Abstract
BACKGROUND AND PURPOSE: This study investigated the role of CT as an early predictor of outcome prognosis after glue embolization of spinal dural arteriovenous fistulas (SDAVF).
METHODS: Over a 13-year period, 26 patients underwent glue embolization of SDAVF and were retrospectively reviewed. Immediately after embolization, each patient had CT evaluation of cast position. Mean follow-up angiography was 23.4 months (range, 1–87 months; median, 21 months). Both MR images and clinical data (e.g., gait and micturition disabilities according to the Aminoff and Logue disability scale, deep and superficial sensitivity) were analyzed, with a mean follow-up of 37.7 months (range, 12–98 months; median, 28 months). Data were tested by univariate analysis by using Fisher’s exact test and the Kruskall Wallis test, depending on the order of the qualitative variables.
RESULTS: Glue was found in the dura mater on CT in 19 (73%) patients. None of these patients had a recanalized fistula on angiography, and the clinical status of all of them improved. Glue was observed in or proximal to the foramen on CT in seven (27%) patients. In five of them, the fistula was recanalized within a mean period of 9 months (range, 3–24 months; median, 6 months) and their clinical status worsened. All five required surgical treatment. On angiography, the absence of fistula recanalization was observed in 21 (81%) patients and correlated with improvements in gait (P = .016), sensitivity (P = .030), and micturition (P = .080). It also correlated with a decrease in the extent of the abnormally high intramedullary T2 signal intensity (P = .002), a decrease in spinal cord diameter (P = .017), and the resolution of prominent perimedullary vessels (P < .001). The presence of glue within the dura mater on CT correlated with the absence of fistula recanalization (P = .045) and with overall improvement in clinical status, including gait and/or sensitivity and/or micturition (P = .042).
CONCLUSION: CT evaluation of embolization cast position immediately after embolization may constitute an early and reliable tool for predicting permanent fistula occlusion and the prognosis for outcome.
A major problem in the management of spinal dural arteriovenous fistulas (SDAVFs) treated by embolization is to provide a reliable tool for predicting the outcome immediately after embolization, to establish that the shunt has been definitely occluded and that there is no further risk of recanalization. The failure of embolization must be documented as quickly as possible, to allow surgical management and prevent clinical deterioration (1–3). So far, clinical outcome, MR imaging, or spinal angiography immediately after embolization have failed to predict final recovery. The only way of doing this was by follow-up (1–12). Today, most authors agree that locating an embolization cast within the arteriovenous shunt and the initial part of its venous drainage network ensures that the malformation has been completely eliminated and that there is no further risk of its recurrence by the development of anastomosis (8–11) as well as the interruption of the intradural draining vein in surgical management (13, 14). These anatomic criteria can be reliably supported by plain-film angiograms, on which glue can be observed in the intradural draining vein. In certain other cases, however, occlusion of the shunt cannot be ascertained, even when total disappearance has been documented (8–11). In a preliminary report (15), we stressed the advantage of CT evaluation after embolization for clear visualization of the exact position of the cast (outside or inside the dura mater). We present here a retrospective study of CT evaluation performed immediately after embolization. The aim of the study was to define an immediate and reliable way of predicting complete and definitive SDAVF occlusion.
Methods
Patient Population
Between 1987 and 2000, 26 patients with angiographically proved SDAVF were managed in our department by endovascular treatment and subsequent CT evaluation of the results. Four of the patients were women, and 22 were men. Their mean age at the time of diagnosis was 62 years (range, 38–87 years; median, 62.5 years). The mean period from onset to diagnosis was 25 months (median, 18 months). All patients (Table 1) underwent clinical evaluation before and after treatment by using the Aminoff and Logue disability scale (12, 14). For sensory disturbance analysis, data for two types of lesion were collected: lesions of the posterior column and lesions of the spinothalamic tract. For the latter, the superior level of sensory disturbance was evaluated. Mean clinical follow-up after treatment was 37.7 months (range, 12–98 months; median, 28 months).
