (28–62)‡ | 42 (17–67) | 31 (12–50) | I vs II†, II vs III†, I vs III† |
| Men (n = 111) | |||||
| MCA | 64 (32–96)** | 71 (40–102) | 65 (32–98) | 56 (31–81) | I vs III† |
| ICA | 34 (20–48)* | 35 (21–49) | 34 (20–48) | 34 (20–48) | Not significant |
Age dependence, P < .05.
Age dependence, P < .001.
All P < .05.
Sex dependence, P < .05.
Values of mean VMCA, VICA, and VMCA/VICA were normally distributed. Because no significant differences were noted in any of these parameters on the basis of laterality (P = .1), reference ranges for the index were calculated by using values from both sides.
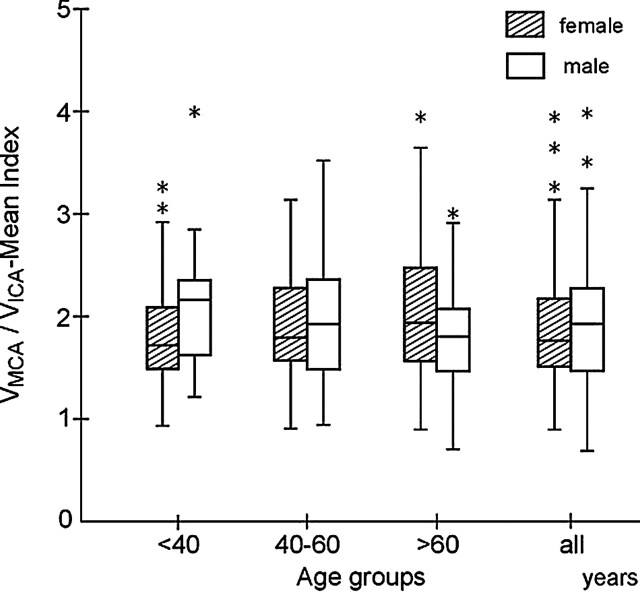
The VMCA/VICA index substantially increased with age (Fig 1). The greatest age dependency was noted in the VMCA/VICA PS index (P < .05), where VMCA/VICA PS index = 1.57 + 0.004(age). We noted weaker age relationships for the VMCA/VICA mean index (P = .02), where VMCA/VICA mean index = 1.82 + 0.002(age), and for the VMCA/VICA ED index (P = .1), where VMCA/VICA ED index = 1.85 + 0.003(age). Regression associated with age explained 1.8% of the variance in each of the indices, as determined by R2. The age dependency of the index was not significant in men but was significant in women (P < .01), where VMCA/VICA PS index = 1.46 + 0.006(age), VMCA/VICA mean index = 1.67 + 0.05(age), and VMCA/VICA ED index = 1.69 + 0.006(age).
Fig 1.
Distribution of values of the VMCA/VICA index, calculated from the mean MCA and ipsilateral extracranial ICA velocities in women and men in three age groups (<40, 40–60, and >60 years) and in 304 healthy subjects. In women, median value of the index increases with age to 60 years, whereas in men, it is relatively stable. The minimum and maximum values of the variable are indicated by the whiskers (solid lines) of each diagram. The median value is indicated by a central horizontal and the lower and upper qualities by the corresponding horizontal ends of the box. *Indicates outlying value, inconsistent with other points in the sample.
The curves obtained for distance-weighted, least-squares smoothing of the individual values of MCA and ICA flow velocities were computed to reveal the points of deflection of the age-flow dependence relation. To match the dynamics of age dependency, all subjects were divided into three age groups: I, younger than 40; II, 40–60; and III, older than 60 years. This separation enabled us to calculate normal reference values for the relevant age limits (15). Table 1 provides details about the mean values and reference ranges across all subjects and separated by sex. Differences in mean flow velocities in the MCA and ICA were statistically significant between age groups. The between-group differences in the VMCA/VICA indices, calculated for all subjects and for men, were insignificant. Differences between group I and group III were substantial in women, for indices calculated on the basis of peak-systolic and end-diastolic velocities (Table 2).
