Abstract
BACKGROUND AND PURPOSE:We objectively assessed surface structural changes of the hippocampus in mesial temporal sclerosis (MTS) and assessed the ability of large-deformation high-dimensional mapping (HDM-LD) to demonstrate hippocampal surface symmetry and predict group classification of MTS in right and left MTS groups compared with control subjects.
METHODS:Using eigenvector field analysis of HDM-LD segmentations of the hippocampus, we compared the symmetry of changes in the right and left MTS groups with a group of 15 matched controls. To assess the ability of HDM-LD to predict group classification, eigenvectors were selected by a logistic regression procedure when comparing the MTS group with control subjects.
RESULTS:Multivariate analysis of variance on the coefficients from the first 9 eigenvectors accounted for 75% of the total variance between groups. The first 3 eigenvectors showed the largest differences between the control group and each of the MTS groups, but with eigenvector 2 showing the greatest difference in the MTS groups. Reconstruction of the hippocampal deformation vector fields due solely to eigenvector 2 shows symmetrical patterns in the right and left MTS groups. A “leave-one-out” (jackknife) procedure correctly predicted group classification in 14 of 15 (93.3%) left MTS subjects and all 15 right MTS subjects.
CONCLUSION:Analysis of principal dimensions of hippocampal shape change suggests that MTS, after accounting for normal right-left asymmetries, affects the right and left hippocampal surface structure very symmetrically. Preliminary analysis using HDM-LD shows it can predict group classification of MTS and control hippocampi in this well-defined population of patients with MTS and mesial temporal lobe epilepsy (MTLE).
MR imaging-based hippocampal volumetric measurements are useful in the diagnosis of mesial temporal lobe epilepsy (MTLE). In a patient with a history of epileptic seizure compatible with MTLE, a significant hippocampal volume asymmetry is predictive of mesial temporal sclerosis (MTS)1,2 and a favorable outcome after epilepsy surgery.3–7 Most prior studies used manual segmentation of the hippocampus from MR images to determine hippocampal volumes.8
Computational anatomy, general pattern theory,9 and other mathematical principles provide an analytic framework and tools for studying structures, such as the hippocampus, using large-deformation high-dimensional mapping (HDM-LD).9 HDM-LD is a semiautomated technique that can generate highly reproducible results, showing a percentage overlap between segmentations of 92.8% in patients with MTLE and pathologic examination-verified MTS.10 HDM-LD shows accentuated areas of deformation in regions shown on histopathologic examination to be most affected in MTS.11 Past investigators have used HDM-LD to characterize specific patterns of hippocampal deformation in patients with mesial temporal sclerosis associated with MTLE,11 schizophrenia,12 Alzheimer disease,13 and depression.14 Such studies suggest that unique patterns of hippocampal deformation are present in different clinical disease states and therefore may provide useful clinical information in diagnosis of CNS diseases that affect the hippocampus. Quantitative measurements of hippocampal deformation, using eigenvector analysis, provide an objective method of assessing patterns that are possibly disease specific.
The major aim of our study was to objectively document the symmetry of hippocampal deformation change in MTS by grouping subjects with right MTS and subjects with left MTS separately and comparing them with a group of normal subjects to eliminate changes due to normal right-left asymmetries. Analyzing the right and left MTS groups separately allows the use of eigenvector analysis to document the degree of deformation symmetry between the groups and to assess the specificity of the deformation pattern in MTS.
As an initial measure of the ability of HDM-LD to predict group changes in the patients with MTS compared with control subjects, we compared patients in MTS groups with control subjects using a “leave-one-out” (jackknife) procedure. These findings give initial information about the use of HDM-LD analysis to predict pattern specific changes in MTS.
Methods
Subject Selection
Patients were identified retrospectively from consecutive cases from the epilepsy surgery series at Saint Louis University, as outlined in our Institutional Review Board protocol. Patients were included in the study if they had a clinical history of focal seizures consistent with MTLE, including seizures with arrested activity, impairment of consciousness, automatisms, and postictal confusion. Duration of epilepsy at the time of MR scanning was noted. All patients underwent video electroencephalographic (EEG) telemetry monitoring to confirm the semiology and EEG localization of their seizures.15 Pathologic specimens from the anterior and mesial temporal lobe were collected from all patients. A neuropathologist macroscopically and microscopically assessed all specimens and identified substantial neuronal loss in hippocampal subregions of CA4 and Sommer sector, with relative sparing of CA2 neurons. All patients had postsurgical pathologic confirmation of MTS. As a further criterion for inclusion in the study, all patients underwent quantitative hippocampal volume measurements and were required to have asymmetry of MR imaging-based hippocampal volume loss (with a greater than 10% hippocampal asymmetry) with the smaller hippocampus concordant with the side of pathologically documented MTS.