TABLE 1:
Aminoff & Logue’s disability scale
| Gait | Micturition | Bowel function |
|---|---|---|
| G0 Normal | M0 Normal | B0 Normal |
| G1 Leg weakness, abnormal gait or stance, restricted activity | M1 infrequent hesitancy or urgency, altered sensation, but continent | B1 Moderate constipation |
| G2 Restricted activity but no support required | M2 Occasional urinary incontinence or retention | B2 Severe constipation or occasional incontinence |
| G3 One stick required for walking | M3 Total incontinence or persistent retention | B3 Total incontinence |
| G5 Two sticks, crutches, or frame required for walking | ||
| G6 Confined to wheelchair |
Imaging Data
Spinal MR imaging examinations were performed before and after treatment in all patients, with the 1.5T GE system from 1987 to 2000 (Sigma 5X and LX, General Electric Medical Systems, Milwaukee, WI). Mean follow-up was 37.7 months (range, 12–98 months; median, 28 months), as well as the clinical follow-up (both were performed at the same time). The examinations included sagittal T1-weighted sequences (380–450/15–20/2 [TR, ms/TE, ms/NEX[) and T2-weighted sequences consisting of a spin-echo protocol (1,500–45/85–1/1) or a fast spin-echo protocol (3,700–4,300/90–105/2), and axial T2-weighted sequences consisting of a gradient echo (400–20/4; flip angle,15°) or fast spin-echo (3700–4300/90–105/2). Gadolinium-enhanced (DOTAREM, Guerbet, France) sagittal T1-weighted sequences were performed for all 26 patients. The following features were assessed: cord swelling (normal/reduced/increased), the extent of the abnormally high T2 signals within the spinal cord, and the extramedullary enhancement of the coronal venous plexus network on T1-weighted images after gadolinium enhancement. The extent of all signal intensity abnormalities was measured by the number of vertebral body levels.
Spinal angiography was also performed before and after treatment in all patients, with a mean follow-up of 23.4 months (range, 1–87 months; median, 21 months). It included a study of the venous drainage of the artery of Adamkiewicz, a search for its delayed venous filling, and evaluation of the fistula. Digital subtraction angiography (DSA) runs were performed to improve fistula visualization, characterize venous drainage (upward, downward, or both), and identify any contraindications of embolization, such as the presence of an anterior or posterior spinal artery arising from the same pedicle or a neighboring one to form a common trunk.
Procedure
All patients underwent embolization under neuroleptanalgesia, as intravenous administration of alfentanyl at the rate of 20 μg/kg/h. As far as possible, the embolization procedure was performed during the same run of DSA (i.e., immediately after the completion of spinal angiography). With the microcatheter positioned as close to the fistula as possible, blood-flow characteristics (vessel diameter and curve, distance from the catheter to the shunt) were evaluated by multiple test injections with contrast material, to determine whether glue could safely reach the proximal intradural draining vein. The aim of this procedure was to fill the radiculomeningeal artery, the arteriovenous shunt itself and the initial part of the vein of drainage without involving the medullary veins. When all the technical conditions for embolization were considered safe, 0.2–0.3 mL of a 25–75%-diluted N-Butyl 2-Cyanoacrylate Lipiodol mixture was injected under fluoroscopic guidance. After NBCA delivery, the microcatheter was quickly removed to prevent its adhesion to the vessel wall. Control spinal angiography was performed bilaterally, from at least two levels above the shunt level to at least two levels below it. All these procedures were carried out by the same operator.
Immediate postembolization CT (Elscint CT from 1987 to 1997, and Siemens Somatom +4 since 1997) was performed at shunt level, together with axial images (section thickness, 1 mm) and coronal reconstruction. The presence of the embolization cast on the inner surface of the dura mater (i.e., the initial venous compartment) was noted (yes/no), whatever the height of column of the cast.
