Abstract
BACKGROUND AND PURPOSE: Mitochondrial dysfunction has been reported in HIV-negative children perinatally exposed to zidovudine, a drug often used in HIV-seropositive mothers during pregnancy. The purpose of this study was to determine the incidence of cerebral MR imaging findings in HIV-uninfected children exposed to zidovudine who present with unexplained neurologic symptoms.
METHODS: Two expert groups conducted a systematic, retrospective review of all cerebral MR images available in a multicentric, nationwide French prospective cohort of children born to HIV-seropositive mothers to identify imaging abnormalities. Experts were blinded to each others’ interpretations, to the children’s neurologic symptoms, and to laboratory evidence of mitochondrial dysfunction. The incidence of abnormalities was determined and compared with the neurologic presentation and laboratory evidence of mitochondrial dysfunction.
RESULTS: MR images from 49 HIV-uninfected children (mean age, 26 months) were available for study. All children were perinatally exposed to zidovudine. Twenty-two had probable or established mitochondrial dysfunction according to their symptoms and laboratory data. Twenty-seven children without mitochondrial dysfunction presented with unexplained neurologic symptoms (n = 14) or nonneurologic symptoms (n = 7), and six were asymptomatic. Sixteen of 22 MR images in children with mitochondriopathy were considered abnormal in both independent analyses. Diffuse hyperintensity in the supratentorial white matter (n = 9) and in the tegmentum pons (n = 9) were the most frequent anomalies. Imaging abnormalities were often multifocal (n = 10) and sometimes associated with necrotic areas (n = 3) and volume loss (n = 8). Although 19 of 27 MR images of children without mitochondrial dysfunction were mainly normal, abnormal images were observed in five of 14 children with unexplained neurologic symptoms and in three of six asymptomatic children.
CONCLUSION: Images observed in children with antiretroviral-induced mitochondrial dysfunction are similar to those observed in congenital mitochondrial diseases. These images were also observed in symptomatic or asymptomatic children without evidence of systemic mitochondrial dysfunction.
In the absence of prophylaxis, approximately 80%–85% of children born to HIV-1–seropositive mothers are uninfected. After 20 years of the HIV epidemic, no specific morbidity has been described in these HIV uninfected children, other than diseases induced by other frequently associated conditions, such as prematurity or maternal drug addiction. The effectiveness of preventing mother-to-child transmission of HIV is now well established and one of the major successes of antiretroviral therapy (1). The risk of the child becoming infected, at least in industrialized countries, has decreased to 1–2%. Although tolerance to antiretroviral agents, such as zidovudine, is generally accepted as being low for most exposed children (2), their long-term effects are not completely known (3).
We previously reported several observations in HIV-uninfected children born to seropositive mothers and perinatally exposed to zidovudine who had persistent mitochondrial dysfunction with neurologic symptoms (4). Although the results in that small cohort were initially contested (5), the association was later confirmed in a larger study (6).
The purpose of this pilot retrospective study was twofold. First, we sought to review all available cerebral MR images from the nationwide French prospective cohort of children born to HIV-seropositive mothers. Second, we stratified the results according to a probability scale of mitochondrial dysfunction. Our working hypothesis was that HIV-uninfected children who present with unexplained neurologic symptoms and laboratory evidence of mitochondrial dysfunction, and who are perinatally exposed to zidovudine have an increased incidence of MR imaging findings.
Methods
Each center participating in the nationwide French prospective study of children born to HIV-seropositive mothers—the Enquête Périnatale Française (EPF) (7, Appendix)—were asked to provide the coordinating center with all cerebral MR images obtained in a subgroup of HIV-exposed but uninfected children who presented with unexplained symptoms and who were at least 6 months of age. The follow-up protocol for this study did not require systematic collection of imaging data, and the indications for such examinations were left to the discretion of the attending physicians. In addition to the symptomatic children, six asymptomatic uninfected children from two centers were also included. These two centers had decided to systematically perform MR imaging for the past 2 years at the end of their follow-up for a study of children aged 18–24 months. The decision to systematically perform MR imaging was made after our observations, which we described in our first report of abnormal MR imaging findings in uninfected children without neurologic symptoms.