The volunteers for control MR studies had no history of central nervous system disease, significant head trauma, or alcohol abuse. Subjects were recruited and included consecutively in the control MR group. No elimination of control subjects or statistical corrections of subject parameters were used in the comparisons for the study.
MR scanning was performed with a 1.5T Signa scanner (General Electric, Milwaukee, Wis). For subjects with epilepsy, whole-brain acquisitions were obtained in the course of their clinical evaluation in the coronal plane with a fast spoiled gradient technique (FSPGR), with 1 of 2 protocols:
Protocol 1.
TR = 14 ms, TE = 3 ms, flip angle = 30°. Voxel dimensions were 0.859 × 0.859 × 1.5 mm. FOV was 22 × 22 cm. Matrix size was 256 × 256.
Protocol 2.
TR = 8.8 ms, TE = 1.8 ms, flip angle = 30°. Voxel dimensions were 0.742 × 0.742 × 1.5 mm. FOV was 38 × 38 cm. Matrix size was 512 × 512.
At Saint Louis University Hospital, epilepsy protocol MR studies were changed to a higher matrix size to improve resolution of images, which explains the 2 different MR protocols. All healthy control subjects had MR studies using protocol 2. All studies, for both subjects with epilepsy and control subjects, were performed on the same MR imaging scanner.
Each group was matched for age and intracranial size.11 Measurement of the intracranial area in the midsagittal plane was performed as described by Free et al,16 tracing along the inner limit of the subcutaneous fat over the convexity, along the margins of the cerebral hemispheres at the base of the brain, and including the brain stem to the foramen magnum. Intracranial area was used as a surrogate measure for intracranial volume.
HDM-LD Segmentation of the Hippocampus
We performed hippocampal deformation segmentations.10 Deformation of individual hippocampi involved a global registration of the entire cerebral volume, followed by an elastic transformation. Total cerebral volumes (excluding the brain stem and cerebellum) were derived using a landmark-based elastic transformation of the template scan, so that comparisons of hippocampal volume, symmetry, and shape could be performed using total cerebral volume as a covariate. The cerebral brain has been previously outlined manually in the template scan. Then, the template scan was globally registered with the MR image of each target using a landmark-based transformation with the global landmarks, followed by an elastic transformation (with 8 basis vectors having 2187 basis coefficients).17 This procedure matched the templates for the cerebral brain (including the hippocampus) onto the target scans.
To quantify hippocampal volume and shape differences between patients with left MTS, those with right MTS, and control subjects, a surface triangulated graph was superimposed onto the hippocampus segmentation in the template and then carried along each deformation of the template to the subjects. The triangulation was obtained using the marching cubes algorithm,18 the surface points being the vertices of the triangulated graph. Left and right hippocampal volumes for each subject were calculated as the volumes enclosed by the transformed surfaces.19
Hippocampal Volume Comparison
For hippocampal volume analysis, the right and left MTS groups were separately compared with the control group. Because of the need to account for right-left hippocampal asymmetries, a repeated-measures analysis of variance (ANOVA) was used with group as the main effect and hemisphere as repeated factors. Group × hemisphere interaction was used to test for volume asymmetry differences.
Hippocampal Shape Comparison
For simultaneous left and right hippocampal shape comparison, the subject surfaces were first registered via a 6-parameter rigid-motion transformation (3 for translation and 3 for rotation). Registration between surfaces is based on the principle that the corresponding surface points after registration have the smallest minimum mean squared error modulo rotation and translation.19 Statistical analyses were performed on the registered surfaces as follows: 1) an overall mean surface was computed for the entire population of registered subject surfaces; 2) vector fields describing hippocampal shape variation within this population were characterized by the covariance matrix of the vector fields; and (3) the dimensionality of the covariance matrix was reduced by computing a complete orthonormal set of eigenvectors via singular value decomposition.19 These eigenvectors are comparable with eigenfunctions identified using principal components analysis and more conventional numeric datasets in that they represent the principal dimensions of statistical variation among the subject datasets and are orthogonal (ie, not correlated) to each other. However, the principal dimensions described by eigenvectors represent variation along various continua of geometric shape variation within a population. Maximal values, positive and negative, of these eigenvector coefficients represent the extremes of such shape variation, and can be used to visualize the manner in which the particular dimension of shape variation (ie, individual eigenvector) alters the hippocampal surface. The first 9 eigenvectors, explaining 75% of the total variance, were used in a multivariate ANOVA to detect overall group difference in hippocampal shape, given by Wilks λ.20 Between-group differences were given by appropriate post hoc contrasts. From these 9 eigenvectors, logistic regression procedures were used to select a subset of eigenvectors that maximally discriminate either MTS group from the control subjects.