Statistical Analysis
The variables tested were clinical, CT, MR imaging, and angiographic characteristics. The clinical variables were gait disability, superior level of superficial sensitivity disability, and micturition disability. The CT variable was the presence of glue inside or outside the dura mater. The MR imaging variables were the extent of the abnormally high intramedullary T2 signal intensity, spinal cord diameter, and the enhancement or nonenhancement of perimedullary veins on coronal T1-weighted images after gadolinium processing. The angiographic variable was the presence or absence of a fistula. Improvement or degradation of gait and micturition was noted when gait and micturition disabilities changed by at least one grade on the Aminoff and Logue scale. Changes in the upper level of deficient superficial sensitivity were also noted. Changes in overall clinical status were recorded when at least one clinical variable (e.g., gait, the upper level of superficial sensitivity, or micturition) changed.
Data were tested by univariate analysis, by using Fischer’s exact test or the Kruskall Wallis test, depending on the order of the qualitative variables. P values <.05 were considered significant.
Results
All 26 patients were treated by embolization by using glue. Twenty-one (81%) patients underwent embolization of their fistula during the same course of spinal angiography. For the remaining five patients, embolization was delayed and was performed during the next 5 days, because of renal malfunction (n = 3) or spasm of the SDAVF vessel to be filled (n = 2). For all the patients treated, embolization succeeded, and, as shown by angiography, the shunt had disappeared at the end of the procedure. In five cases, the presence of the cast in the initial part of the venous drainage network was assessed at the end of the procedure on plain films and confirmed by CT in each case.
The presence of glue inside the dura mater on CT was observed in 19 (73%) patients (Table 2). On angiography, the fistula was not recanalized in any of these patients (Fig 1), and their clinical status, including gait, the upper level of deficient superficial sensitivity, or micturition improved in all 19 cases. For deep sensitivity, the clinical status did not change in any case, after versus before treatment.
TABLE 2:
Position of the cast relative to the dura mater
| Number of patients | Cast position on CT relative to the dura mater | Number of follow-up angiograms/results |
|---|---|---|
| 19 | Inside | 12/0 recurrence |
| 7 | Outside | 7/5 recurrence |
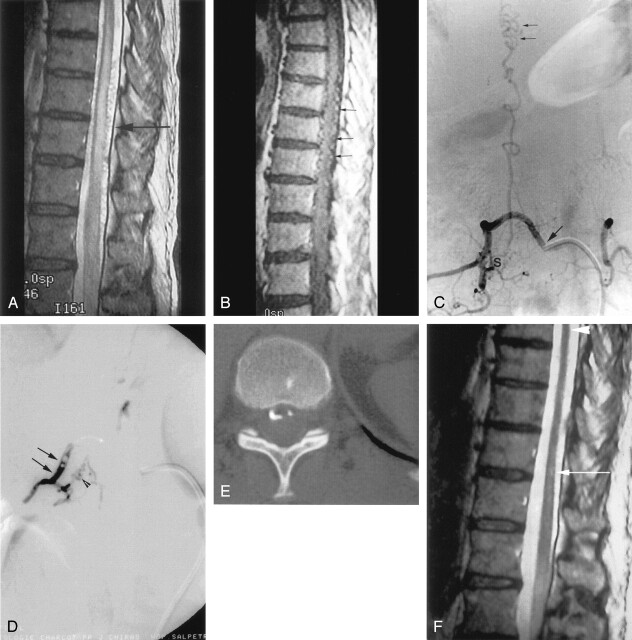
Fig 1.
A, Sagittal T2-weighted MR image cord swelling and central increased signal intensity into the conus, as well as enlarged pial vessels (arrow). B, Sagittal contrast-enhanced T1-weighted MR image shows enhancing enlarged pial vessels (arrows). C, Anterioposterior view of spinal angiogram (injection of intercostal artery) shows filling of an SDAVF with pial drainage (arrowheads); arising from right T9 pedicle (arrow indicates catheter tip). D, Final angiogram control shows embolization cast (arrows) until the initial part of the venous drainage (arrowheads) and no more evidence of the SDAVF or the pial drainage. E, Axial CT control shows clearly embolization cast within the dura mater and into the proximal venous drainage. F, Posttreatment (15 months) sagittal T2-weighted MR image with no more evidence of cord swelling (arrowhead); central increased signal intensity has dramatically decreased, but still remains visible (long arrow). G, Sagittal contrast-enhanced T1-weighted MR image shows no more enhancement of pial vessels previously observed on Figure 1B.