The coordinating center (Appendix) received cerebral MR images for 54 children, which formed the basis of this review. MR images in five subjects were excluded because of their poor technical quality. Eleven children underwent at least two MR imaging examinations. All but four children described in the first two reports of mitochondrial toxicity of antiretrovirals were included in this analysis (4, 6).
MR Imaging
Because the study was undertaken retrospectively and spanned 3 years, a common MR imaging protocol was unavailable. Imaging was performed at a variety of centers with differing facilities (eg, different manufacturers and field strengths). In all cases, T2-weighted images were obtained with different sequence designs (TRs and TEs). A fluid-attenuated inversion recovery (FLAIR) sequence was performed in all but two subjects. Most images were obtained in the axial plane, coronal plane, or both. T1-weighted imaging was performed in every case but was not consistently useful for evaluating tissue characteristics. Diffusion-weighted imaging (DWI) and spectroscopy, which were not routinely available until recently, were not performed in any patient.
Images were interpreted in two independent and blinded reviews (Appendix). In the first review, a group of pediatric neurology experts and two pediatric neuroradiologists (C.R., F.B.) independently examined all images in two separate sessions. The observers interpreted the images knowing the age of the child on examination, but they were blinded to the child’s symptoms and the results of an ongoing mitochondrial laboratory evaluation. Their conclusions were based on a consensus opinion and classified by using a scheme established from all of the observed anomalies. The following features were noted: 1) abnormalities involving the supratentorial white matter with respect to the localization and intensity of abnormal signal intensity (ie, diffuse or posterior and areas abnormal hyperintensity); 2) abnormalities involving the brainstem, more precisely, the tegmentum pons; 3) abnormalities involving the basal ganglia; 4) volume loss (global or limited to the corpus callosum); and 5) other findings. Because the normal variability of hyperintensity in the posterior regions in young children, isolated hyperintensity limited to this area and not associated with other abnormal images was classified as normal.
A third independent pediatric neuroradiologist (W.B.) conducted the second review. This observer also read the images in a random order, blinded to the conclusions of the first analysis and isolated from the first expert group. His interpretations were classified as described before.
The interobserver agreement between the first observer group and the second observer was determined by calculating the κ coefficient (8). In cases of divergent interpretations by the two groups, the least severe interpretation was used in the final analysis of the results. Thus, an MR image that one group judged to be normal and that the other considered abnormal was considered normal, regardless of the number and intensity of anomalies observed. If both groups considered the MR imaging findings to be abnormal, only the anomalies that both groups observed were included in the analysis.
Neurologic Assessment
A neuropediatrician evaluated all children with neurologic symptoms; their neurologic findings were recorded in detail. Cognitive development was assessed by using the Denver or Brunet Lezine scale (in children younger than 5 years). Children with well-defined hereditary or acquired neurologic disease were excluded from the study.
Mitochondrial Dysfunction Diagnostic Scale
A detailed description of the scale we used and its validation are described elsewhere (6). In summary, the criteria were the same as those used for constitutional mitochondrial disease. Established mitochondrial dysfunction was diagnosed when profound deficit of at least one complex of the respiratory chain and/or a major histologic finding on muscular biopsy were present. Probable mitochondrial dysfunction was defined as compatible symptoms, persistent hyperlactatemia (≥2.5 mmol/L), and at least two minor histologic findings on biopsy. For this analysis, the two levels of probability were grouped together.
Results
Demographic and Clinical Description of the Children
The mean age of the 49 children was 26 months (range, 10–44 months) at the time of their first MR imaging examination. All of the HIV-uninfected children had been exposed to at least one antiretroviral agent before, during, and/or after birth. Table 1 lists their general physical characteristics, which were consistent with those of the French cohort as a whole.
TABLE 1:
Clinical and demographic characteristics of mothers and children
| Characteristic | No. |
|---|---|
| Children | 49 |
| Girls | 20 |
| Boys | 29 |
| Mode of mother’s infection | |
| Past drug addiction | 5 |
| Transfusion | 1 |
| Sexual | 40 |
| Mother’s geographic origin* | |
| Europe | 14 |
| Sub-Saharan Africa | 26 |
| North Africa | 4 |
| Other | 2 |
| Premature births | |
| <32 wk | 0 |
| <37 wk | 9 |
| ≥37 wk | 40 |
| Birth weight | |
| <2500 g | 11 |
| ≥2500 g | 34 |
| Not known | 4 |
| Birth | |
| Vaginal delivery | 30 |
| Cesarean delivery | 18 |
| Not known | 1 |
| Perinatal prophylactic treatment | |
| Zidovudine | 13 |
| Zidovudine + lamivudine | 19 |
| Zidovudine + other | 2 |
| Zidovudine + lamivudine + other | 15 |
All of the children were born in France.
