Abstract
BACKGROUND AND PURPOSE: To evaluate predictors of recanalization and a favorable neurologic outcome in patients with acute vertebrobasilar occlusion (VBO) treated with local intra-arterial fibrinolysis (LIF).
METHODS: The multicentric data of 180 patients with acute VBO treated with LIF were retrospectively evaluated. The modified Rankin scale (mRS) was used to evaluate the neurologic status before LIF and at the time of discharge. Patient’s sex, age, etiology of VBO, recanalization, symptom duration before LIF, and pretreatment mRS were correlated with posttreatment mRS. Multiple logistic regression analysis was used to identify independent variables for recanalization and neurologic outcome.
RESULTS: The overall mortality was 43%. Complete recanalization was achieved in 99 (55%) patients and a partial recanalization in 35 (19%) patients, respectively. Recanalization was significantly associated with a favorable outcome (P < .001). The success of recanalization was negatively correlated with the volume of the thrombus (P < .001). No correlation was found between site and etiology of VBO and recanalization. Neurologic outcome correlated strongly with the pretreatment mRS (P < .001) and also with age (P < .02). Coma lasting less than 4.5 hours led to a positive trend toward a better outcome after univariate testing (P < .001).
CONCLUSIONS: Success of recanalization and neurologic status before treatment predict neurologic outcome in patients with VBO. Thrombus volume has an adverse effect on the recanalization success.
Mortality of patients with acute vertebrobasilar occlusion (VBO) treated with nonthrombolytic drugs is 80%–90%.1–7 After the introduction of selective local intra-arterial fibrinolysis (LIF),8,9 the mortality of this devastating disease could be lowered to approximately 42%–65%.5,6,9–15 Thus, complete recanalization could be identified as an important variable for the neurologic outcome. However, there is still no consensus regarding which other factors predict survival and a favorable outcome in patients with VBO selected for treatment with LIF.
Our purpose was to find independent clinical and angiographic variables with a prognostic impact in patients with VBO. Therefore, we addressed the relationship between pretreatment clinical findings, such as patient’s age and sex, severity and duration of brain stem symptoms, and posttreatment neurologic outcome in 180 patients. Furthermore, we correlated angiographic findings such as site of occlusion, thrombus volume, and etiology of VBO with the recanalization rate and subsequent clinical outcome.
Methods
Patients
The data of 180 adult patients (124 men, 56 women; mean age, 57.9 ± 13.6 [SD] years; range, 22–83 years) with angiographically confirmed VBO treated with LIF at 5 German stroke centers were retrospectively evaluated. The cases were consecutively collected at center A from 1986 through 1997 (n = 41 patients), at center B from 1987 through 1995 (n = 30 patients), at center C from 1990 through 1995 (n = 41 patients), at center D from 1992 through 1996 (n = 20 patients), and at center E from 1996 through 2003 (n = 48 patients).
All patients underwent detailed neurologic examination revealing symptoms consistent with acute progressive thrombosis of the vertebrobasilar system. Presence of coma, locked-in-syndrome, brain stem symptoms, as well as symptom duration until the beginning of treatment, were recorded. The neurologic status was rated by an experienced vascular neurologist according to the modified Rankin scale (mRS) before treatment and at the time of discharge or transfer of the patient (mRS 0–6: 0 = no symptoms at all; 1 = no significant disability despite symptoms, able to carry out all usual duties and activities; 2 = slight disability, unable to carry out all previous activities but able to look after own affairs without assistance; 3 = moderate disability, requiring some help but able to walk without assistance; 4 = moderately severe disability, unable to walk without assistance and unable to attend to own bodily needs without assistance; 5 = severe disability, bedridden, incontinent, and requiring constant nursing care and attention; 6 = dead). Presence of an intracranial hemorrhage on CT scan or MR imaging was an exclusion criterion for LIF. Patients were not excluded from LIF by the presence of small- to medium-sized ischemic lesions due to the natural poor prognosis of acute VBO. Informed consent was obtained from patients and/or their families before treatment. At least 1 CT scan was performed within 72 hours after LIF. Furthermore, several follow-up MR imaging or CT scans were done, especially in patients with neurologic deterioration after LIF, to rule out intracranial bleeding or malignant cerebellar infarction.
