Abstract
BACKGROUND AND PURPOSE: Cerebral white matter (WM) hyperintensities are a frequent finding in elderly people, and lowering of cerebral magnetization transfer ratio (MTR) has been observed. The aim of this study was to assess the relationship between age-related WM hyperintensities and MTR changes in the brain.
METHODS: We performed MR imaging in a group of young subjects, a group of elderly individuals with minimal WM hyperintensities, and a group of elderly individuals with abundant WM hyperintensities. In addition, we performed volumetric MTR analysis of the whole brain and of the normal-appearing WM (NAWM) in these groups.
RESULTS: Volumetric MTR parameters differed between elderly and young patients. Mean MTR ± standard error of the mean (SEM) was 34.0% ± 0.12% in the young, 33.0% ± 0.08% in the elderly with minimal WM hyperintensities, 32.8% ± 0.09%) in the group with abundant WM hyperintensities. Peak height (number of voxels ± SEM) was 122 ± 1.2 in the young, 99 ± 1.5 in the elderly with minimal WM hyperintensities, and 98 ± 1.6 in the group with abundant WM hyperintensities. Mean MTR of NAWM was lower in the elderly compared with the young (36.7% ± 0.12%) but did not differ between subjects with minimal (36.0% ± 0.11%) and those with abundant WM hyperintensities (35.9% ± 0.13%).
CONCLUSION: Our results show that aging gives rise to changes in normal-appearing brain tissue. These changes, which can be detected on magnetization transfer imaging, seem to have no relationship with age-related WM hyperintensities and might have a different etiology.
White matter (WM) hyperintensities are often observed on T2-weighted images of the brain in elderly subjects. WM hyperintensities occur in about 30% of healthy subjects older than 60 years, and the prevalence of these lesions rises steadily with increasing age (1). Apart from age, other established risk factors are female sex (2), aortic atherosclerosis (3), and elevated systolic blood pressure (4). Furthermore, subjects with vascular dementia have more WM hyperintensities than age-matched subjects with good cognitive function (1).
Magnetization transfer imaging (MTI) is a MR technique that exploits the transfer of magnetization between a pool of protons bound to macromolecules and a pool of free protons (5). The amount of transfer of magnetization between these pools can be measured and quantified by calculating the magnetization transfer ratio (MTR) (6). Demyelinating disorders have been associated with low MTR values (5). Hence, the myelin components in the brain are the macromolecules thought to be responsible for the MTR abnormalities (7).
Low MTR values have been found in age-related WM hyperintensities (8–10). Moreover, in brain areas with a normal appearance on conventional MR images, MTR values are lower in the elderly compared with younger subjects (11). However, whether age-related WM hyperintensities and changes in normal-appearing brain tissue are different manifestations of the same underlying pathology or whether they reflect different neurodegenerative processes is unknown.
The aim of this study was to assess the relationship between age-related WM hyperintensities and changes in normal-appearing brain tissue. We examined a group of young subjects, a group of elderly subjects with minimal WM hyperintensities, and a group elderly subjects with abundant WM hyperintensities to determine 1) whether young subjects have whole-brain MTR values different from those of elderly subjects, 2) whether elderly subjects with minimal WM hyperintensities have whole-brain MTR values different from those of elderly subjects with a large load WM hyperintensities, and 3) whether normal-appearing WM (NAWM) in elderly subjects with minimal WM hyperintensities has MTR values different from those of NAWM in elderly subjects with a large load WM hyperintensities.
Methods
Subjects
We recruited 11 young, healthy volunteers without a history of physical or mental disease. Elderly subjects were selected from the first 225 participants of a randomized clinical trial, the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) study (12). Inclusion criteria for this study were age between 70 and 82 years, increased cholesterol level, cardiovascular event more than 6 months before inclusion, and risk factors for vascular disease (smoking, hypertension, or known diabetes). This study was approved by our institutional review board, and written consent was obtained from all participants.