The investigators in charge of the care of each of the children noted no obstetric or infectious history likely to cause neurologic dysfunction. None of the children had been exposed to any treatment other than the antiretroviral agent(s) and standard iron and/or vitamin supplements during pregnancy.
Twenty-two children (45%) had a diagnosis of mitochondrial dysfunction (established in 11, probable in 11), with neurologic symptoms consisting of cognitive delay (n = 15), motor dysfunction (n = 6), and/or nonfebrile seizure (n = 6) (Tables 1 and 2). Fourteen children had unexplained neurologic symptoms but without sufficient evidence of mitochondrial pathology (n = 14). Seven had undergone MR imaging as part of a general examination motivated by unexplained nonneurologic symptoms. Six asymptomatic children underwent MR imaging as part of a systematic screening program at the end of a follow-up examination for children aged 18–24 months (Table 2) who were born to HIV-seropositive mothers.
TABLE 2:
Clinical symptoms
| Children | No. |
|---|---|
| With mitochondrial dysfunction | 22 |
| Cognitive delay | 15 |
| Dystonia | 6 |
| Nonfebrile seizures | 6 |
| Severe malaise | 3 |
| Nystagmus | 2 |
| Other | 4 |
| Without mitochondrial dysfunction | 27 |
| Neurologic symptoms | 14 |
| Cognitive delay | 5 |
| Nonfebrile seizures | 3 |
| Repeated febrile seizures | 2 |
| Dystonia | 2 |
| Nystagmus | 1 |
| Acute idiopathic polyneuritis | 1 |
| Other | 2 |
| Persistent biochemical perturbations | 7 |
| Asymptomatic | 6 |
Analysis of MR Images
Agreement in the interpretations of the two groups was achieved in 43 of 49 children. One-half (50%) of all MR images were scored as unequivocally abnormal in both independent expert analyses. The imaging findings mostly consisted of diffuse hyperintensity in the white matter (n = 13) and/or anomalies in the brainstem (n = 14), which manifested as hyperintensity in the tegmentum pons (Table 3). In four children, abnormality was observed in the basal ganglia (n = 4). Ten children were classified as having cerebral volume loss (supposed atrophy). In three children, experts observed manifestations of necrosis in the white matter, evident as liquefaction of the white matter on T1- and T2-weighted images. Disagreement remained in six children: In three, both groups considered the MR images abnormal, but the type or intensity of the findings was scored differently. In three children, the groups disagreed about the presence or absence of notable lesions, which involved diffuse atrophy in one child, brainstem hyperintensity associated with hyperintensity in the posterior white matter in another child, and diffuse white matter hyperintensity in the last child. The κ coefficient of concordance was highly significant for each of the qualitative parameters tested (Table 3).
TABLE 3:
κ Coefficient for the two groups of experts
| Finding | κ |
|---|---|
| Normal vs abnormal* | 0.83 ± 0.16 |
| Diffuse anomaly of the white matter | 0.93 ± 0.15 |
| Anomaly of the brainstem | 0.87 ± 0.14 |
| Anomaly of the basal ganglia | 0.78 ± 0.15 |
| Supratentorial atrophy | 0.82 ± 0.15 |
Note.—Data are the mean ± standard deviation.
Normal MR images included those showing an isolated anomaly of the posterior white matter.
Stratification According to Potential Mitochondrial Dysfunction
Children with Mitochondrial Dysfunction.—
Twenty-two children (45%) had established or probable evidence of mitochondrial dysfunction. Sixteen of them had MR images that were considered abnormal, showing the following patterns: diffuse hyperintensity of the white matter greater than that expected for the child’s age (n = 9) and hyperintensity in the tegmentum pons (n = 9). Ten of the 16 children with abnormal MR images had a combination of diffuse hyperintensity in the white matter and in the tegmentum pons. Eight children also had evidence of volume loss (ie, atrophy), and three had evidence consistent with areas of necrosis (Table 4, Figs 1 and 2).