Hippocampal Volume Asymmetry Comparison
The asymmetry in the volumes of the hippocampus was studied by quantifying the group × hemisphere interaction in the above repeated-measures ANOVA of hippocampal volumes. The methodology for hippocampal surface asymmetry analysis has been developed previously.9
Hippocampal Shape Asymmetry Comparison
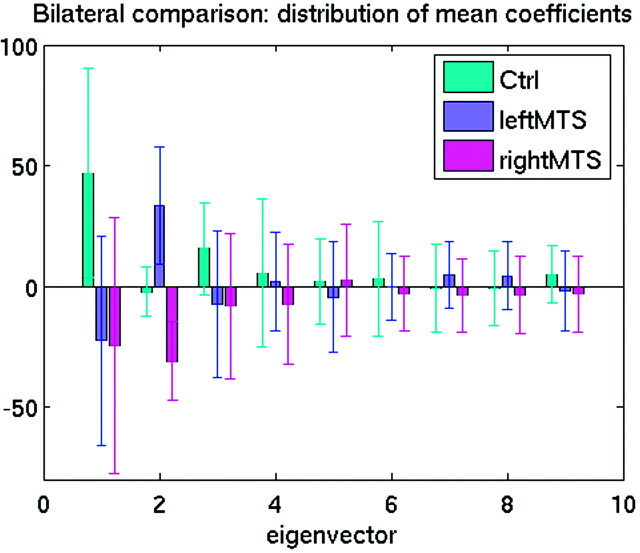
The asymmetry in the shape of the hippocampus was studied by forming asymmetry vector fields based on flipping the right-side hippocampal surface across the midsagittal plane to the left side in each subject. The asymmetry vector field was then characterized by its covariance structure, the dimensionality of which was again reduced by computing a complete orthonormal set of eigenvectors via singular value decomposition.9 The first 9 eigenvectors, explaining 75% of the total variance, were used to compute asymmetry measures for each group. Mean coefficients associated with the first 9 eigenvectors for all groups were plotted, as displayed in Fig 1.
Fig 1.
Mean coefficients associated with the first 9 eigenvectors for all groups. The displacement of each graphic point on the hippocampal surface from template (or atlas, which was a manually outlined hippocampus of a healthy subject not otherwise included in this study) to target (or subject) is represented by a vector. Crosshairs represent the standard deviation of each vector. The figure shows the first 9 eigenvectors, which explained 75% of the total variance and were used to compute asymmetry measures for each group. Of the first 3 eigenvectors, eigenvectors 1 and 3 showed large differences between the control group and each of the MTS groups but showed very similar values for the MTS groups. Eigenvector 2 showed large differences between the control and MTS groups, as well as between the MTS groups. Because eigenvectors 1 and 3 show only minimal differences between the MTS groups, they minimally contributed to discriminating shape differences between the MTS groups. Eigenvector 2, for the MTS groups, showed similar absolute magnitudes. However, the left MTS groups showed a positive value, whereas the right MTS group showed a negative value. This indicates that the shape changes represented by eigenvector 2, comparing the right and left MTS hippocampi, were highly symmetrical.
Asymmetry measures quantify deviation away from perfect symmetry (or zero asymmetry). Normal brain structures may have nonzero but nonsignificant asymmetry measures (ie, small and nonsignificant deviation from zero). A large asymmetry measure with significance would indicate large and significant deviation from zero. The statistical differences in the asymmetric shape of the hippocampus were also detected based on these 9 asymmetry eigenvectors in a multivariate ANOVA to detect overall group difference in hippocampal shape asymmetry, given by Wilks λ.9
Results
There were 15 subjects in both the right and left MTS groups. The right MTS group included 7 women and 8 men with an average duration of epilepsy of 28.4 years. The left MTS group included 8 women and 7 men with an average duration of epilepsy of 25.3 years.
Comparisons of Mean Age and Intracranial Size
The mean age ± SD (P value compared with control group) for the control group was 35.7 ± 10.4 years, for the right MTS groups, 35.7 ± 8.7 years (P = .999), and for the left MTS groups, was 34.7 ± 8.6 years (P = .754). The mean intracranial size ± SD (P value compared with control group) for the control group was 188.1 ± 9.9 cm2, for the right MTS group, 192.8 ± 11.9 cm2 (P = .2497), and for the left MTS group, 185.0 ± 7.7 cm2 (P = .3871).
Hippocampal Volume Comparison
The mean ± SD hippocampal volume for the control group was 3185 ± 413 mm3 on the right and 2803 ± 330 mm3 on the left; for the right MTS group, it was 1953 ± 403 mm3 on the right and 2567 ± 362 mm3 on the left; and for the left MTS group, it was 2765 ± 467 mm3 on the right and 1555 ± 215 mm3 on the left. Repeated-measures ANOVA revealed significant group effect (F = 26.1; df = 2, 42; P < .0001).