Glue was observed in foraminal locations before or on CT in seven (27%) patients. Five of these patients developed anastomosis and recanalized their fistula within a mean period of 9 months, as shown on follow-up spinal angiography (Fig. 2). The clinical status of these five patients deteriorated, however, and all required surgical treatment. In the remaining two of the seven patients with glue in foraminal locations, the fistula was not recanalized on angiography, and their clinical status improved.
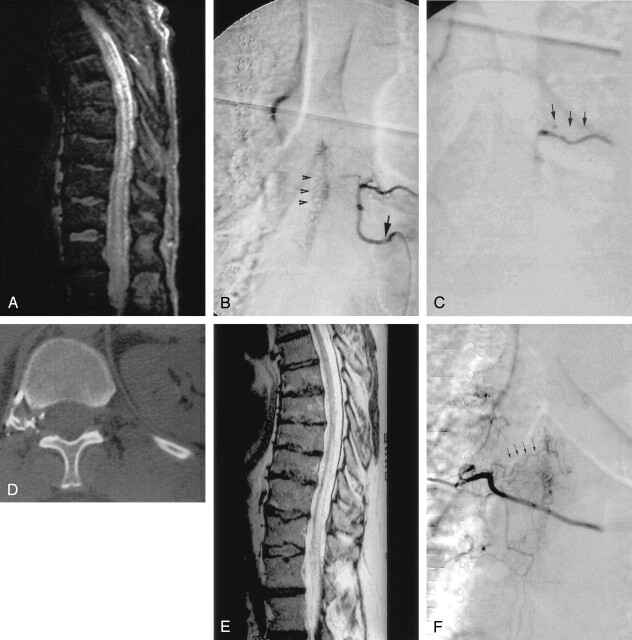
Fig 2.
A, Sagittal T2-weighted MR image shows cord swelling and central increased signal intensity into the conus, as well as enlarged pial vessels. B, Anterioposterior view of spinal angiogram (injection of intercostal artery) shows filling of an SDAVF with pial drainage (arrowheads), arising from left T6 pedicle (arrow indicates catheter tip). C, Angiogram control shows embolization cast (arrowheads) and no more evidence of the SDAVF nor the pial drainage. D, Axial CT control shows embolization cast in foraminal location, but not within the dura mater. E, Posttreatment (8 months) sagittal T2-weighted MR image (patient with clinical worsening after an initial period of improvement). Cord swelling and central increased signal intensity into the conus are remaining, as well as enlarged pial vessels. F, Anterioposterior view of control spinal angiogram shows recurrence of SDAVF arising from right T6 pedicle via retrocorporeal anastomosis (arrowheads).
Overall, the fistula was not recanalized on angiography in 21 (81%) patients after a mean follow-up of 11 months. MR imaging findings improved in all 21 patients, with a decrease in the extent of the abnormally high T2 signal intensity within the spinal cord, but not complete disappearance of the abnormality, and a decrease in spinal cord diameter, which returned to normal in all cases. Disappearance of prominent perimedullary vessels was observed during follow-up in all 21 patients. In the five remaining patients whose fistulas were recanalized, however, the prominent perimedullary vessels persisted, and there was no decrease in the extent of the abnormally high intramedullary T2 signal intensity, or in spinal cord edema.
The absence of fistula recanalization correlated significantly with gait improvement (P = .016), sensitivity improvement (P = .030), and, with borderline significance, the improvement in micturition (P = .080). It also correlated with the decrease in the extent of the abnormally high intramedullary T2 signal intensity (P = .002), the decrease in spinal cord diameter (P = .017), and the disappearance of prominent perimedullary vessels (P < .001). The presence of glue within the dura mater on CT correlated with the absence of fistula recanalization (P = .045) and with the overall improvement in clinical status, including gait, sensitivity, or micturition (P = .042), but not with the MR imaging findings (P > .05 for all these findings).