TABLE 4:
Findings observed on MR images
| Finding | Children with Mitochondrial Dysfunction (n = 22) | Children without Mitochondrial Dysfunction (n = 27) | Total (n = 49) |
|---|---|---|---|
| Normal | 6 | 19 | 25 |
| Abnormal | 16 | 8 | 24 |
| White matter | |||
| Necrosis | 3 | 0 | 3 |
| Diffuse | 9 | 4 | 13 |
| Posterior | 4 | 1 | 5 |
| Brainstem | 9 | 5 | 14 |
| Basal ganglia | 4 | 0 | 4 |
| Supratentorial atrophy | 8 | 2 | 10 |
| Other* | 1 | 2 | 3 |
Vermian atrophy, n = 1; hyperintensity in one cerebellar hemisphere, n = 1; and hyperintensity in both cerebellar hemispheres, n = 1.
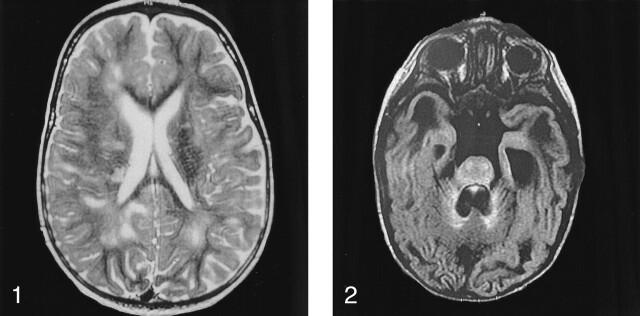
Fig 1.
Image in a boy aged 1 year 5 month with cognitive delay, dystonia, and nystagmus. Results of muscular biopsy established mitochondrial dysfunction. Axial T2-weighted MR image shows diffuse hyperintensity in the periventricular white matter. Note bilateral areas of necrosis.
Fig 2.
Image in a boy aged 10 months with severe cognitive delay and repeated seizures. Results of muscular biopsy established mitochondrial dysfunction. Axial FLAIR MR image shows diffuse hyperintensity in the tegmentum pons, basilar pons, and cerebellar vermis.
Children without Mitochondrial Dysfunction.—
Of the remaining 27 children without evidence of mitochondrial dysfunction, eight had MR images that were considered abnormal. In four children, images showed diffuse hyperintensity in the white matter, and in five, images showed abnormalities in the brainstem similar to those described for children with mitochondrial dysfunction. A combination of hyperintensity in both the white matter and the tegmentum pons were observed in four of eight children with abnormal MR images, and no necrotic areas were detected in this group. Three of six asymptomatic children in this group who underwent imaging as part of routine monitoring were judged to have notable abnormalities on MR imaging. Two children had diffuse hyperintensity of the white matter, one of whom also had hyperintensity in the tegmentum pons. In the third child, T2-weighted images showed hyperintensity in the tegmentum pons in association with hyperintensity in the posterior white matter (Table 4, Figs 3 and 4).
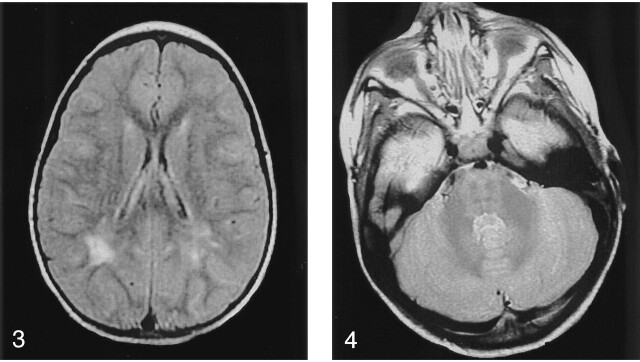
Fig 3.
Image in an asymptomatic boy aged 2 years. Axial FLAIR MR image shows hyperintensity in the bilateral periventricular white matter in the posterior trigonal regions.
Fig 4.
Image in an asymptomatic boy aged 2 years. Axial T2-weighted MR image shows hyperintensity of the brainstem and around the dentate nuclei.