Angiography
Selective diagnostic 4-vessel intra-arterial digital subtraction angiography was performed in all cases. All angiographic films were analyzed by an experienced neuroradiologist blinded to clinical findings before and after endovascular treatment. Recanalization was assessed on the control angiogram after LIF and classified according to Thrombolysis in Myocardial Infarction (TIMI) grades.16 For statistical analysis, TIMI grades 0 and 1 were combined, leading to 3 categories: No recanalization (TIMI 0 and 1), partial recanalization (TIMI 2), and complete recanalization (TIMI 3). The TIMI classification was used only for the perfusion of the distal vertebral arteries and basilar artery. Occlusion of the posterior inferior cerebral artery (PICA), anterior inferior cerebral artery (AICA), superior cerebellar artery (SCA), and the distal posterior cerebral artery (PCA) was not considered in our analysis.
The site of occlusion was classified according to Archer and Horenstein17 after the 3 anatomic segments of the basilar artery (BA) and intracranial vertebral artery (VA). Furthermore, we defined the “cervical segment” as a fourth pathoanatomic segment, including the whole extracranial vertebral artery (VA) (V0–V3 segment).
The type of occlusion was based solely on the results of the angiographic study. We subdivided the type of occlusion into 3 categories: (1) atherothrombotic, (2) artery-to-artery embolism from the proximal VA (V0–V2-segments), and (3) embolism from the heart or aortic arch. Atherothrombotic category included occlusion of the BA in conjunction with a hemodynamically significant stenosis (>50% lumen diameter reduction) of the intracranial vertebrobasilar circulation (ie, distal vertebral artery [V4 segment], vertebrobasilar junction, or the midbasilar artery) due to dissection or atherosclerosis. Embolism from the proximal vertebral artery was assumed if a stenosis (>50% lumen diameter reduction) of the extracranial cervical segment of the VA was present and the contralateral vertebral artery was occluded or hypoplastic. Embolism from the heart or aortic arch was assumed in all other cases.
The thrombus volume was calculated using the intraluminal visible radiopaque tip of the microcatheter (Target Therapeutics, Fremont, Calif; Tracker 18 = 0.9 mm) as a reference. For the determination of the length of the thrombus, the carotid arteries were injected. In case of bilateral absent or hypoplastic posterior communicating arteries, the length of the thrombus was measured by microcatheter injection. The diameter was determined by measuring the proximal or distal stump of the basilar artery. A formula ([diameter of BA/2]2 × π × length of the thrombus) was used to calculate thrombus volume. Then, the thrombus volume was categorized as follows: <100 μL, 100–300 μL, and >300 μL.
Endovascular Procedure
LIF was performed under general anesthesia. After placing a guiding catheter in the dominant vertebral artery, a microcatheter was advanced to the proximal portion of the occluding thrombus (Fig 1). Recombinant tissue-type plasminogen activator (rtPA) (n = 97 patients), urokinase (n = 75 patients), or streptokinase (n = 8 patients) was administered. Because the length of the thrombus differed markedly and the multicentric data were retrospectively evaluated, dosage of the thrombolytic drug as well as the duration of LIF varied substantially. Dosage varied widely because of the thrombus volume treated: 25–160 mg for rtPA, 150,000–1,700,000 IU for urokinase, and 110,000–250,000 IU for streptokinase, respectively. The duration of fibrinolytic infusion was generally confined to 2 hours but was prolonged if a beginning recanalization was visible on the control angiogram. LIF was most often stopped when the V4 segments, the entire BA, and the precommunicating segments of the PCAs were recanalized, even if PICA, AICA, SCA, and the postcommunicating segments of the PCAs remained occluded. In all cases, an initial bolus of 5000 IU of heparin was given before the angiography. The fibrinolytic treatment was carried out under systemic heparin administration, which was maintained after the procedure for 2 weeks, except for patients with intracranial hemorrhage. Acetylsalicylic acid was not regularly applied before treatment but some patients were on aspirin at admission to the hospital.