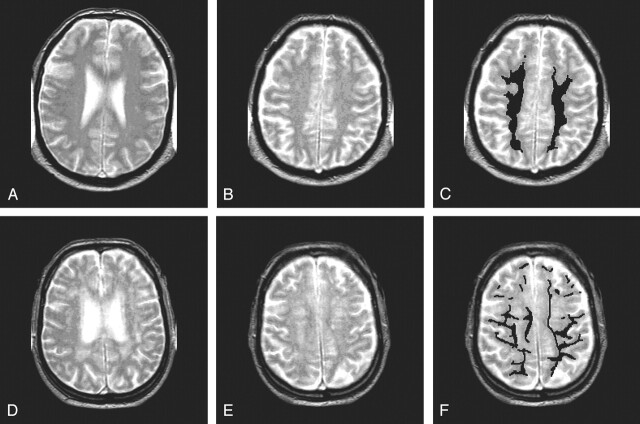
In all subjects, we performed MTI and T2-weighted MR imaging. In the young subjects, no WM hyperintensities were observed. In all elderly subjects, an experienced rater (M.A.v.B.) assessed the Scheltens score (13) of the periventricular and deep WM by using a T2-weighted sequence. The rating for the periventricular region was extended so that lesions larger than 10 mm were given a score of 3 points. The scores for all subdivisions were summed, resulting in a possible maximum score of 33 points. From the 225 elderly subjects, we selected a group with minimal WM hyperintensities (Scheltens score, 5 or less) and a group with a large load WM hyperintensities (Scheltens score, 13 or higher). Figure 1 shows an example of minimal WM hyperintensities and an example of more profound WM hyperintensities. Subjects with unusual age-related changes (eg, excessive atrophy) or subjects with lacunar infarctions with a diameter larger than 5 mm were excluded. Fifty-one subjects with minimal WM hyperintensities and 41 subjects with a large load WM hyperintensities were identified and included in this study.
Fig 1.
T2-weighted images in two subjects. Segmentation was done on a section above the ventricles (B and E). In C and F, segmentation of NAWM is shown.
A–C, Subject with few WM hyperintensities.
D–F, Subject with more extensive WM hyperintensities.
MR Imaging
To assess the extent of WM hyperintensities, a dual fast spin-echo sequence was performed (TR/TE1/TE2 =3000/27 and 120, echo train length = 10, 3-mm section thickness, no intersection gap, 256 matrix, 220-mm FOV, 80% scanning percentage). MTI was performed by using a three-dimensional (3D) gradient-echo pulse sequence (TR/TE = 106/6, 12° flip angle, 5-mm section thickness, 256 matrix, 220-mm FOV, 50% scanning percentage) (14). Two consecutive sets of axial images were acquired: The first was obtained without a radio-frequency saturation pulse, and the second was obtained in combination with a radio-frequency saturation pulse (sinc-shaped, 1100 Hz downfield of H2O resonance).
Postprocessing
For postprocessing of MT images, 3DVIEWNIX semiautomated software (Department of Radiology, Hospital of the University of Pennsylvania, Philadelphia) was used. A detailed description of this procedure has been published (6). In short, the following steps were performed: semiautomated segmentation of the intracranial volume from the 3D MTI study, automated calculation of MTR values for each intracranial voxel, and automated display of all voxels representing the brain as an MTR histogram. MTR was defined as the percentage of change in signal intensity between the images with (Ms) and those without (M0) the saturation pulse by using the following equation: MTR = [(M0 − MS)/M0] × 100%.
Pixels with an MTR value lower than 20% were defined as belonging to the CSF (15). From the MTR histogram the peak height was derived. In addition to lesion load, this parameter depends on physiologic differences in brain size; therefore, it was normalized for brain volume by dividing the individual MTR histogram bins by the total number of brain voxels (16). The normalized MTR histogram peak height (number of voxels) was presumed to represent the integrity of the brain, irrespective of physiologic and pathologic differences in brain size (17).