Repeat Examinations
Eleven of 49 children underwent at least two examinations: Eight were symptomatic, and three, asymptomatic. All had an initial MR image that was abnormal. The mean time between the first and second examinations was 18 months (range, 5–41 months). All repeat MR images were scored as abnormal. Informative comparisons of image intensity were considered impossible because of the effects of myelin maturation between the examinations.
Discussion
Both independent expert groups unambiguously scored one-half of the MR images collected for this study as abnormal. The pathologic feature of hyperintensity in the white matter is always difficult to establish in children younger than 2 or even 3 years because of myelin maturation. Because it was impossible to constitute an appropriate control group, we performed two independent analyses. The agreement between the two analyses was excellent, with a κ coefficient of 0.8 overall and above 0.9 for diffuse abnormalities in the supratentorial white matter. However, abnormalities in signal intensity are not specific, and they can be the consequence of numerous physiopathologic processes. This finding can be associated with diverse causes of perinatal injury; maternal drug abuse; and various viral infections, including cytomegaloviral infection. Nevertheless, we were able to exclude all of the known causes that were likely to induce similar clinical and radiologic symptoms, as found in this study population. In the context of maternal HIV infection, particular attention was given to possible drug addiction of the mother during pregnancy, the devastating effect of which on the fetus is well known (9, 10). All children born to a drug-addicted mother who used or potentially used illicit drugs during pregnancy were excluded from the analysis.
The only exposure that was common to all these children was antiretroviral prophylaxis with zidovudine (Retrovir) either alone or in association with other antiretroviral agents. A link between this prophylaxis and MR imaging results is plausible, but it remains a hypothesis in the absence of appropriate controls. Zidovudine neurotoxicity in HIV-infected patients has not clearly been demonstrated (11). The mitochondrial toxicity of zidovudine is well established, but its main target is muscle (12). Neurologic involvement with clinical symptoms and massive white matter hyperintensity on MR imaging has been described during a severe episode of lactic acidosis induced by a nucleoside analog, a rare complication of a regimen involving zidovudine (13). However, the neurotropism of HIV, as well as the numerous neurologic opportunistic infections observed in HIV-infected patients, makes it difficult to accurately assess a potential neurologic toxicity associated with antiretroviral treatment. Perinatally exposed neonates are in a different situation. They are not infected by HIV, partly because of prophylaxis, and their time of exposure to zidovudine is limited. The drug is generally prescribed during the second half of the pregnancy and then to the neonate for 6 weeks after birth (14). Although the treatment is now often more complex because of combination drug therapy and although administration continues throughout pregnancy in some cases, zidovudine alone or in combination remains the most frequently prescribed drug for this application in both North America and Europe.
In rat and mouse models, in utero exposure to zidovudine leads to abnormal behavior of unknown pathophysiology and of unknown relevance to man (15). Closer to the human model at birth, monkeys exposed in utero have unambiguous morphologic and functional abnormalities in the mitochondria of various organs, including the brain (16, 17). Recent work in a monkey model, revealed a profound depletion of brain mitochondrial DNA after in utero exposure to a combination drug regimen that included zidovudine (18). The potential clinical effect and the reversibility of these findings remain to be established, and even the extent to which they can be extrapolated to the human neonate is unclear.
However, growing evidence now suggests that human neonates exposed to zidovudine also have mitochondrial damage, at least transiently. During the first months of life, approximately one-third of children have substantial hyperlactatemia, sometimes persisting until the age of 6–12 months (19). Mitochondrial DNA depletion and ultrastructural mitochondrial pathology have also been described in peripheral blood lymphocytes of exposed neonates (20, 21). Despite such evidence, the long-term consequences of this mitochondrial dysfunction must be rigorously evaluated and determined. Several observations of mitochondrial dysfunction with neurologic expression in children exposed to zidovudine in utero have been described (4). These led to a specific screening program to detect and record symptoms consistent with mitochondrial dysfunction in HIV-uninfected children in the French nationwide cohort (6). An 18-month incidence of 0.3% in 2644 children exposed to zidovudine was reported for those with established mitochondrial dysfunction, and 0.8% was reported for those with probable mitochondrial disease. No such cases were observed among the 1748 infants not exposed to antiretroviral agents. These findings are to be compared with the general rate of 0.01% for pediatric neuromitochondrial disease in the general population (22, 23).