TABLE 2:
Normal reference values for the VMCA/VICA index by age group
| VMCA/VICA Index | Age Group |
Between-Group Differences | |||
|---|---|---|---|---|---|
| All | I (<40 y) | II (40–60 y) | III (>60 y) | ||
| All subjects (n = 304) | |||||
| Peak systole | 1.73 (0.72–2.74)* | 1.68 (0.76–2.60) | 1.78 (0.76–2.80) | 1.77 (0.60–2.94) | Not significant |
| Mean | 1.91 (0.81–3.01) | 1.88 (0.86–2.90) | 1.96 (0.84–3.10) | 1.90 (0.67–3.13) | Not significant |
| End diastole | 1.99 (0.73–3.15) | 1.94 (0.80–3.08) | 2.06 (0.65–3.47) | 2.00 (0.68–3.32) | Not significant |
| Women (n = 193) | |||||
| Peak systole | 1.70 (0.74–2.66)* | 1.62 (0.76–2.44)† | 1.78 (0.96–2.60) | 1.89 (0.51–3.27) | I vs III‡ |
| Mean | 1.87 (0.82–2.92)* | 1.82 (0.88–2.76)† | 1.91 (0.94–2.88) | 2.06 (0.59–3.53) | I vs III‡ |
| End diastole | 1.93 (0.78–3.08)* | 1.86 (0.84–2.88)† | 1.96 (0.82–3.10) | 2.17 (0.66–3.68) | I vs III‡ |
| Men (n = 111) | |||||
| Peak systole | 1.77 (0.69–2.85) | 1.85 (0.82–2.88) | 1.78 (0.53–3.01) | 1.68 (0.72–2.64) | Not significant |
| Mean | 1.98 (0.80–3.16) | 2.10 (0.96–3.24) | 2.04 (0.71–3.37) | 1.78 (0.81–2.75) | Not significant |
| End diastole | 2.08 (0.66–3.50) | 2.20 (0.83–3.57) | 2.19 (0.51–3.87) | 1.86 (0.85–2.87) | Not significant |
Age dependence, P < .001.
Sex dependence, P < .05.
P < .05.
The VMCA/VICA index tended to be higher in men younger than 60 years than in others (Fig 1), but substantial sex differences were noted only in group I (Table 2). The range of reference values was wider in men than in women in age groups I and II, whereas in group III, the range was higher in women than in men.
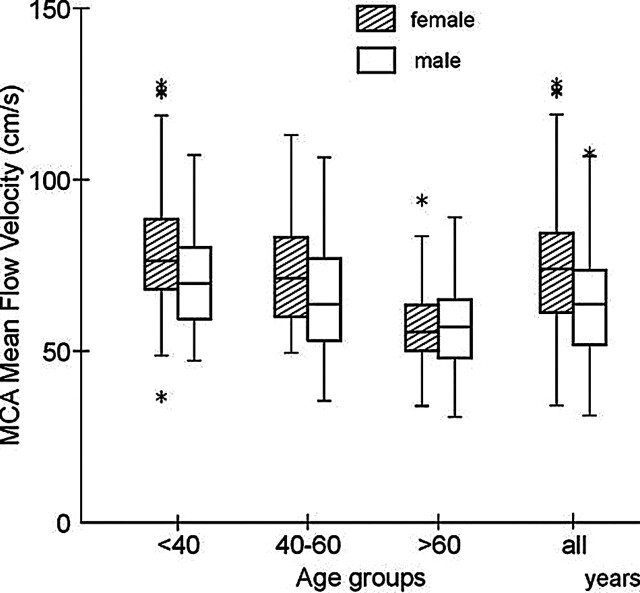
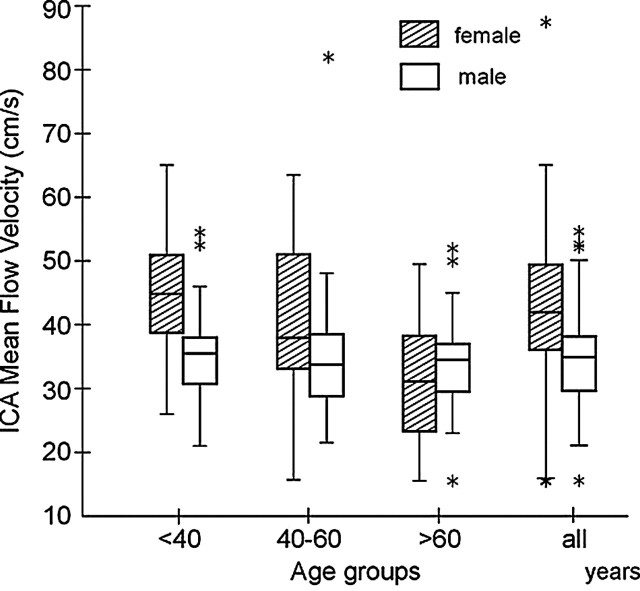
Mean blood flow velocities in the MCA and ICA decreased substantially with age (Figs 2 and 3). In the youngest group, mean flow velocities in both vascular segments were significantly higher in women than in men (Table 1). In group II, velocities were also higher in women than in men, but this difference was not significant. In group III, no significant differences were noted in velocities based on sex. In women older than 60 years, VMCA was about 28% less than the VMCA in those younger than 40 years; this age-related decline in VMCA amounted to 21% in men. Similar age-related comparisons in those older than 60 years versus those younger than 40 years demonstrated a 31% decline in VICA in women and a 3% decline in men.