Besides the volumetric MTR analysis of the whole brain, we also performed a regional analysis of the NAWM. We analyzed the centrum semiovale in the second section above the lateral ventricles to prevent the inclusion of CSF in the ventricles and sulci due to partial volume effects. We selected NAWM by manually segmenting out WM hyperintensities. Figure 1 shows two examples of this segmentation. In the NAWM, we assessed the mean MTR because the relative low number of voxels incorporated in this regional analysis peak height could not be assessed reliably. The reproducibility of mean MTR measurements in NAWM by using the manual outlining method was assessed by calculating the intraclass correlation coefficient by performing the outlining in 10 subjects three times. The intraclass correlation coefficient for this method of selecting the NAWM was 0.95.
Statistical Analysis
To assess differences of volumetric and regional MTR parameters between study groups, unpaired t tests were used. SPSS (SPSS, Inc., Chicago, IL) statistical software was used for all tests. P values < .05 were considered to indicate a significant difference.
Results
Table 1 shows the baseline characteristics of the three study groups. Among the elderly, 51 subjects had a Scheltens score of 5 or less, and 41 subjects had a Scheltens score of 13 or more. As expected, we found a small difference in age between the group of elderly with minimal WM hyperintensities and the group of elderly with a large load of WM hyperintensities.
TABLE 1:
Descriptive details of the study population
| Detail | Young Subjects (n = 11) | Elderly Subjects |
|
|---|---|---|---|
| Minimal WM Hyperintensities(n = 51) | High WM Hyperintensities (n = 41) | ||
| Mean age ± SD(y) | 25.9 ± 4.0 | 73.8 ± 3.4 | 75.6 ± 2.9 |
| Sex | |||
| Men | 2 (18) | 18 (35) | 24 (59) |
| Women | 9 (82) | 33 (65) | 17 (41) |
| Median Scheltens score | 0 | 3 (0–5) | 20 (13–33) |
Note.—Data in parentheses are the percentage or range. SD = standard deviation.
In young subjects, the normalized peak height of the MTR histogram was 122. In elderly subjects with minimal WM hyperintensities, the peak height was 99, and in elderly subjects with extensive WM hyperintensities, it was 98 (Table 2). Differences in peak height between the young subjects and both groups of elderly subjects were significant (both P < .005). However, no difference in peak height was observed between the elderly group with minimal WM hyperintensities and the elderly group with extensive WM hyperintensities (99 vs 98, P = .37). We observed similar differences between the groups with respect to the mean MTR value of the whole brain (Table 2).
TABLE 2:
MTR details of the study population
| MTR | Elderly Subjects |
||
|---|---|---|---|
| Young Subjects (n = 11) | Minimal WM Hyperintensities(n = 51) | High WM Hyperintensities(n = 41) | |
| Whole brain | |||
| Peak height (voxels ± SEM) | 122 (1.2) | 99 (1.5)* | 98 (1.6)* |
| Mean MTR (% ± SEM) | 34.0 (0.12) | 33.0 (0.08)* | 32.8 (0.09)* |
| NAWM mean (% ± SEM) | 36.7 (0.12) | 36.0 (0.11)* | 35.9 (0.13)* |
Significant difference between group and young subjects (P < .05).
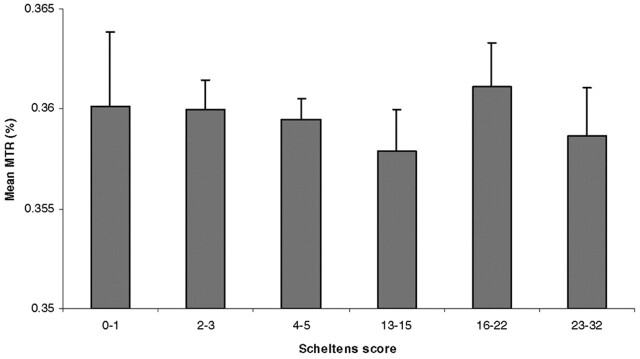
In young subjects, the mean (± standard error of the mean)MTR value of the NAWM was 36.7% ± 0.12%. In elderly subjects with minimal WM hyperintensities, the mean value was 36.0% ± 0.11%, and in elderly subjects with extensive WM hyperintensities, it was 35.9% ± 0.13% (Table 2). Differences between the young subjects and the two groups of elderly subjects were significant (both P < .005). However, the difference between the two elderly groups was not significant (P = .93). In an additional analysis, we assigned each of the elderly subjects in one of three subgroups according to Scheltens score. We found no differences in the mean MTR of the NAWM among any of the subgroups (Fig 2).