Hippocampal Shape Comparison
Multivariate ANOVA on the coefficients from the first 9 eigenvectors (accounting for 75% of the total variance) showed highly significant overall group effect (F = 11.2, df = 18,68, P < .0001) as well as highly significant post hoc between-group differences (left MTS versus control: F = 10.3, P < .0001; right MTS versus control: F = 8.3, P < .0001). In the comparison of the left MTS group with the control group, eigenvectors 1, 2, and 3 were selected by a logistic regression procedure (likelihood ratio: χ2 = 32.0, df = 3, P < .0001). A “leave-one-out” (jackknife) procedure correctly predicted group classification in 14 of 15 (93.3%) MTS subjects and in 14 of 15 (93.3%) control subjects. In the comparison of the right MTS group with the control group, eigenvectors 1, 2, and 3 were selected by a logistic regression procedure (likelihood ratio: χ2 = 41.3, df = 3, P < .0001). A “leave-one-out” (jackknife) procedure correctly predicted group classification in all 15 MTS subjects and all 15 control subjects. Figure 1 shows the mean coefficient associated with the first 9 shape eigenvectors for each group. In the first 3 eigenvectors, there were large (by visual analysis) differences between the control group and each of the MTS groups; however, the largest difference among the 2 MTS groups was found in the second eigenvector and the MTS groups were rather similar in the first and third eigenvectors. This suggested that the laterality of the MTS was largely symmetric in the diseased side hippocampus and was characterized by eigenvector 2. The reconstruction of the hippocampal deformation vector fields due solely to eigenvector 2 was depicted in Figs 2 and 3 (color scale hippocampi) to demonstrate this. Figures 2 and 3 also show the hippocampi surfaces of subjects affected by MTS, applying all vector fields.
Fig 2.
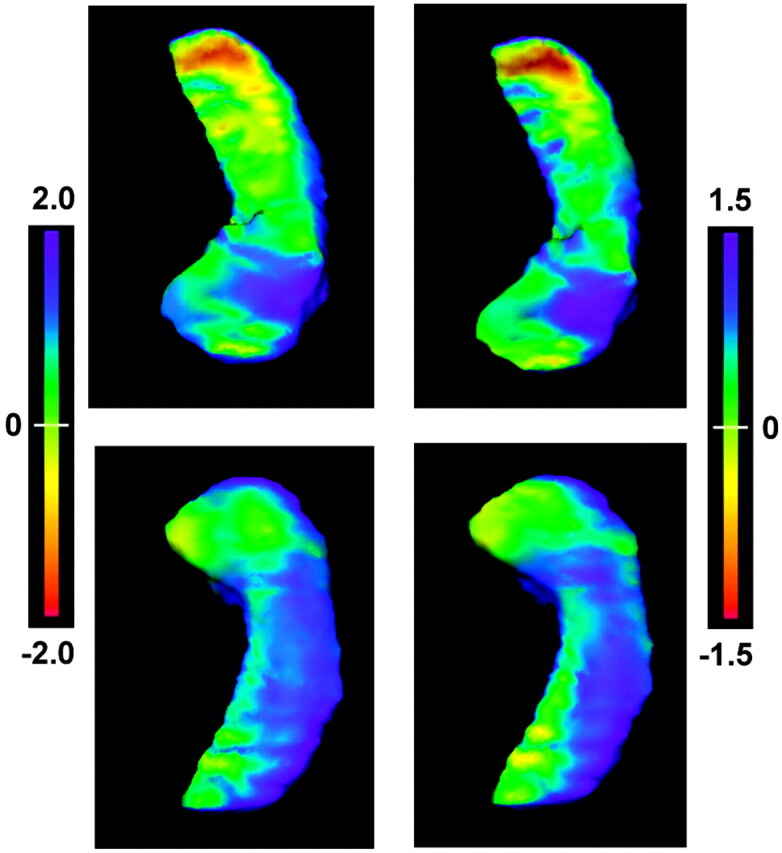
The flame-scale hippocampus panels that display deformation patterns of the left MTS hippocampi. The left column shows the deformation pattern using all eigenvector coefficients, whereas the right column shows the deformation pattern after applying only the extreme positive coefficient from eigenvector 2. Top row, view from above. Bottom row, view from below. The deformation patterns are projected on the surfaces of the control hippocampi, with the flame scale representing (in millimeters) the surface differences between the MTS hippocampi and control hippocampi. The patterns of deformation show marked similarity, demonstrating that the positive component of eigenvector 2 largely represents the deformation changes accounting for differences in the left MTS hippocampi.
Fig 3.