Fig 2.
Distribution of mean blood flow velocities in the MCA for women and men in the three age groups and in 304 healthy subjects. Mean velocity is higher in women up to the age of 60 years, and in older subjects, mean velocities are substantially lower and similar in both sexes. A description of box-and-whisker plot is provided in Figure 1.
Fig 3.
Distribution of mean blood flow velocities in the extracranial ICA for women and for men in the three age groups and in all 304 healthy subjects. Mean velocity is substantially high in women up to the age of 60 years; after this time, velocity decreases significantly. A different pattern of age dependence is seen in men: flow velocity is almost stable throughout the life span. A description of box-and-whisker plot is provided in Figure 1.
The VMCA/VICA index was about 13%–17% higher in women older than 60 years than in those younger than 40 years, whereas the index insignificantly decreased by about 8–15% in men.
Discussion
The present study was designed to establish normal reference ranges for the VMCA/VICA index, as determined with transcranial color-coded duplex and carotid duplex sonography in a large population of healthy subjects. The upper reference limit may be used in clinical practice to differentiate mild vasospasm from hyperemia, a common finding in young healthy women, in patients with disturbed cerebrovascular reactivity, and in patients receiving triple-H therapy (10,11,20). In such cases, a normal VMCA/VICA index may be used to exclude vasospasm. In addition, the blood flow velocity in a narrowed MCA may be normal in patients with increased intracranial pressure or constriction of the cerebral microvasculature (8,9,21), though a high VMCA/VICA index may indicate vasospasm. The establishment of reference values for the VMCA/VICA index and the upper limits of the reference range based on age and sex may improve the diagnostic accuracy of sonography for cerebral vasospasm.
Lindegaard et al (14) proposed a threshold value of 3.0 for the VMCA/VICA index to differentiate mild vasospasm from hyperemia. The upper limit of the reference range for the index, as determined in our study, was above this value, particularly in older women and younger men. Therefore, the clinical application of a 3.0 threshold value for the index may falsely suggest cerebral vasospasm. However, the discrepancy between our results and the proposed 3.0 threshold value may be partially explained by differences in technique. Our VMCA/VICA index was determined on the basis of color-coded duplex sonography, whereas Lindegaard et al (14) used a conventional blind TCD technique. TCCS enables the determination of angle-corrected blood flow velocities in the MCA, which are more accurate than velocities measured with conventional TCD (4,22). In general, angle-corrected velocities are about 10%–30% higher than velocities measured with the blind TCD technique (15,23). In our study, VMCA values were about 20% higher than the velocities in the small group of patients Aaslid et al (13) and Lindeegard et al (14) examined, whereas velocities in the ICA were similar. It should be noted, however, that these authors examined neurosurgical patients in whom flow velocities might be lower than those in healthy volunteers. Because the threshold value of 3.0 for the VMCA/VICA index, which is widely used to detect vasospasm, considerably overlaps with our normal reference ranges, novel diagnostic thresholds for MCA vasospasm must be determined by using TCCS combined with carotid duplex sonography in a larger population of patients after subarachnoid hemorrhage.