Fig 2.
Mean MTR values in elderly study subjects, stratified by Scheltens score. Error bars = SEM.
Discussion
To our knowledge this is the first report of an association between WM hyperintensities and MTR abnormalities in normal-appearing brain tissue in the elderly. We made three observations. First, volumetric MTR values of the whole brain differed between young subjects and elderly subjects. Second, using this volumetric approach, we found no differences between elderly subjects with minimal WM hyperintensities and elderly subjects with a high load of WM hyperintensities. Third, performing regional MTR analysis of NAWM, we observed no differences between the two groups of elderly subjects with different loads of WM hyperintensities. If WM hyperintensities were responsible for the age-related volumetric MTR changes that we observed in the brain, differences in MTR values would have been expected between the elderly subjects with extensive and those with minimal WM hyperintensities. Because we did not observe such a difference, the presence of WM hyperintensities cannot explain these volumetric MTR changes. Also, because MTR measurements in NAWM reproduced the difference between young individuals and elderly individuals, it is clear that changes outside the WM hyperintensities—and thus located in normal-appearing brain tissue—must be responsible for age-related changes in MTR. Furthermore, the lack of association between the load of WM hyperintensities and the amount of abnormalities in NAWM suggests that WM hyperintensities and abnormalities in NAWM have different etiologies.
Young versus Old Subjects
Several other authors have described the difference in MTR values of the NAWM between young subjects and elderly subjects (11,18–20). In a group of healthy volunteers aged 16–55 years, Silver et al (11) found a small but significant age-related decline in MTR in the corpus callosum and the hemispheric WM. Their study was carefully conducted to ensure that WM hyperintensities or gray matter were not included in the MTR measurement. Three other studies (28) revealed a decline in volumetric MTR with increasing age in. However, whether WM hyperintensities were incorporated in the analysis or not was unclear.
WM Hyperintensities
The etiology of age-related WM hyperintensities is incompletely understood. Apart from age, other established risk factors include aortic atherosclerosis (3) and elevated systolic blood pressure (4). These risk factors corroborate the view that vascular factors lead to hypoperfusion and ischemia, which eventually cause WM hyperintensities (1). Interestingly, pathology studies have shown that WM hyperintensities that look homogeneously on MR imaging have different histologic features. Braffman et al (10) found that WM hyperintensities consist of WM infarctions, WM gliosis, and plaques of demyelination. Fazekas et al (9) found a spectrum of perivascular tissue damage, including perivascular demyelination, fibrosis, and edema. In patients with Alzheimer disease, Scheltens et al (8) found loss of myelinated axons in the WM hyperintensities. Moreover, the observation that the MTR of WM hyperintensities is lower than the MTR of NAWM (21–25), indicates a lower concentration of macromolecules in the WM hyperintensities, which is consistent with the various histologic findings.
Normal-Appearing WM
Our finding of a lower MTR in the NAWM is in agreement with the findings of Bronge et al (26). They compared histologic sections with MR sections and observed that abnormal areas on histologic sections were 54% larger than the area with increased signal intensity on T2-weighted MR images. The histologic changes in the area with a normal signal intensity on T2-weighted images were less severe than those observed in the area with increased signal intensity. Still, a lower intensity of myelin staining was observed in the area that appeared normal on MR imaging. Because MTI is more sensitive to demyelinating lesions than conventional MR imaging techniques, it comes as no surprise that MTI seems capable of depicting such demyelinating lesions in the NAWM in the elderly.