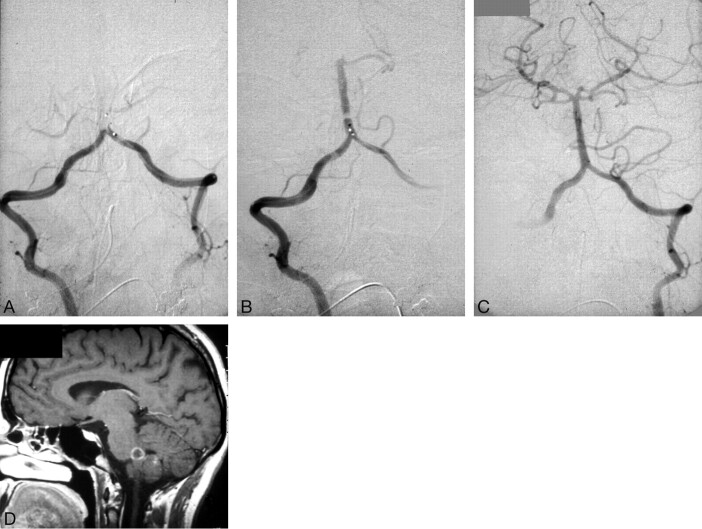
Fig. 1.
Case of a 30-year-old woman comatous for 2 hours upon arrival at the hospital and during the angiography.
A, The anteroposterior view of the angiogram demonstrates the complete caudal occlusion of the BA. A microcatheter was placed in both VAS, and 140 mg of rtPA was administered.
B, An interim result showing partial recanalization.
C, The final result showing complete recanalization of the vertebrobasilar system. The patient was asymptomatic at first but developed a palsy of the right VI and VII cranial nerves 1 day later.
D, Follow-up MR imaging (T1-weighted, contrast-enhanced) confirms the subacute ischemic infarction in the right PICA territory and at the pontomedullary junction, where the nuclei of the VI and VII cranial nerves are located.
Statistics
Multiple logistic regression analysis was used to identify independent variables for recanalization and neurologic outcome. The odds ratio within the 95% confidence interval was determined. Furthermore, Fisher exact test and χ2 test were used for univariate analysis. Significance was declared at the P < .05 level. This analysis is part of a more extended research project investigating factors influencing the outcome in acute VBO treated with LIF.
Results
In 180 patients, angiographic findings before and after treatment were obtainable. The neurologic outcome could be determined in 176 of 180 patients. Control CT scans or MR imaging after LIF was available in 145 patients. In 43 (30%) of the patients, intracranial bleedings were observed (n = 22 hemorrhagic transformations and n = 21 parenchymal hematomas).
Recanalization
Forty-three (24%) patients had a rostral occlusion, 50 (28%) a midbasilar occlusion, 62 (34%) a caudal occlusion, and 25 (14%) had a cervical occlusion, respectively. A complete recanalization was achieved in 99 (55%) patients, a partial recanalization in 35 (19%) patients, and no recanalization in 46 (26%) patients, respectively. In Table 1, recanalization is correlated with the thrombus volume, the etiology of VBO, and the site of occlusion. The most important variable influencing the recanalization rate was the thrombus volume (P < .001). If it was less than 100 μL (which roughly corresponds to a thrombus length of 10 mm), representing mostly a circumscribed rostral or midbasilar thrombus, a complete recanalization was achieved in 81% of the patients. On the other hand, a thrombus volume of more than 300 μL led to a complete recanalization in only 26% of the cases. Most often, the thrombus volume was between 100 and 300 μL; in this group, complete recanalization was achieved in slightly more than half of the patients (52%).
Table 1:
Relationship between thrombus volume, etiology, site of occlusion, and recanialization
| No Recanalization (%) | Partial Recanalization (%) | Complete Recanalization (%) | n | |
|---|---|---|---|---|
| Thrombus volume (P < .001) | ||||
| <100 μl | 10 | 9 | 81 | 57 |
| 100-300 μl | 26 | 22 | 52 | 81 |
| >300 μl | 45 | 29 | 26 | 42 |
| Etiology (not significant) | ||||
| Cardioembolism | 27 | 20 | 52 | 99 |
| Embolism from VA | 21 | 5 | 74 | 19 |
| Atherothrombotic | 24 | 23 | 53 | 62 |
| Site of occlusion (not significant) | ||||
| Midbasilar & rostral | 22 | 17 | 61 | 93 |
| Caudal | 24 | 23 | 53 | 62 |
| Cervical | 40 | 20 | 40 | 25 |
Note:—VA indicates vertebral artery.
Patients with partial or complete recanalization had a significantly better neurologic outcome than nonrecanalized patients (P < .001; Table 2). For 38 (86%) of 44 patients who had no recanalization, the posttreatment mRS was 5 or 6; such a poor outcome was present in only 32 (33%) of 97 patients with full recanalization and in 43% of the patients with partial recanalization. The etiology of VBO and the site of occlusion did not affect the recanalization rate, as shown in Table 1.