The clinical relevance of the lower reference limit of the VMCA/VICA index is not currently defined. However, low values may be expected in patients with low-flow MCA infarcts and preserved collateral flow, in patients with occlusions of large or small branches of the artery, or even in patients with M1 occlusion during an early phase of recanalization (20,24,25). The potential use of this index in these clinical settings should be evaluated in future studies.
We noted a minimal yet significant increase in the VMCA/VICA index with age. This finding must be interpreted separately for women and for men, as disparate trends were observed. An age-dependent decrease in flow velocity in both the MCA and the ICA was observed in the entire group, but we noted velocities in women as old as 60 years that were higher than those of age-matched men; this finding is in accordance with those of other reports (15,26–28). Blood flow velocities in the ICA were also substantially higher in young women than in age-matched men. Our findings are consistent with a report that showed that cerebral blood flow is higher in women during menarche than in men of the same age (15,28–30). After menopause, however, cerebral blood flow in women declines and equals that of age-matched men; this change may be partly related to a decline in plasma estrogen levels (31–33). However, such changes in cerebral blood flow should uniformly affect velocity in the MCA and ipsilateral ICA, leaving the VMCA/VICA index constant. The divergent age-related trends in VMCA and VICA, manifested in our study as substantial fluctuations of the VMCA/VICA index, suggest that different age-related changes in the diameter of the intracranial and extracranial arteries may occur in women compared with men.
The number of extreme values for the VMCA/VICA index and flow velocities was highest in subjects younger than 40 years and in those older than 60 years. These values may have been related to anatomic variants, measurement errors, or even disease that might have be undetected on the basis of our selection criteria. In older subjects, atherosclerotic disease of the MCA can lead to the vascular narrowing and increased MCA velocity. Consequently, the VMCA/VICA index is high. Blood flow velocity in the MCA can also be elevated as a result of atherosclerotic narrowing of the terminal portion of the ICA, because the blood flow jet can be present in the proximal part of the MCA. Furthermore, atherosclerotic vessels can be tortuous, making proper adjustment of the electronic cursor on the display to the long axis of the vessel difficult. Usually, the angle between the affected vessel and the ultrasound beam is high (22). Measurement errors increase with increasing angles of insonation. However, the probability of atherosclerotic disease is low in young subjects, and the functional state of brain vessels, particularly in young women (20), seems to be more diverse than the states of the brain vasculature in older subjects. Those who use the technique in everyday practice should take these situations, which represent potential pitfalls of the sonographic technique, into account. Therefore, the normal reference ranges can be helpful in making clinical valuable conclusions.
Mechanical behavior of an artery is dependent on the relative amount of collagen and elastin in the arterial wall and on the thickness of the wall, or the thickness-radius ratio (17). The collagen-elastin ratio and the thickness-radius ratio increase with age, accompanying stiffness. It has been reported that the elastic properties and diameter of the intracranial and extracranial arteries demonstrate different trends associated with age (17). Stiffness of the intracranial arteries increases from birth, while less rigid extracranial arteries exhibit minimal age-related changes up to 40 years. The thickness-radius ratio of the intracranial arteries is small to around 40 years, whereas the wall thickness and diameter of extracranial arteries increases more rapidly with age. At a constant level of cerebral blood flow, these progressively disparate changes in stiffness and diameter of the intracranial and extracranial arteries may lead to an increase in the VMCA/VICA index.
Several studies have demonstrated sex-related differences in the elastic properties of arterial walls across the human lifespan (18,34–36). Hansen et al (18) noted that the diameter of the carotid artery is significantly larger in men than in women from the age of 25, with a substantial increase to the age of 40–45 years and a steeper increase in women with age after this point. Our results were consistent with those observations, as the mean blood flow velocity in the ICA decreased with age only minimally in men, whereas the decrease was substantial in women. These differences were responsible for increased VMCA/VICA indices in men younger than 60 years compared with age-matched women. In older subjects, the index substantially increased in women, surpassing values observed in age-matched men. This phenomenon may be explained by the relatively greater dilatation of the ICA compared with MCA in women. This could be related to divergent trends in the age-related distention of the two vessels due to the physiologic effect of the smaller body size of women (36). Alternatively, it may be related to larger intracranial/extracranial differences in arterial stiffness after the cessation of ovarian function, because female sex hormones, in contrast to testosterone, lead to a decrease in the collagen-elastin ratio (35). The argument favoring greater dilatation of the ICA is strengthened by the rapid age-related decline in ICA velocity compared with MCA velocity in women. This observation is consistent with the results of Jonason et al (37) and Hansen et al (18), which demonstrated that postmenopausal women have carotid arteries substantially larger than those of younger women. The possibility that this phenomenon is related to the effects of estrogen on the arterial tree before menopause or concomitant effects of aging alone is speculative.