Discussion
The concept of subacute progressive transverse myelopathy, which was first introduced by Foix and Alajouanine (16) and later substantiated by Lhermitte et al (17) has been considered to constitute the pathophysiology of the spinal dural fistulas. Progressively increased pressure in the perimedullar venous network leads to both gradual ascending thrombosis of epidural exit veins and venous ischemia of the spinal cord (18). Therefore, although SDAVFs are curable, they may often lead to a state of longstanding myelopathy, which in many cases is due to repeatedly delayed diagnosis, despite increased clinical awareness of the disease. Consequently, certain clinical symptoms may reach a stage at which they are not completely reversible.
Until now, surgery has been considered to be the criterion standard, because of its high success rate (1, 19, 20). Polyvinyl alcohol particles provide only temporary occlusion, as the studies of Nichol et al and Hall et al clearly indicate (2–4), and therefore are no longer used for SDAVF embolization (2, 3, 5). On the other hand, by using glue for endovascular treatment during the same course of initial spinal angiography may cure the fistula completely and permanently, thus avoiding general anesthesia and the high cost of hospitalization. Nevertheless, two important reservations must be considered: first, the insufficient success rate of endovascular treatment compared with surgery must, as far as possible, lead to the improvement of the technique of complete shunt occlusion. There are anatomic limitations to embolization, which are mostly the result of an anterior spinal artery arising from the same pedicle, or of unfavorably small or tortuous angioarchitecture, either of which prevents safe catheter positioning. Usually, diagnostic catheters (e.g., Cobra and Mapper) are used throughout the procedure, which sometimes makes catheterization more difficult and leads to the spasm of the pedicle, so the procedure could to be delayed. At present, the recurrence rate is estimated to range from 15–20% (6, 8), with an initial apparent success rate of 75–90%.
Second, ensuring permanent occlusion of the fistula needs immediate, reliable, and predictable reasons for definitive occlusion of the shunt. Until now, only angiography showing its disappearance at the end of the procedure in case of embolization, or repeat angiography after surgical management, proved that the shunt occlusion had held. We indeed know that such occlusion may only be temporary (6, 8), which means that only clinical outcome and repeat MR imaging during the months after treatment can show its true results.
All the patients included in this study initially exhibited apparently “adequate” embolization, as defined by Niimi et al (8) (e.g., penetration of the glue into the fistula or draining vein or both, angiographic disappearance of the fistula after embolization or fistula drainage, as determined by bilateral angiography of two pedicles below the shunt, two above, and two at shunt level, and finally no defect in the venous drainage of the spinal cord after embolization).
In our series, dilution of histoacryl with the lipidol was increased when the distance between the catheter tip and the shunt was longer, the radiculomeningeal vessel, afferent to the shunt, was tortuous and with a smaller caliber, and the hemodynamic flow of the fistula was lower, to obtain a migration of the embolization material up to the fistula and the initial part of the draining vein, without distal migration within perimedullar veins. The presence of the embolization cast on the inner surface of the dura mater, documented by CT, correlated with the absence of recanalization of the fistula. In all the patients whose fistula was not recanalized, we observed clinical improvement, which included significantly improved gait disability, improvements in sensitivity and micturition, and significant decreases in the extent of the high intramedullary signal intensity and in cord swelling, on T2-weighted images, as well as the disappearance of tortuous dilated subarachnoid vessels on gadolinium-enhanced T1-weighted images, as previously reported by Cognard et al (15). The fundamental postulate concerning definitive occlusion of the shunt is anatomic: the initial venous drainage of the shunt is located inside the inner surface of the dura mater. Because this venous drainage is unique, its exclusion by glue may signify permanent occlusion of the shunt, without creation of any colateral anastomosis. The main problem is how to assess this anatomic feature by imaging. Contrast resolution of digital spinal angiography, in the present series between the vascular or venous compartment and the dura mater, is clearly insufficient, as suggested by our results, because in 14 patients (14/19 [74%]) the presence of the embolization cast was documented on CT but was not previously identified on angiography. Therefore, in these cases, the occlusion of the shunt observed at the end of the procedure could not be considered definitive. This point underlines the limitations of repeat angiography for confirming the permanence of shunt occlusion. CT provides enough contrast resolution to establish whether the NBCA cast has entered the inner surface of the dura mater at the level of the fistula. Consequently, the criteria of “adequate” embolization defined by Niimi et al (8) can be fully and reliably assessed. Here, therefore, CT did not “miss” any satisfactorily treated patient. The height of the column of the cast, whatever its location, however, is not related to outcome in our series and was observed to vary in the different groups of patients.