Table 2:
Relationship between recanalization, etiology, VBO, site of occlusion, and posttreatment score
| Posttreatment Scores |
n | |||
|---|---|---|---|---|
| mRS 0-2 (%) | mRS 3-4 (%) | mRS 5-6 (%) | ||
| Recanalization (P < .001) | ||||
| None | 2 | 12 | 86 | 44 |
| Partial | 34 | 23 | 43 | 35 |
| Complete | 29 | 38 | 33 | 97 |
| Etiology (not significant) | ||||
| Cardioembolism | 22 | 30 | 48 | 98 |
| Embolism from VA | 22 | 39 | 39 | 18 |
| Atherothrombotic | 25 | 23 | 52 | 60 |
| Pretreatment score (P < .001) | ||||
| mRS 3 | 71 | 29 | 0 | 14 |
| mRS 4 | 28 | 30 | 42 | 60 |
| mRS 5 | 14 | 27 | 59 | 102 |
Note:—VBO indicates vertebrobasilar occlusion; mRS, modified Rankin Scale; VA, vertebral artery.
Neurologic Outcome
Seventy-seven (43%) of the patients died. Table 2 shows the relationship between recanalization, etiology of VBO, pretreatment score, age, and posttreatment score. A relatively favorable pretreatment score (mRS 3–4) was significantly correlated with a good to moderate clinical outcome (mRS 0–4). However, 60 of 102 patients with a pretreatment mRS of 5 had a posttreatment score of mRS 5–6. After univariate testing, a trend toward a better neurologic outcome was found in patients with shorter-lasting brain stem symptoms, including comatose status (P < .02; Table 3). The neurologic prognosis worsened if the progressive brain stem stroke symptoms lasted more than 8 hours. Furthermore, we found a univariately significant correlation between the duration of a pretreatment coma and the posttreatment score in 86 patients. However, after multivariate testing, neither the duration of the brain stem symptoms nor the duration of the coma was an independent variable for the neurologic outcome. Patients being awake but with quadriplegia had a bad prognosis, independent from the duration of this symptoms (n = 10 patients); 70% of them died or remained in the locked-in-syndrome. Patients younger than 40 had a higher rate of good outcomes; patients older than 70 had a higher rate of mortality (P < .020; Table 3). The neurologic outcome was not affected by the sex of the patient or by the etiology of the occlusion.
Table 3:
Relationship between duration of brain stem symptoms, coma duration, patient age, and posttreatment score
| Posttreatment Scores |
n | |||
|---|---|---|---|---|
| mRS 0–2 (%) | mRS 3–4 (%) | mRS 5–6 (%) | ||
| Duration of brain stem symptoms (P < .020*) | ||||
| 0–4 hours | 31 | 28 | 41 | 51 |
| 4.5–8 hours | 22 | 29 | 49 | 63 |
| 8.5–12 hours | 16 | 22 | 62 | 32 |
| >12 hours | 11 | 33 | 56 | 18 |
| Coma duration (P < .001*) | ||||
| 0.5–4 hours | 22 | 24 | 53 | 45 |
| 4.5–8 hours | 10 | 26 | 65 | 31 |
| 8.5–12 hours | 0 | 20 | 80 | 10 |
| Patient’s age (P < .020) | ||||
| 22–39 years | 42 | 25 | 33 | 24 |
| 40–69 years | 22 | 31 | 47 | 118 |
| >70 years | 15 | 21 | 65 | 34 |
Note:—*indicates univariable testing; mRS, modified Rankin scale.
Comparing the clinical outcome of 176 patients treated from 1986 through 1994 (n = 107 patients) and from 1995 through 2003 (n = 69 patients), no significant difference was found between these 2 groups.
Discussion
To the best of our knowledge, the present study is the largest series on patients with angiographically proven acute VBO treated with LIF to date. After multivariate testing, we observed that the pretreatment neurologic score and the recanalization of the VBO were independent variables of the clinical outcome (both P < .001). Furthermore, another independent variable, and thus the most important factor for the success of the endovascular recanalization, was the volume of the thrombus (P < .001). However, we found that site and etiology of the occlusion did not significantly influence the success of LIF. We could not establish a time window that would definitely exclude selected patients from intra-arterial fibrinolysis.