A limited number of TCD reports of reference values for the VMCA/VICA index have been published (13,14). Our study was based on a large sample of healthy subjects across a wide age range and included statistical analyses that may have established normal reference data for the VMCA/VICA index. Appropriate age and sex matching of the VMCA/VICA index obtained with TCCS and carotid sonography is a prerequisite for drawing clinically valuable conclusions.
References
- 1.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recordings of flow velocity in basal cerebral arteries. J Neurosurg 1982;57:769–774 [DOI] [PubMed] [Google Scholar]
- 2.Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg 1984;60:37–41 [DOI] [PubMed] [Google Scholar]
- 3.Bogdahn U, Becker G, Winkler J, Greiner K, Perez J, Meurers B. Transcranial color-coded real time sonography in adults. Stroke 1990;21:1680–1688 [DOI] [PubMed] [Google Scholar]
- 4.Krejza J, Mariak Z, Melhem ER, Bert RJ. A guide to the identification of major cerebral arteries with transcranial color Doppler sonography. AJR Am J Roentgenol 2000;174:1297–1303 [DOI] [PubMed] [Google Scholar]
- 5.Lysakowski C, Walder B, Costanza MC, Tramer MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke 2001;32:2292–2298 [DOI] [PubMed] [Google Scholar]
- 6.Mariak Z, Krejza J, Swiercz M, Kordecki K, Lewko J Accuracy of transcranial color Doppler ultrasonography in the diagnosis of middle cerebral artery spasm determined by receiver operating characteristic analysis. J Neurosurg 2002;96:323–330 [DOI] [PubMed] [Google Scholar]
- 7.Krejza J, Baumgartner RW. Clinical applications of transcranial color-coded duplex sonography. J Neuroimaging 2004;14:215–225. [DOI] [PubMed] [Google Scholar]
- 8.Ohkuma H, Manabe H, Tanaka M, Suzuki S. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2000;31:1621–1627 [DOI] [PubMed] [Google Scholar]
- 9.Yundt KD, Grubb RLJ, Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab 1998;18:419–424 [DOI] [PubMed] [Google Scholar]
- 10.Manno EM, Gress DR, Schwamm LH, Diringer MN, Ogilvy CS. Effects of induced hypertension on transcranial Doppler ultrasound velocities in patients after subarachnoid hemorrhage. Stroke 1998;29:422–428 [DOI] [PubMed] [Google Scholar]
- 11.Romner B, Bellner J, Kongstad P, Sjoholm H. Elevated transcranial Doppler flow velocities after severe head injury: cerebral vasospasm or hyperemia? J Neurosurg 1996;85:90–97 [DOI] [PubMed] [Google Scholar]
- 12.Meixensberger J, Hamelbeck B, Dings J, Ernemann U, Roosen K. Critical increase of blood flow velocities after subarachnoid haemorrhage: vasospasm versus hyperaemia. Zentralbl Neurochir 1996;57:70–75 [PubMed] [Google Scholar]
- 13.Aaslid R, Huber P, Nornes H. A transcranial Doppler method in the evaluation of cerebrovascular spasm. Neuroradiology 1986;28:11–16 [DOI] [PubMed] [Google Scholar]
- 14.Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir (Wien) 1989;100:12–24 [DOI] [PubMed] [Google Scholar]
- 15.Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. Am J Roentgenol 1999;172:213–218 [DOI] [PubMed] [Google Scholar]
- 16.Forteza AM, Krejza J, Koch S, Babikian VL. Ultrasound Imaging of Cerebrovascular Disease. In: Babikian VL, Wechsler L, Higashida RT, eds. Imaging Cerebrovascular Disease. Philadelphia: Butterworth-Heinemann;2003. :3–35
- 17.Hayashi K, Handa H, Nagasawa A, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomechanics 1980;13:175–184 [DOI] [PubMed] [Google Scholar]
- 18.Hansen F, Mangell P, Sonesson B, Lanne T. Diameter and compliance in the human common carotid artery-variations with age and sex. Ultrasound Med Biol 1995;21:1–9 [DOI] [PubMed] [Google Scholar]