Etiology
Why do our findings suggest that WM hyperintensities and the changes on MTR in the NAWM have a different etiology? If one etiology causes WM hyperintensities and MTR changes in the aging brain, the presence of WM hyperintensities and MTR changes should be correlated. Because we did not find such a correlation, we believe that each of these changes must have a different etiology. Firbank et al (28) found an association between the burden of WM hyperintensities and the diffusivity of the NAWM, which suggests a common etiology of WM hyperintensities and changes in NAWM. This observation is not in line with our findings. A couple possible explanations might be responsible for this apparent discrepancy. First, the technique of their segmentation differed from ours. They mentioned a possible inclusion of WM hyperintensities in the segmented NAWM, whereas we did not include any WM hyperintensities in the NAWM. Second, their population differed from ours in that our cohort included more people with a high burden of WM hyperintensities. Therefore, we were able to compare two extreme groups, which made our data more robust.
The group of elderly with minimal WM hyperintensities was 1.8 years younger than the group of elderly with extensive WM hyperintensities. We believe that this difference did not influence our findings. If the difference in age between these two groups influenced our findings, the older age in the elderly group with extensive WM hyperintensities would have caused the mean MTR of the NAWM to be lower than that of a younger group of elderly people with minimal WM hyperintensities. Because such a difference was not observed, the small age difference between these two groups seemed irrelevant to our findings and conclusions.
Conclusion
Age-related WM hyperintensities and age-related MTR changes in NAWM are likely to have different etiologies. Histologic bases, risk factors, and functional correlates of the MTR changes in the NAWM remain to be determined. Our findings may lead to a better understanding of changes in the brain associated with aging.
References
- 1.Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. J Neurol Sci 1992;110:1–7 [DOI] [PubMed] [Google Scholar]
- 2.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study—the Rotterdam scan study. J Neurol Neurosurg Psychiatry 2001;70:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Leeuw FE, de Groot JC, Oudkerk M, et al. Aortic atherosclerosis at middle age predicts cerebral white matter lesions in the elderly. Stroke 2000;31:425–429 [DOI] [PubMed] [Google Scholar]
- 4.Longstreth WT Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 1996;27:1274–1282 [DOI] [PubMed] [Google Scholar]
- 5.Grossman RI, Gomori JM, Ramer KN, Lexa FJ, Schnall MD. Magnetization transfer: theory and clinical applications in neuroradiology. Radiographics 1994;14:279–290 [DOI] [PubMed] [Google Scholar]
- 6.van Buchem MA, Udupa JK, McGowan JC, et al. Global volumetric estimation of disease burden in multiple sclerosis based on magnetization transfer imaging. AJNR Am J Neuroradiol 1997;18:1287–1290 [PMC free article] [PubMed] [Google Scholar]
- 7.Kucharczyk W, Macdonald PM, Stanisz GJ, Henkelman RM. Relaxivity and magnetization transfer of white matter lipids at MR imaging: importance of cerebrosides and pH. Radiology 1994;192:521–529 [DOI] [PubMed] [Google Scholar]
- 8.Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology 1995;45:883–888 [DOI] [PubMed] [Google Scholar]
- 9.Fazekas F, Kleinert R, Offenbacher H, et al. The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol 1991;12:915–921 [PMC free article] [PubMed] [Google Scholar]
- 10.Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology, II: hyperintense white-matter foci in the elderly. AJR Am J Roentgenol 1988;151:559–566 [DOI] [PubMed] [Google Scholar]