Compared with the exhaustive clinical screening data previously described, results of present retrospective analysis of MR images can be considered only preliminary evidence of neuroradiologic findings in children born to HIV-infected mothers. This study had several weaknesses. For instance, the indications for MR imaging differed from center to center, and the severity of neurologic symptoms leading to MR imaging were different across the study population. Furthermore, two centers performed MR imaging in asymptomatic children. Finally, the variety of scanners and pulse sequences potentially limit definitive conclusions. Analysis of the MR imaging data was nevertheless informative, revealing consistent abnormalities in 16 of 22 children with mitochondrial disease. Although the images were not specific, they were entirely consistent with those observed in cases of congenital or constitutional mitochondrial disease (24–27). Particularly notable were the anomalies of the brainstem (ie, hyperintensities in the tegmentum pons), which were observed in nearly one-half the cases. Most of the MR imaging data in children without clear evidence of mitochondrial dysfunction were normal. Nevertheless, in some children, MR images showed abnormalities similar to those observed in children with mitochondrial dysfunction, though the magnitude of the changes in signal intensity was less pronounced, and no evidence for necrosis was observed. The similarity of these anomalies, particularly in the brainstem, suggested that a similar pathologic process might have been occurring, even in asymptomatic subjects; mitochondrial dysfunction could be limited to the brain or transient leading to a white matter sequela, however, this is only speculation.
Newer MR imaging technologies such diffusion-weighted imaging, diffusion tensor imaging, and spectroscopy might be useful for further investigation. Proton MR spectroscopy has proved to be helpful in the assessment of several groups with known mitochondrial metabolic disease (28, 29). Because nucleoside analogues alter mitochondrial functions, specific abnormalities, such as a lactate peak, on both the long- and short TE-spectra could be expected. Also, because several interpretations of abnormal signal intensity in the white matter are possible, spectral patterns might help in identifying the pathophysiologic cause of the imaging abnormalities. Finally, studies in an animal model could allow for a placebo arm for comparison. In children with unexplained neurologic symptoms and/or biochemical disturbances, we propose careful evaluation by using newer MR imaging technologies to better delineate the spectrum and importance of abnormalities found on MR imaging.
Conclusion
Images observed in children with antiretroviral-induced mitochondrial dysfunction are similar to those observed in patients with congenital mitochondrial diseases. The most frequent abnormalities consisted of hyperintensity in the central white matter and in the tegmentum pons. Similar findings were also observed in symptomatic or asymptomatic children without mitochondrial dysfunction. Neurologic toxicity of in utero zidovudine exposure must be carefully evaluated.
Appendix
French Expert Group for the First Review
Marc Tardieu, co-coordinator (Neurology, Hôpital Bicêtre AP-HP, Le Kremlin Bicêtre); Francis Brunelle, co-coordinator (Neuroradiology, Hôpital Necker AP-HP, Paris); Béatrice Husson (Radiology, Hôpital Bicêtre AP-HP, Le Kremlin Bicêtre); Isabelle Desguerres (Neurology, Hôpital St Vincent de Paul AP-HP, Paris); Catherine Adamsbaum (Radiology, Hôpital St Vincent de Paul AP-HP, Paris); Charles Raybaud (Neuroradiology, Hôpital de la Timone, Marseille); and Thierry Billette de Villemeur (Neurology Hôpital Trousseau AP-HP, Paris).
Foreign Expert for the Second Review
William Ball, Neuroradiology, Children’s Hospital Medical Center Cincinnati, OH.