- 19.Horn PS, Pesce AJ. Reference intervals: un update. Clin Chim Acta 2003;334:5–23 [DOI] [PubMed] [Google Scholar]
- 20.Krejza J, Mariak Z, Huba M, Wolczynski S, Lewko J. Effect of endogenous estrogen on blood flow through carotid arteries. Stroke 2001;32:30–36 [DOI] [PubMed] [Google Scholar]
- 21.Hassler W, Steinmetz H, Gawlowski J. Transcranial Doppler ultrasonography in raised intracranial pressure and in intracranial circulatory arrest. J Neurosurg 1988;68:745–751 [PubMed] [Google Scholar]
- 22.Krejza J, Mariak Z, Babikian VL. Importance of angle correction in the measurement of blood flow velocity with transcranial Doppler sonography. AJNR Am J Neuroradiol 2001;22:1743–1747 [PMC free article] [PubMed] [Google Scholar]
- 23.Schoning M, Buchholz R, Walter J. Comparative study of transcranial color duplex sonography and transcranial Doppler sonography in adults. J Neurosurg 1993;78:776–784 [DOI] [PubMed] [Google Scholar]
- 24.Alexandrov AV, Bladin CF, Norris JW. Intracranial blood flow velocities in acute ischemic stroke. Stroke 1994;25:1378–1383 [DOI] [PubMed] [Google Scholar]
- 25.Babikian VL, Gomes J, Krejza J. Assessment of cerebrovascular pathophysiology. In: Pinsky RM, ed. Cerebral Blood Flow: Mechanisms of Ischemia, Diagnosis and Therapy. Berlin: Springer-Verlag;2002. :201–216
- 26.Muller M, Schimrigk K. A comparative assessment of cerebral hemodynamics in the basilar artery and carotid territory by transcranial Doppler sonography in normal subjects. Ultrasound Med Biol 1994;20:677–687 [DOI] [PubMed] [Google Scholar]
- 27.Ringelstein EB. Kahlscheuer B, Niggemeyer E, Otis SM. Transcranial Doppler sonography: anatomical landmarks and normal velocity values. Ultrasound Med Biol 1990;16:754–761 [DOI] [PubMed] [Google Scholar]
- 28.Martin PJ, Evans DH, Naylor AR. Transcranial color-coded sonography of the basal cerebral circulation: reference data from 115 volunteers. Stroke 1994;25:390–396 [DOI] [PubMed] [Google Scholar]
- 29.Scheel P, Ruge C, Petruch UR, Schoning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke 2000;31:147–150 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez G, Wartenkin S, Risberg J, Rosadini G. Sex differences in regional blood flow. J Cereb Blood Flow Metab 1988;8:783–789 [DOI] [PubMed] [Google Scholar]
- 31.Coffey CE, Lucke JF, Saxton JA, et al. Sex differences in brain aging - A quantitative magnetic resonance imaging study. Arch Neurol 1998;55:169–179 [DOI] [PubMed] [Google Scholar]
- 32.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999;340:1801–1811 [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS. The molecular and neuroanatomical basis for estrogen effects in the central nervous system. J Clin Endocrinol Metab 1999;84:1790–1797 [DOI] [PubMed] [Google Scholar]
- 34.Sonesson B, Lanne T, Vernersson E, Hansen F. Sex difference in the mechanical-properties of the abdominal aorta in human beings. J Vasc Surg 1994;20:959–969 [DOI] [PubMed] [Google Scholar]
- 35.Fischer GM, Bashey RI, Rosenbaum H, Lyttle CR. A possible mechanism in arterial-wall for mediation of sex difference in atherosclerosis. Exp Mol Pathol 1985;43:288–296 [DOI] [PubMed] [Google Scholar]
- 36.Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol 2001;37:1374–1380 [DOI] [PubMed] [Google Scholar]
- 37.Jonason T, Henriksen E, Kangro T, Vessby B, Ringqvist I. Menopause is associated with the stiffness of the common carotid artery in 50-year-old women. Clin Physiol 1998;18:149–155 [DOI] [PubMed] [Google Scholar]