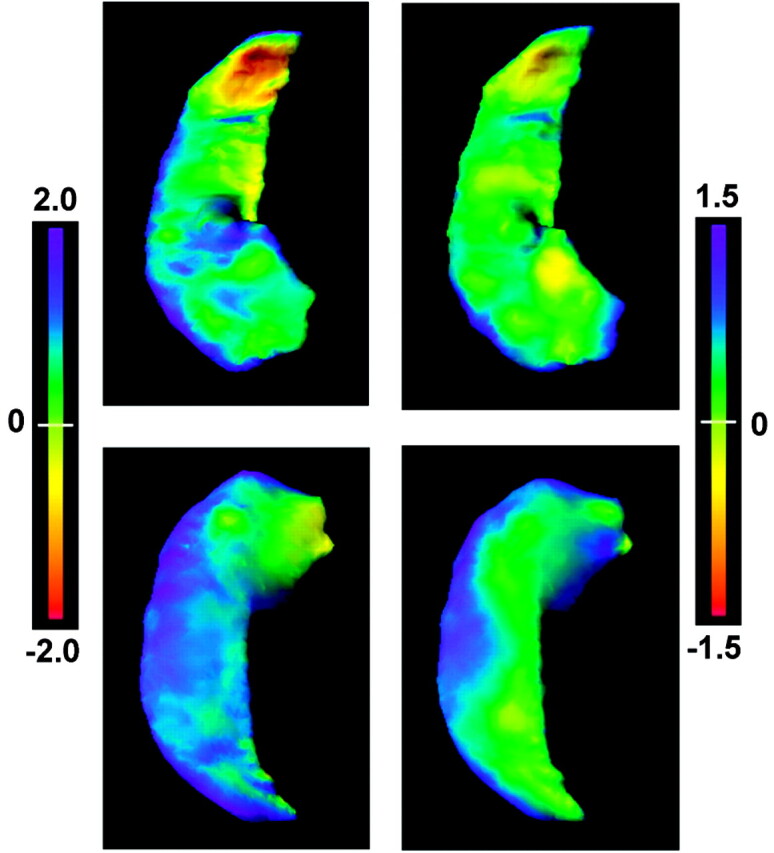
The flame scale hippocampus panels, presented in a similar fashion as in Fig 2, showing deformation patterns of the right MTS hippocampi. The left column shows the deformation pattern with application of all eigenvector coefficients, whereas the right column shows the deformation pattern after applying only the extreme negative coefficient from eigenvector 2. Flame scale units are measured in millimeters. As with the positive component of eigenvector 2 for the left MTS hippocampal deformation pattern, the negative component of eigenvector 2 largely represents the deformation changes accounting for the differences in the right MTS hippocampi.
Hippocampal Volume Asymmetry Comparison
Repeated measures ANOVA revealed significant hemisphere-by-group interaction (F = 147; df = 2,42; P < .0001).
Hippocampal Shape Asymmetry Comparison
The control group had an asymmetry measure of 1.7 that was nonsignificant. The asymmetry measure for the left MTS groups was 5.2 (P < .001) and for the right MTS group, 2.4 (P = .046). Both left MTS and right MTS were shown to be equally different from the healthy control subjects in the multivariate analysis of the asymmetry eigenvectors. Using the first 9 eigenvectors, multivariate ANOVA showed an overall group effect (F = 10.4; df = 18,68; P < .0001) as well as between-group differences (left MTS versus control: F = 11.0; df = 9,20; P < .0001; right MTS versus control: F = 11.9; df = 9,20; P < .0001). The shape asymmetry measure is a unitless term.
Discussion
HDM-LD provides reproducible and precise segmentation of the hippocampus in healthy control subjects,21 and in patients with MTS due to MTLE.10 Test-retest intrasubject overlap of HDM-LD hippocampal segmentations in subjects with MTS was 92.8%.10 Previous studies have documented HDM-LD hippocampal mapping patterns in MTS due to MTLE by using intrasubject comparison with the contralateral hippocampus,22 as well comparison of hippocampi from subjects with MTS with hippocampi of matched control subjects.11 Hippocampi affected by MTS show accentuated hippocampal surface anatomy changes in hippocampal subregions of CA1 and the adjacent subiculum (Sommer sector),11 which are histopathologically well-established regions of preferential involvement in MTS.23–26
Using HDM-LD to directly compare patients with MTS with control subjects eliminates difficulties caused by bilateral involvement of MTS and normal hippocampal asymmetries. Hippocampal surface anatomic changes of the affected hippocampi, comparing the right and left MTS groups by visual analysis, were suggestive that regions of involvement were similar.11 Further objective comparison of changes in the right and left MTS groups by using eigenvector analysis, comparing the groups separately with the control group, allows for more precise definition of possible right-left asymmetries caused by MTS, and a general assessment of the specificity of HDM-LD-defined hippocampal surface structure change in subjects with MTS and MTLE.