Improvements in gait, sensitivity, or micturition were predicted by the presence of the embolization cast on the inner surface of the dura mater; however, no correlation emerged between the presence of the cast of glue within the dura mater and the improvement in MR imaging parameters. This may be partly due to the absence of fistula recanalization in patients with a cast outside the dura mater, as observed for two patients (2/21, 9%) in our series. When a follow-up CT showed that we could not be sure of the presence of glue within the dura mater (i.e., in the proximal part of the draining vein), the patient should be advised that the next follow-up MR images are of great importance. If those MR images show no regression of spinal cord edema or an increase in the extent of the abnormally high intramedullary signal intensity, on T2-weighted images, or residual tortuous vessels within the spinal cord, spinal angiography must be recommended.
Clinical improvement may occur, even transiently, in cases of incomplete shunt embolization, for instance, the initial disappearance of the fistula at the end of the procedure on angiography, without involving the initial venous drainage shown on the CT control. Such was the case for five patients (5/21 [24%]) in our series. For all of them, recanalization was due to the development of new colateral vessels, because it was documented on control angiographies. It never occurred through the glue cast after its potential resorption. Until the occurrence of anastomosis, the lowering of venous pressure leads to an improvement in cord functions similar to that observed in patients with a cast inside the dura mater. After the anastomosis development, recanalization of the fistula leads to progressive reascent of blood pressure into the venous network, with secondary deterioration of the clinical status. At the same time, anastomosis may never occur, as was the case in the present study for the two patients mentioned above who had a cast outside the dura mater.
It is also noteworthy that for the patients in the “fully cured” group, long-term follow-up, even after 36 months (n = 7), showed that the clinical improvement was stable, with no significant regression of gait, sensitivity, or bladder or bowel status, as previously reported by Tacconi et al (9), Behrens et al (10) and Symon et al (11), who studied surgical series with follow-ups ranging from 3 to 24 years. They found that 18–36 months after surgery, clinical improvement (especially of gait) and the stabilization of the improvement in total disability (gait, micturition, and bowel control) was documented in 84% of patients. At the last follow-up (mean, 147 months), however, 35% of patients had improved or stabilized. In the series reported by Tacconi et al and by Symon et al (9, 11), only overall disability was assessed, and its components were not investigated separately. Moreover, in connection with the advanced mean age of the patients in these two series at the time of diagnosis, and even more so at the time of the latest follow-up, it should be remembered that intercurrent disease may interfere with the course of the initial disease, especially complaints such as arthritis, neurodegenerative disease, and prostate adenoma or carcinoma, thus leading to the overestimation of malfunctions due to SDAVF itself.
Conclusion
Our study suggests that the presence of a glue cast within the dura mater, as observed on postembolization CT, is strongly predictive of permanent occlusion of the fistula. This observation, made immediately after the procedure, can guide long-term follow-up by clinical tests, MR imaging, and spinal angiography. Then, endovascular management of SDAVF may constitute a reliable alternative to surgery.