The first larger series in 43 patients treated with LIF after acute VBO showed a dramatic improvement regarding mortality and morbidity after successful recanalization5,6: all patients without recanalization died, whereas 14 of the 19 recanalized patients survived, 10 of them with a favorable neurologic outcome. This result has been confirmed by other studies.10–15,17–19 In addition, our results emphasize the idea that complete or at least partial recanalization of the VBO is indispensable for a favorable neurologic outcome in VBO (Table 2). Nevertheless, in contrast to previous reports,6,14,15 we differentiated not only between “recanalization” and “no recanalization” but also defined “partial recanalization” (ie, TIMI grade 2) as an angiographic result after LIF. We found that even a partial recanalization leads to a relatively satisfactory posttreatment mRS of 0–4 in 57% of the patients versus 14% in nonrecanalized patients (Table 2). Ezaki et al,18 however, found significantly improved neurologic outcome only in patients with complete recanalization, whereas 11 of 15 patients with partial recanalization had a poor outcome.
According to our data, the thrombus volume seems to be the most important parameter for the success of the LIF (Table 1). If the thrombus volume exceeded 300 μL, the recanalization rate decreased significantly. Novel promising recanalization techniques, such as mechanical recanalization,20,21 might be helpful in cases of caudal VBO with large thrombus masses to improve the completeness and rapidity of recanalization in future.
Previous studies10,13 subdivided the etiology of VBO into 2 different mechanism types. Occlusion in the vertebrobasilar junction combined with intracranial stenosis were classified as an “atherothrombotic occlusion.” Absence of atherosclerosis in conjunction with an occlusion in the distal segment of the BA was defined as an “embolic occlusion.” In both studies, the authors found a higher recanalization rate (66% and 72% in embolic occlusion versus 19% and 60% in atherothrombotic occlusion, respectively) as well as a better neurologic outcome in embolic occlusion than in atherothrombotic occlusion. In the present study, we used a similar subdivision for defining the etiology of VBO, but we could not determine a significant difference in the recanalization rate and outcome between these subgroups (Tables 1 and 2). Nevertheless, divergent definitions used by the different groups, as well as missing data regarding pre-existing embolic and arteriosclerotic risk factors in the (most often) retrospective study designs, might be responsible for the disagreeing results.
There is little consensus in the literature regarding the influence of the site of occlusion on the recanalization rate and the neurologic outcome. Similar to our findings, some investigators observed no association between a particular occlusion pattern and these parameters.6,15,18 On the contrary, several groups11,12,14 demonstrated a lower recanalization rate and a worse outcome in caudal VBO compared with distal clot location. The reason for these different findings might be that 4-vessel angiography to determine the length (and the volume) of the thrombus was not performed in all previous studies.
To reduce the rate of secondary bleeding into the ischemic area and to thus decrease poor outcome, it is well accepted to restrict intravenous thrombolysis to the first 3 hours of symptom onset in the anterior circulation and intra-arterial fibrinolysis to the first 3–6 hours.22,23 Eckert et al10 found a significantly better clinical outcome in patients with acute VBO treated within 6 hours after symptom onset than in patients treated after more than 6 hours. They concluded that performing LIF is not reasonable if the patient suffers from severe neurologic deficit for more than 6 hours. However, our data do not suggest such a rigid exclusion of patients with acute VBO from LIF, because we found that neither the duration of brain stem stroke symptoms nor the coma duration was an independent variable for a favorable neurologic outcome (Table 3). This in accordance with the study of Arnold et al,15 who found no significant association between time to treatment and clinical outcome in 40 patients treated with LIF within 12 hours after symptom onset.
Good clinical outcome was present in 42% of the patients between 22 and 39 years (Table 3). In contrast, the highest mortality was observed in the group of patients older than 70 years with 65%; this is the double compared with the young patient group. Despite this, older patients had still a rate of good outcome that was comparable with the medium-aged group (Table 3). This result implies that exclusion of older VBO patients from LIF should not be too restrictive. Furthermore, the pretreatment score was worse in older patients, which might influence their worse outcome. Moreover, older patients have more frequent arterial stenosis caused by atherosclerotic plaques; therefore, they might be more often in danger of suffering a rethrombosis.24 Considering the limited experience, percutaneous transluminal angioplasty, alone or stent-assisted, might become a beneficial procedure by which to treat intracranial vertebrobasilar stenosis even in acute VBO.10,25 Thus, LIF could be performed in combination with angioplasty and/or stent placement of the underlying stenosis in these patients to prevent acute reocclusion.