In healthy control subjects, the right hippocampus is typically larger than the left hippocampus.13 Eigenvector amplitudes account for this asymmetry. The left MTS group had an apparent exaggeration of normal asymmetry (Fig 1). The asymmetry measure of lower magnitude and significance in the right MTS group probably occurred because asymmetry measures quantified deviation away from zero-asymmetry (not normal asymmetry) and because the diseased hippocampus in the right MTS group was on the right side (asymmetry vector fields were formed by flipping from right to left) and so reversed the normal pattern of asymmetry. To objectively evaluate for symmetry of involvement of the MTS hippocampi in the right and left MTS groups, we used eigenvector function analysis, as depicted in Figs 2 and 3. Previous studies have demonstrated that hippocampal surface structural changes in subjects with MTS as a result of MTLE are most accentuated in the Sommer sector.11,22 The deformation views in Figs 2 and 3 again confirm this finding, showing the most accentuated deformation patterns in the lateral aspect of the body of the hippocampus, which is the surface region of the Sommer sector.11
Several aspects of the results support the accuracy of HDM-LD in depicting the changes in hippocampal shape associated with MTS. First, our results confirm previous findings that there are specific regions of volume loss within the hippocampus that correlate with the well-established histopathologic pattern of MTS.24 Second, even though we statistically considered the right and left MTS groups separately, after accounting for normal variations in the size and shape of the right and left hippocampi, the results of eigenvector-defined shape changes of the MTS hippocampi in the right and left MTS groups were very similar. The pattern and symmetry of involvement of the MTS hippocampi in the right and left MTLE groups takes on further significance when considering challenges in study design of subjects with MTLE due to MTS. MTLE due to MTS is relatively rare, making selection bias of subjects a concerning issue. In the current study, we performed a consecutive review of subjects undergoing epilepsy surgery at a tertiary referral center, whereas control subjects were selected from the general population. Therefore, factors associated with selection of the patient groups could explain some of the differences in our findings. In light of the pattern and symmetry of involvement of the MTS hippocampi, however, we would consider such selection bias an unlikely explanation for our findings.
From a clinical perspective, an asymmetry of hippocampal volumes is very important in the diagnosis of temporal lobe epilepsy, correlating with lateralization of EEG onset of seizures,27–30 postsurgical pathologic verification of MTS,1,5,31,32 and good outcome after epilepsy surgery.3,33 Past studies have used different criteria to define hippocampal asymmetry.28,29,34 However, hippocampal volume asymmetry may be insensitive in detecting some cases of MTS because of the relatively common occurrence of bilateral hippocampal atrophy.34–37 Quigg et al,35 evaluating 40 consecutive patients undergoing temporal lobe epilepsy surgery, found highly significant volume loss, compared with control subjects, in hippocampi contralateral to the side of temporal lobe resection. To detect bilateral hippocampal volume loss, past investigators used groups of control subjects to establish normal ratios of hippocampal volumes to intracranial or brain parenchymal volume,34,38–40 setting a limit of 2 SD below the normal distribution as a significant degree of hippocampal volume loss.40–42
Given that HDM-LD and other computational tools may detect more subtle changes in regional hippocampal anatomy, a potential application of such methods in epilepsy is in the evaluation of patients with temporal lobe epilepsy and symmetrical hippocampal volumes. Our current method measures regional surface displacement differences over the hippocampus, showing marked differences in specific hippocampal surface regions. These same regions would theoretically be involved, to a lesser degree, in patients with MTS and symmetrical volumes. In our current analysis we used a “leave-one-out” (jackknife) procedure, which uses eigenvector functions (which quantify shape differences), to predict group classification. The procedure correctly predicted 14 of 15 in the right MTS subjects and control subjects, and 15 of 15 in the left MTS and control subjects. By using HDM-LD to compare patients with MTS and symmetrical hippocampal volumes with control subjects, we may detect subtle but significant regions of hippocampal shape change. Past investigators have described abnormally formed hippocampi in patients with partial epilepsy, with associated normal total volumes, and have noted abnormal orientation of the hippocampi in 3D space.43 First-degree relatives of patients with familial MTLE also show hippocampi with “irregular” shape.40 Although our current deformation technique shows regional surface displacement, measurement of other parameters, such as rotation around a specified axis, is also possible with further development of our deformation technique. Measurement of 3D spatial relationships of hippocampal structure may help to improve quantitation and detection of these shape changes in patients with MTLE and symmetrical hippocampal volumes. Our current study has several limitations in assessing whether HDM-LD can independently predict hippocampal shape differences in MTS, including limited sample size and selection of a well-defined group of subjects with hippocampal volume asymmetry and MTS. Further study using larger groups of patients and MTS subjects without hippocampal volume asymmetry will be necessary to verify our preliminary findings.