Centers and Investigators of Enquête Périnatale Française Participating in the Study
Centre Hospitalier Amiens (Brigitte Pautard); Centre Hospitalier Argenteuil (Dominique Brault, Christine Alisy); Hôpital Bicêtre AP-HP (Marc Tardieu); Hôpital Jean Verdier AP-HP, Bondy (Eric Lachassinne); Centre Hospitalier d’Evry (Adrien May); Hôpital Louis Mourier AP-HP, Colombes (Corinne Floch, Fabienne Mazy); Centre Hospitalier de Marseille (Isabelle Thuret, Brigitte Chabrol); Hôpital Necker AP-HP, Paris (Stéphane Blanche, Pierre Quartier); Hôpital Robert Debré AP-HP, Paris (Martine Levine, Nathalie Leblanc); Hôpital St Vincent de Paul AP-HP, Paris (Ghislaine Firtion, Isabelle Desguerres); Centre Hospitalier d’Annemasse (Hervé Testard); Centre Hospitalier de Saint-Brieux (Claire de Barrace); Centre Hospitalier de Saint Laurent du Maroni, French Guyana (Anne-Marie Zoccarato); Hôpital Trousseau AP-HP, Paris (Catherine Dollfus, Thierry Billette de Villemeur); Hôpital de Bayonne (Xavier Hernandorena); Hôpital Lariboisière AP-HP, Paris (Nicole Ciraru-Vigneron, Claudine Brunner); and Hôpital Antoine Béclère AP-HP, Clamart (Michèle Vial).
Coordinating Team
Béatrice Barret and Marie-Jeanne Mayaux (Inserm U569 Hôpital Bicêtre AP-HP, Le Kremlin Bicêtre), Marc Tardieu (Service de Neurologie Pédiatrique, Hôpital Bicêtre AP-HP, Le Kremlin Bicêtre), and Stéphane Blanche (Unité d’Immunologie-Hématologie Pédiatrique, Hôpital Necker AP-HP, Paris).
Footnotes
Supported by the Agence Nationale de Recherche sur le SIDA, Paris, France, and performed in coordination with the Réseau de Pharmacovigilance Français (F. Bavoux, MD, Hôpital St Vincent de Paul, APH-HP, Paris, France).
References
- 1.Mofenson L, McIntyre J. Advances and research directions in the prevention of mother to child HIV-1 transmission. Lancet 2000;355:2237–2244 [DOI] [PubMed] [Google Scholar]
- 2.Taylor GP, Low-Beer N. Antiretroviral therapy in pregnancy: a focus on safety. Drug Safety 2001;24:683–702 [DOI] [PubMed] [Google Scholar]
- 3.Culnane M, Fowler M, Lee SS, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women: Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. JAMA 1999;281:151–157 [DOI] [PubMed] [Google Scholar]
- 4.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet 1999;354:1084–1089 [DOI] [PubMed] [Google Scholar]
- 5.The Perinatal Safety review working group. Nucleoside exposure in the children of HIV-infected women receiving antiretroviral drugs: absence of clear evidence for mitochondrial disease in children who died before 5 years of age in five United States cohorts. J Acquir Immune Defic Syndr 2000;25:261–268 [DOI] [PubMed] [Google Scholar]
- 6.Barret B, Tardieu M, Rustin P, et al. Mitochondrial dysfunction in HIV uninfected children. AIDS 2003;17:1769–1785 [DOI] [PubMed] [Google Scholar]
- 7.Blanche S, Rouzioux C, Moscato ML, et al. A prospective study of infants born to women seropositive for human immunodeficiency virus type 1: HIV Infection in Newborns French Collaborative Study Group. N Engl J Med 1989;320:1643–1648 [DOI] [PubMed] [Google Scholar]
- 8.Nelson JC, Pepe MS. Statistical description of interrater variability in ordinal ratings. Stat Methods Med Res 2000;9:475–496 [DOI] [PubMed] [Google Scholar]
- 9.Filley CM, Kleinschmidt-Demasters BK. Toxic leukoencephalopathy. N Engl J Med 2001;345:425–432 [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Calamandrei G. Delayed developmental effects following prenatal exposure to drugs. Curr Pharm Des 2001;7:859–880 [DOI] [PubMed] [Google Scholar]
- 11.Wynn HE, Brundage RC, Fletcher CV. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs 2002;16:595–609 [DOI] [PubMed] [Google Scholar]
- 12.Brinkman K, Ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effect of reverse transcriptase inhibitors: mitochondrial toxicity as a common path way. AIDS 1998;12:1735–1744 [DOI] [PubMed] [Google Scholar]