Our study has some limitations: we are aware of the fact that the use of stroke scales other than the mRS (eg, National Institutes of Health Stroke Scale) have been more accurate for the determination of the pretreatment score. Unfortunately, these data were not available in our retrospective study design. Because of the clinical features of acute VBO and lack of information by the relatives, it was difficult to identify the exact time of symptom onset in several cases. Thus, similar to a previous study,7 the determination of a well-defined time window for LIF in acute VBO is not possible despite the large number of patients in the present study.
Lindsberg et al26 reported favorable clinical outcome and recanalization rate in patients treated with intravenous rtPA after acute VBO. BA occlusion was diagnosed by MR angiography alone in 45 of 50 patients in this study; therefore, extension of thrombosis could not be defined. Furthermore, the overall recanalization rate was markedly lower in the study by Lindsberg et al26 compared with our study (52% versus 74%). Eckert et al27 recently reported a comparison of the combination of systemic glycoprotein IIb/IIIa receptor inhibitor and low-dose intra-arterial rtPA (median dose, 20 mg) with the intra-arterial rtPA monotherapy. Despite an increase of hemorrhagic complications, the combined therapy led to a better neurologic outcome than intra-arterial rtPA alone.27 In our opinion and according to our data, the future treatment has to be adapted to the site of occlusion, volume of the thrombus, and the presence of underlying extracranial or intracranial stenosis. Thus, the primary intravenous use of glycoprotein IIb/IIIa receptor inhibitors or rtPA may be helpful for bridging the interval until referral to an experienced stroke center.27 Thereafter, the rapidity and completeness of the recanalization, as well as the prevention of a reocclusion, should be the main goals independent from the therapeutic approach used.
Conclusion
In conclusion, our results suggest that rapid and complete restoration of the blood flow is crucial for a favorable prognosis of acute VBO. Because the recanalization rate is strongly associated with the thrombus volume, novel recanalization techniques should be considered in extensive thrombus masses. For the clinical outcome, the pretreatment score and the recanalization rate are the most important factors. Although the prognosis of older patients seems to be worse, age is not a definite exclusion criterion from LIF per se.
Acknowledgments
We thank Mr. Peter Dirschedl and Mr. Ralf Strobl (Department of Statistics, University of Munich, Germany) for their help in the statistical evaluation.
Footnotes
The work was presented in part at the 13th European Stroke Conference, May 12–15, 2004, Mannheim, Germany, and at the 29th Congress of the European Society of Neuroradiology, Sep 8–11, 2004, Aachen, Germany.
References
- 1.Caplan LR. Occlusion of the vertebral or basilar artery. Follow-up analysis of some patients with benign outcome. Stroke 1979;10:277–82 [DOI] [PubMed] [Google Scholar]
- 2.Caplan LR. Bilateral distal vertebral artery occlusion. Neurology 1983;33:552–58 [DOI] [PubMed] [Google Scholar]
- 3.Labauge R, Pages M, Blard JM. Survie prolongee apres occlusion du tronc basilaire. Rev Neurol 1981;145:789–94 [PubMed] [Google Scholar]
- 4.Labauge R, Pages M, Marty-Double C, et al. Occlusion du tronc basilaire. Rev Neurol 1981;137:545–71 [PubMed] [Google Scholar]
- 5.Brückmann H, Ferbert A, del Zoppo GJ, et al. Acute vertebral-basilar thrombosis. Angiologic-clinical comparison and therapeutic implications. Acta Radiologica Suppl 1986;369:38–42 [PubMed] [Google Scholar]
- 6.Hacke W, Zeumer H, Ferbert A, et al. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke 1988;19:1216–22 [DOI] [PubMed] [Google Scholar]
- 7.Ferbert A, Brückmann H, Drummen R. Clinical features of proven basilar artery occlusion. Stroke 1990;21:1135–42 [DOI] [PubMed] [Google Scholar]