References
- 1.Lee T, Gromelski E, Bowen B, Green B. Diagnostic and surgical management of spinal dural arteriovenous fistulas. Neurosurgery 1998;43:242–247 [DOI] [PubMed] [Google Scholar]
- 2.Nichols DA, Rufenacht DA, Jack CRJ, Forbes G. Embolization of spinal dural arteriovenous fistula with polyvinyl alcohol particles: experience in 14 patients. AJNR Am J Neuroradiol 1992;13:933–940 [PMC free article] [PubMed] [Google Scholar]
- 3.Hall WA, Oldfield EH, Doppman J. Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg 1989;70:714–720 [DOI] [PubMed] [Google Scholar]
- 4.Tadavarthy SM, Moller JH., Amplatz K. Polyvynil alcohol (Ivalon): a new embolic material. AJR AM J Roentgenol 1975;125:609–616 [DOI] [PubMed] [Google Scholar]
- 5.Gobin YP, Houdart E, Casasco A, Merland J. Endovascular therapy for arteriovenous malformations and fistulae in the spinal cord. Semin Intervent Radiol 1993;510:227–242 [Google Scholar]
- 6.Joon K, Song Y, Gobin P. n-butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae. AJNR Am J Neuroradiol 2001;22:40–47 [PMC free article] [PubMed] [Google Scholar]
- 7.Barth MO, Chiras JRM, Vega Molina J, Bories. J Results of embolization of spinal dural arteriovenous fistulas with perimedullary venous drainage. Neurochirurgie 1984;30:381–386 [PubMed] [Google Scholar]
- 8.Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery 1997;40:675–682; discussion 682–683 [DOI] [PubMed] [Google Scholar]
- 9.Tacconi L, Lopez Izquierdo BC, Simon L. Outcome and prognostic factors in the surgical treatment of spinal dural arteriovenous fistulas: a long-term study. Neurosurgery 1997;11:298–305 [DOI] [PubMed] [Google Scholar]
- 10.Behrens S, Thron A. Long-term follow-up and outcome in patients treated for spinal dural arteriovenous fistula. J Neurol 1999;246:181–185 [DOI] [PubMed] [Google Scholar]
- 11.Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine: clinical features and surgical results in 55 cases. J Neurosurg 1984;60:238–247 [DOI] [PubMed] [Google Scholar]
- 12.Aminoff MJ, Logue V. The prognosis of patients with spinal vascular malformations. Brain 1974;97:211–218 [DOI] [PubMed] [Google Scholar]
- 13.Afshar. JK, Doppman. JL, Oldfield E. Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas. J Neurosurg 1995;82:196–200 [DOI] [PubMed] [Google Scholar]
- 14.Logue V, Aminoff MJ, Kendall B. Results of surgical treatment for patients with a spinal angioma. J Neurosurg Psychiatry 1974;37:1074–1081 [Google Scholar]
- 15.Cognard C, Miaux Y, Pierot L, et al. The role of CT in evaluation of the effectiveness of embolisation of spinal dural arteriovenous fistulae with n-butyl cyanoacrylate. Neuroradiology 1996;38:603–608 [DOI] [PubMed] [Google Scholar]
- 16.Foix C, Alajouanine T. Necrotizing subacute myelitis [in French]. Rev Neurol 1926;46:1–42 [Google Scholar]
- 17.Lhermitte J, Fribourg-Blanc A, Kyriaco N. Angeio-hypertrophic gliosis of spinal cord [in French]. Rev Neurol 1931;2:37–53 [Google Scholar]
- 18.Merland JJ, Riche MC, Chiras J. Intraspinal extramedullary arteriovenous fistulae draining into the medullary veins. J Neuroradiol 1980;7:271–320 [PubMed] [Google Scholar]
- 19.Mourier KL, Gelbert F, Rey A. Spinal dural arteriovenous malformations with perimedullary drainage: indications and results of surgery in 30 cases. Acta Neurochir (Wien) 1989;100:136–141 [DOI] [PubMed] [Google Scholar]
- 20.Oldfield EH, Di Chiro G, Quindlen EA, Rieth K, Doppman J. Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dural fistula. J Neurosurg 1983;59:1019–1030 [DOI] [PubMed] [Google Scholar]