- 13.Church JA, Mitchell WG, Gonzalez-Gomez I, et al. Mitochondrial DNA depletion, near fatal metabolic acidosis and liver failure in an HIV infected child treated with combination antiretroviral therapy. J Pediatr 2001;138:748–751 [DOI] [PubMed] [Google Scholar]
- 14.Loutfy MR, Walmsley SL. Treatment of HIV infection in pregnant women: antiretroviral management options. Drugs 2004;64:471–488 [DOI] [PubMed] [Google Scholar]
- 15.Venerosi A, Calamendrei G, Alleva E. Animal models of antiHIV drugs exposure during pregnancy: effect on neurobehavioral development. Prog Neuropsychopharmacol Biol Psychiatry 2002;26:747–761 [DOI] [PubMed] [Google Scholar]
- 16.Gerschenson M, Erhart SW, Paik CY, et al. Fetal mitochondrial heart and skeletal muscle damage in Erythrocebus patas monkeys exposed in utero to 3′-azido-3′-deoxythymidine. AIDS Res Hum Retroviruses 2000;16:635–644 [DOI] [PubMed] [Google Scholar]
- 17.Ewings EL, Gerschenson M, St Claire MC, et al. Genotoxic and functional consequences of transplacental zidovudine exposure in fetal monkey brain mitochondria. J Acquir Immune Defic Syndr 2000;24:100–105 [DOI] [PubMed] [Google Scholar]
- 18.Gerschenson M, Nguyen V, Ewings EL, et al. Mitochondrial toxicity in fetal Erythrocebus patas monkeys exposed transplacentally to zidovudine + lamivudine. AIDS Res Huma Retroviruses 2004;20:91–100 [DOI] [PubMed] [Google Scholar]
- 19.Alimenti A, Burdge D, Ogilvie A, et al. Lactic acidemia in HIV infants exposed to perinatal antiretroviral therapy. Pediatr Inf Dis J 2003;22:782–789 [DOI] [PubMed] [Google Scholar]
- 20.Shiramizu B, Shikuma KM, Kanemoto L, et al. Placenta and cord blood mitochondrial DNA toxicity in HIV-infected women receiving nucleoside reverse transcriptase inhibitors during pregnancy. J Acquir Immune Defic Syndr 2003;32:370–374 [DOI] [PubMed] [Google Scholar]
- 21.Poirier MC, Divi RL, Al-Harti L, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr 2003;33:175–183 [DOI] [PubMed] [Google Scholar]
- 22.Uusimaa J, Remes AM, Rantala H, et al. Childhood encephalopathies and myopathies: a prospective study in a defined population to assess the frequency of mitochondrial disorders. Pediatrics 2000;105:598–603 [DOI] [PubMed] [Google Scholar]
- 23.Darin N, Oldfors A, Mosleni AR, Holme E, Tulinius M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological biochemical and DNA abnormalities. Ann Neurol 2001;49:377–383 [PubMed] [Google Scholar]
- 24.Valanne L, Ketonen L, Majander A, et al. Neuroradiologic findings in children with mitochondrial disorders. AJNR Am J Neuroradiol 1998;19:369–377 [PMC free article] [PubMed] [Google Scholar]
- 25.Munoz A, Mateos F, Simon R, et al. Mitochondrial diseases in children: neuroradiological and clinical features in 17 patients. Neuroradiology 1999;41:920–928 [DOI] [PubMed] [Google Scholar]
- 26.Arii J, Tanabe Y. Leigh syndrome: serial MR imaging and clinical follow-up. AJNR Am J Neuroradiol 2000;21:1502–1509 [PMC free article] [PubMed] [Google Scholar]
- 27.de Lonlay-Debeney P, Von Kleist-Retzow JC, Hertz-Pannier L, et al. Cerebral white matter disease in children may be caused by mitochondrial respiratory chain deficiency. J Pediatr 2000;136:209–214 [DOI] [PubMed] [Google Scholar]
- 28.Ulmer S, Flemming K, Hahn A, Stephani U, Jansen O. Detection of acute cytotoxic changes in progressive neuronal degeneration of childhood with liver disease (Alpers-Huttenlocher syndrome) using diffusion-weighted MRI and MR spectroscopy. J Comput Assist Tomogr 2002;26:641–646 [DOI] [PubMed] [Google Scholar]
- 29.Abe K, Yoshimura H, Tanaka H, Fujita N, Hikita T, Sakoda S. Comparison of conventional and diffusion-weighted MRI and proton MR spectroscopy in patients with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like events. Neuroradiology 2004;46:113–7 [DOI] [PubMed] [Google Scholar]