- 8.Zeumer H, Hacke W, Kolmann HL, et al. Lokale Fibrinolysetherapie bei Basilaristhrombose. Dtsch Med Wochenschr 1982;107:728–31 [DOI] [PubMed] [Google Scholar]
- 9.Zeumer H, Hacke W, Ringelstein EB. Local intraarterial thrombolysis in vertebrobasilar thromboembolic disease. AJNR Am J Neuroradiol 1983;4:401–04 [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert B, Kucinski T, Pfeiffer G, et al. Endovascular therapy of acute vertebrobasilar occlusion: early treatment onset as the most important factor. Cerebrovasc Dis 2002;14:42–50 [DOI] [PubMed] [Google Scholar]
- 11.Becker K, Monsein L, Ulatowski J, et al. Intraarterial thrombolysis in vertebrobasilar occlusion. AJNR Am J Neuroradiol 1996;17:255–62 [PMC free article] [PubMed] [Google Scholar]
- 12.Cross DT, Moran C, Akins P, et al. Relationship between clot location and outcome after basilar artery thrombolysis. AJNR Am J Neuroradiol 1997;18:1221–28 [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt T, von Kummer R, Muller Kuppers M, et al. Thrombolytic therapy of acute basilar artery occlusion: Variables affecting recanalization and outcome. Stroke 1996;27:875–81 [DOI] [PubMed] [Google Scholar]
- 14.Levy EI, Firlik AD, Wisniewski S, et al. Factors affecting survival rates for acute vertebrobasilar artery occlusions treated with intra-arterial thrombolytic therapy: a meta-analytical approach. Neurosurgery 1999;45:539–48 [DOI] [PubMed] [Google Scholar]
- 15.Arnold M, Nedeltchev K, Schroth G, et al. Clinical and radiological predictors of recanalization and outcome of 40 patients with acute basilar artery occlusion treated with intra-arterial thrombolysis. J Neurol Neurosurg Psychiatry 2004;75:857–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med 1985;312:932–36 [DOI] [PubMed] [Google Scholar]
- 17.Archer CR, Horenstein S. Basilar artery occlusion. Clinical and radiological correlation. Stroke 1977;8:383–90 [DOI] [PubMed] [Google Scholar]
- 18.Ezaki Y, Tsutsumi K, Onizuka M, et al. Retrospective analysis of neurological outcome after intra-arterial thrombolysis in basilar artery occlusion. Surg Neurol 2003;60:423–30 [DOI] [PubMed] [Google Scholar]
- 19.Berg-Dammer E, Felber SR, Henkes H, et al. Long-term outcome after local intra-arterial fibrinolysis of basilar artery thrombosis. Cerebrovasc Dis 2000;10:183–88 [DOI] [PubMed] [Google Scholar]
- 20.Wikholm G. Transarterial embolectomy in acute stroke. AJNR Am J Neuroradiol 2003;24:892–94 [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer TE, Hamann GF, Schulte-Altedorneburg G, et al. Treatment of vertebrobasilar occlusion by using a coronary waterjet thrombectomy device: a pilot study. AJNR Am J Neuroradiol 2005;26:1389–94 [PMC free article] [PubMed] [Google Scholar]
- 22.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–87 [DOI] [PubMed] [Google Scholar]
- 23.del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke 1998;29:4–11 [DOI] [PubMed] [Google Scholar]
- 24.Wallace RC, Furlan AJ, Moliterno DJ, et al. Basilar artery rethrombosis: successful treatment with platelet glycoprotein IIB/IIIA receptor inhibitor. AJNR Am J Neuroradiol 1997;18:1257–60 [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama T, Tanaka K, Kaneko M, et al. Thrombolysis and angioplasty for acute occlusion of intracranial vertebrobasilar arteries: report of three cases. J Neurosurg 1998;88:919–22 [DOI] [PubMed] [Google Scholar]
- 26.Lindsberg PJ, Soinne L, Tatlisumak T, et al. Long-term outcome after intravenous thrombolysis of basilar artery occlusion. JAMA 2004;292:1862–66 [DOI] [PubMed] [Google Scholar]
- 27.Eckert B, Koch C, Thomalla G, et al. Aggressive therapy with intravenous abciximab and intra-arterial rtPA and additional PTA/stenting improves clinical outcome in acute vertebrobasilar occlusion. Combined local fibrinolysis and intravenous abciximab in acute vertebrobasilar stroke treatment (FAST) –Results of a multicenter study. Stroke 2005;36:1160–65 [DOI] [PubMed] [Google Scholar]