Our findings of accentuated volume changes clearly differ from hippocampal shape changes in schizophrenia, Alzheimer disease, and depression. Patients with schizophrenia show minimal volume loss in the lateral hippocampal head and subiculum.12 In very mild Alzheimer-type dementia, subjects show inward deformities in the head of the hippocampus and along the lateral surface of the hippocampal body with substantial overall hippocampal volume loss.13 Finally, patients with depression show a shape deformation of the subiculum without any measurable volume loss.14 These differences in HDM-LD determined hippocampal subregion involvement in different disease states suggests that the regions of involvement may be specific to different pathophysiologic mechanisms associated with different disease states. Our initial results show that HDM-LD techniques can distinguish group changes when comparing MTS patients with control subjects. Therefore, further application of HDM-LD based techniques to distinguish hippocampal changes in different disease states is a possibility.
Conclusion
Eigenvector analysis of principal dimensions of hippocampal shape change suggests that MTS, after accounting for normal right-left asymmetries, affects the right and left hippocampal surface structure in a similar pattern. This preliminary analysis using HDM-LD shows it can predict group classification of MTS and control hippocampi in this well-defined population of patients with MTS and MTLE.
References
- 1.Lencz T, McCarthy G, Bronen RA, et al. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol 1992;31:629–37 [DOI] [PubMed] [Google Scholar]
- 2.Cascino GD. Clinical correlations with hippocampal atrophy. Magn Reson Imaging 1995;13:1133–36 [DOI] [PubMed] [Google Scholar]
- 3.Jack CR Jr, Sharbrough FW, Cascino GD, et al. Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol 1992;31:138–46 [DOI] [PubMed] [Google Scholar]
- 4.Cascino GD. Clinical evaluation and noninvasive electroencephalography. Preoperative evaluation. Neuroimaging Clin N Am 1995;5:547–58 [PubMed] [Google Scholar]
- 5.Kim JH, Tien RD, Felsberg GJ, et al. Fast spin-echo MR in hippocampal sclerosis: correlation with pathology and surgery. AJNR Am J Neuroradiol 1995;16:627–36 [PMC free article] [PubMed] [Google Scholar]
- 6.Arruda F, Cendes F, Andermann F, et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol 1996;40:446–50 [DOI] [PubMed] [Google Scholar]
- 7.Holmes MD, Dodrill CB, Ojemann GA, et al. Outcome following surgery in patients with bitemporal interictal epileptiform patterns. Neurology 1997;48:1037–40 [DOI] [PubMed] [Google Scholar]
- 8.Watson C, Jack CR Jr, Cendes F. Volumetric magnetic resonance imaging. Clinical applications and contributions to the understanding of temporal lobe epilepsy. Arch Neurol 1997;54:1521–31 [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Joshi SC, Miller MI, et al. Statistical analysis of hippocampal asymmetry in schizophrenia. Neuroimage 2001;14:531–45 [DOI] [PubMed] [Google Scholar]
- 10.Hogan RE, Mark KE, Wang L, et al. Mesial temporal sclerosis and temporal lobe epilepsy: MR imaging deformation-based segmentation of the hippocampus in five patients. Radiology 2000;216:291–97 [DOI] [PubMed] [Google Scholar]
- 11.Hogan RE, Wang L, Bertrand ME, et al. MRI-based high-dimensional hippocampal mapping in mesial temporal lobe epilepsy. Brain 2004;127:1731–40 [DOI] [PubMed] [Google Scholar]
- 12.Csernansky JG, Joshi S, Wang L, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci U S A 1998;95:11406–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csernansky JG, Wang L, Joshi S, et al. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Dementia of the Alzheimer type. Neurology 2000;55:1636–43 [DOI] [PubMed] [Google Scholar]
- 14.Posener JA, Wang L, Price JL, et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry 2003;160:83–89 [DOI] [PubMed] [Google Scholar]
- 15.Risinger MW, Engel J Jr, Van Ness PC, et al. Ictal localization of temporal lobe seizures with scalp/sphenoidal recordings. Neurology 1989;39:1288–93 [DOI] [PubMed] [Google Scholar]
- 16.Free SL, Bergin PS, Fish DR, et al. Methods for normalization of hippocampal volumes measured with MR. AJNR Am J Neuroradiol 1995;16:637–43 [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MI, Banerjee A, Christensen GE, et al. Statistical methods in computational anatomy. Stat Methods Med Res 1997;6:267–99 [DOI] [PubMed] [Google Scholar]