- 11.Silver NC, Barker GJ, MacManus DG, Tofts PS, Miller DH. Magnetisation transfer ratio of normal brain white matter: a normative database spanning four decades of life. J Neurol Neurosurg Psychiatry 1997;62:223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepherd J, Blauw GJ, Murphy MB, et al. The design of a prospective study of pravastatin in the elderly at risk (PROSPER): PROSPER study group—Prospective Study of Pravastatin in the Elderly at Risk. Am J Cardiol 1999;84:1192–1197 [DOI] [PubMed] [Google Scholar]
- 13.Scheltens P, Barkhof F, Leys D, et al. A semiquantitative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci 1993;114:7–12 [DOI] [PubMed] [Google Scholar]
- 14.Dousset V, Grossman RI, Ramer KN, et al. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992;182:483–491 [DOI] [PubMed] [Google Scholar]
- 15.Bosma GP, Rood MJ, Zwinderman AH, Huizinga TW, van Buchem MA. Evidence of central nervous system damage in patients with neuropsychiatric systemic lupus erythematosus, demonstrated by magnetization transfer imaging. Arthritis Rheum 2000;43:48–54 [DOI] [PubMed] [Google Scholar]
- 16.van Buchem MA, Grossman RI, Armstrong C, et al. Correlation of volumetric magnetization transfer imaging with clinical data in MS. Neurology 1998;50:1609–1617 [DOI] [PubMed] [Google Scholar]
- 17.van Buchem MA, McGowan JC, Kolson DL, Polansky M, Grossman RI. Quantitative volumetric magnetization transfer analysis in multiple sclerosis: estimation of macroscopic and microscopic disease burden. Magn Reson Med 1996;36:632–636 [DOI] [PubMed] [Google Scholar]
- 18.Hofman PA, Kemerink GJ, Jolles J, Wilmink JT. Quantitative analysis of magnetization transfer images of the brain: effect of closed head injury, age and sex on white matter. Magn Reson Med 1999;42:803–806 [DOI] [PubMed] [Google Scholar]
- 19.Rovaris M, Iannucci G, Cercignani M, et al. Age-related changes in conventional, magnetization transfer, and diffusion-tenser MR imaging findings: study with whole-brain tissue histogram analysis. Radiology 2003;227:731–738 [DOI] [PubMed] [Google Scholar]
- 20.Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain, II: quantitative magnetization transfer ratio histogram analysis. AJNR Am J Neuroradiol 2002;23:1334–1341 [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe JL, Ezekiel F, Jagust WJ, Schuff N, Fein G. Volumetric method for evaluating magnetization transfer ratio of tissue categories: application to areas of white matter signal hyperintensity in the elderly. Radiology 1997;204:570–575 [DOI] [PubMed] [Google Scholar]
- 22.Tanabe JL, Ezekiel F, Jagust WJ, et al. Magnetization transfer ratio of white matter hyperintensities in subcortical ischemic vascular dementia. AJNR Am J Neuroradiol 1999;20:839–844 [PMC free article] [PubMed] [Google Scholar]
- 23.Wong KT, Grossman RI, Boorstein JM, Lexa FJ, McGowan JC. Magnetization transfer imaging of periventricular hyperintense white matter in the elderly. AJNR Am J Neuroradiol 1995;16:253–258 [PMC free article] [PubMed] [Google Scholar]
- 24.Hanyu H, Asano T, Sakurai H, et al. Magnetization transfer ratio in cerebral white matter lesions of Binswanger’s disease. J Neurol Sci 1999;166:85–90 [DOI] [PubMed] [Google Scholar]
- 25.Mehta RC, Pike GB, Enzmann DR. Measure of magnetization transfer in multiple sclerosis demyelinating plaques, white matter ischemic lesions, and edema. AJNR Am J Neuroradiol 1996;17:1051–1055 [PMC free article] [PubMed] [Google Scholar]
- 26.Bronge L, Bogdanovic N, Wahlund LO. Postmortem MRI and histopathology of white matter changes in Alzheimer brains; a quantitative, comparative study. Dement Geriatr Cogn Disord 2002;13:205–212 [DOI] [PubMed] [Google Scholar]
- 27.Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW. Regional and global changes in cerebral diffusion with normal aging. AJNR Am J Neuroradiol 2001;22:136–142 [PMC free article] [PubMed] [Google Scholar]
- 28.Firbank MJ, Minnett T, O’Brien J. Changes in DWI and MRS associated with white matter hyperintensities in elderly subjects. Neurology. 2003;61:950–954 [DOI] [PubMed] [Google Scholar]