- 18.Claudio M, Roberto S. Using marching cubes on small machines. Graphical models and image processing. Graph Model Im Proc 1994;56:182–83 [Google Scholar]
- 19.Joshi S, Miller MI, Grenander U. On the gemometry and shape of brain sub-manifolds. Intern J Pattern Recognit Artif Intell 1997;11:1317–43 [Google Scholar]
- 20.Wang L, Swank JS, Glick IE, et al. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage 2003;20:667–82 [DOI] [PubMed] [Google Scholar]
- 21.Haller JW, Banerjee A, Christensen GE, et al. Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology 1997;202:504–10 [DOI] [PubMed] [Google Scholar]
- 22.Hogan RE, Bucholz RD, Joshi S. Hippocampal deformation-based shape analysis in epilepsy and unilateral mesial temporal sclerosis. Epilepsia 2003;44:800–06 [DOI] [PubMed] [Google Scholar]
- 23.Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropath Exp Neurol 1993;52:433–43 [DOI] [PubMed] [Google Scholar]
- 24.Thom M, Sisodiya SM, Beckett A, et al. Cytoarchitectural abnormalities in hippocampal sclerosis. J Neuropathol Exp Neurol 2002;61:510–19 [DOI] [PubMed] [Google Scholar]
- 25.Babb TL, Brown WJ, Pretorius J, et al. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia 1984;25:729–40 [DOI] [PubMed] [Google Scholar]
- 26.Bratz E. Ammonshornbefunde bei epileptikern. Arch Psychiatr Nervenkr 1899;31:820–35 [Google Scholar]
- 27.Cendes F, Leproux F, Melanson D, et al. MRI of amygdala and hippocampus in temporal lobe epilepsy. J Comput Assist Tomogr 1993;17:206–10 [DOI] [PubMed] [Google Scholar]
- 28.Cook MJ, Fish DR, Shorvon SD, et al. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain 1992;115:1001–15 [DOI] [PubMed] [Google Scholar]
- 29.Jack CR Jr, Sharbrough FW, Twomey CK, et al. Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology 1990;175:423–29 [DOI] [PubMed] [Google Scholar]
- 30.Spencer SS, McCarthy G, Spencer DD. Diagnosis of medial temporal lobe seizure onset: relative specificity and sensitivity of quantitative MRI. Neurology 1993;43:2117–24 [DOI] [PubMed] [Google Scholar]
- 31.Bronen RA, Cheung G, Charles JT, et al. Imaging findings in hippocampal sclerosis: correlation with pathology. AJNR Am J Neuroradiol 1991;12:933–40 [PMC free article] [PubMed] [Google Scholar]
- 32.Cascino GD, Jack CR Jr, Parisi JE, et al. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol 1991;30:31–36 [DOI] [PubMed] [Google Scholar]
- 33.Radhakrishnan K, So EL, Silbert PL, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology 1998;51:465–71 [DOI] [PubMed] [Google Scholar]
- 34.Jack CR Jr, Trenerry MR, Cascino GD, et al. Bilaterally symmetric hippocampi and surgical outcome. Neurology 1995;45:1353–58 [DOI] [PubMed] [Google Scholar]
- 35.Quigg M, Bertram EH, Jackson T, et al. Volumetric magnetic resonance imaging evidence of bilateral hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsia 1997;38:588–94 [DOI] [PubMed] [Google Scholar]
- 36.Barr WB, Ashtari M, Schaul N. Bilateral reductions in hippocampal volume in adults with epilepsy and a history of febrile seizures. J Neurol Neurosurg Psychiatry 1997;63:461–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King D, Spencer SS, McCarthy G, et al. Bilateral hippocampal atrophy in medial temporal lobe epilepsy. Epilepsia 1995;36:905–10 [DOI] [PubMed] [Google Scholar]
- 38.Van Paesschen W, Connelly A, King MD, et al. The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Ann Neurol 1997;41:41–51 [DOI] [PubMed] [Google Scholar]
- 39.Briellmann RS, Jackson GD, Kalnins R, et al. Hemicranial volume deficits in patients with temporal lobe epilepsy with and without hippocampal sclerosis. Epilepsia 1998;39:1174–81 [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi E, Li LM, Lopes-Cendes I, et al. Magnetic resonance imaging evidence of hippocampal sclerosis in asymptomatic, first-degree relatives of patients with familial mesial temporal lobe epilepsy. Arch Neurol 2002;59:1891–94 [DOI] [PubMed] [Google Scholar]
- 41.Liu RS, Lemieux L, Bell GS, et al. The structural consequences of newly diagnosed seizures. Ann Neurol 2002;52:573–80 [DOI] [PubMed] [Google Scholar]
- 42.Van Paesschen W, Revesz T, Duncan JS, et al. Quantitative neuropathology and quantitative magnetic resonance imaging of the hippocampus in temporal lobe epilepsy. Ann Neurol 1997;42:756–66 [DOI] [PubMed] [Google Scholar]
- 43.Baulac M, De Grissac N, Hasboun D, et al. Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol 1998;44:223–33 [DOI] [PubMed] [Google Scholar]