Abstract
BACKGROUND AND PURPOSE:The hippocampal fissure is a fetal sulcus that, except for its most medial part (the superficial hippocampal sulcus), is normally obliterated. Hippocampal cavities are residual cysts attributable to lack of hippocampal fissure obliteration. We hypothesized that either hippocampal sulcus enlargement or an increase in number or size of hippocampal cavities could be associated with medial temporal lobe atrophy (MTA) occurring in Alzheimer disease.
METHODS:Two observers assessed the maximal hippocampal sulcus width by means of the fimbriosubicular distance at the anterior part of the hippocampal body; as well as the occurrence, number, and size of hippocampal cavities; and the visual rating score of MTA on magnified coronal high-resolution T1-weighted MR images of 21 patients with Alzheimer disease and 15 nondemented elderly controls.
RESULTS:Both observers found the maximal hippocampal sulcus width significantly larger in patients with Alzheimer disease than in controls (P < .0001). The interobserver averaged fimbriosubicular distance in patients with Alzheimer disease was 2.84 mm (SD = 0.94), approximately twice that of the corresponding distance in nondemented subjects (1.41 mm; SD, 0.58). Both observers found a significant correlation between the fimbriosubicular distance and MTA score (observer 1, rs = 0.71; observer 2, rs = 0.74; P < .0001). None of the observers found significant differences between patients with Alzheimer disease and nondemented subjects with respect to occurrence, number, or size of hippocampal cavities, nor did they find a significant correlation between the number or size of hippocampal cavities and MTA. Interobserver agreement ranged from moderate to very good.
CONCLUSION:Enlargement of the hippocampal sulcus, assessed by the fimbriosubicular distance, is associated with MTA in Alzheimer disease, but enlargement of the hippocampal cavities is not.
The most common cause of dementia is Alzheimer disease. Neuropathologic changes underlying Alzheimer disease first occur in the medial temporal lobe.1 Therefore, structural neuroimaging in Alzheimer disease is focused on detection of medial temporal lobe atrophy (MTA), particularly of the hippocampus, parahippocampal gyrus (including the entorhinal cortex), and amygdala.2
The hippocampal fissure is a fetal sulcus around which occurs the hippocampal folding. It begins as a small indentation in the primitive hippocampus at approximately the 10th week of fetal development. Later, the sulcus deepens, but between the 18th and the 21st week of fetal development, it is obliterated almost completely by fusion between the cornu ammonis and the dentate gyrus.3 No more than the most medial part of the hippocampal fissure persists as an open and shallow groove between the dentate gyrus and the subiculum, just below the fimbria and the fimbriodentate sulcus, on the medial surface of the hippocampus4,5—the superficial hippocampal sulcus.5 Hippocampal cavities are generally considered residual cysts resulting from lack of hippocampal fissure obliteration. Therefore, hippocampal cavities are most commonly localized laterally, at the apex of the hippocampal fold, between the cornu ammonis and the dentate gyrus. Hippocampal cavities are regularly found in routine MR imaging studies and are believed to represent a normal variant reflecting CSF collection.6,7
Because brain atrophy results in enlargement of the CSF spaces, we hypothesized that either hippocampal sulcus enlargement or an increase in the number or size of hippocampal cavities could be associated with MTA occurring in Alzheimer disease.
Materials and Methods
Subjects
Between 2000 and 2002, 21 patients (7 men, 14 women) fulfilling the National Institute of Neurologic, Communicative Disorders and Stroke (NINCDS)-Alzheimer Disease and Related Disorders Association (ADRDA) criteria for probable Alzheimer disease,8 and 15 elderly nondemented controls (9 men, 6 women) were selected for this study. The patients were randomly selected from the outpatient memory clinic of the Alzheimer Center (VU University Medical Center, Amsterdam, the Netherlands), as part of a prospective study on brain aging and dementia approved by the local ethics committee. Control subjects were relatives of the patients and provided informed consent to be included. To evaluate cognitive function, we administered the Mini-Mental State Examination (MMSE) (possible range of scores, 0–30)9 to all subjects. On the basis of the MMSE, patients with Alzheimer disease were classified as having mild-to-moderate (MMSE scores, ≥10) or severe (MMSE scores, <10) dementia. No patients were under antidementia therapy before they underwent MR imaging examination.
MR Imaging Protocol
MR imaging examinations were performed by using a superconductive magnet operating at 1T (Impact, Siemens, Erlangen, Germany) and a routine imaging protocol for dementia. For this study, we used a high-resolution 3D T1-weighted gradient-echo sequence (TE = 7 ms, TR = 15 ms, NEX = 1, flip angle = 8 °, effective section thickness = 1.49 mm).
Image Assessment
Two observers (A.J.B.-L., J.H.v.W.) rated the images, blinded to clinical information, with the use of digital image files displayed at full resolution, without pixel interpolation.
Because the identifiable part of the hippocampal sulcus is normally a shallow groove just below the fimbria and above the subiculum, we used magnified coronal high-resolution T1-weighted images perpendicular to the long axis of the temporal lobe to better visualize it and took the fimbriosubicular distance as a linear measurement to evaluate its maximal width. We measured this distance perpendicular to the visible longitudinal extent of the hippocampal sulcus on both sides, at the anterior part of the hippocampal body, after choosing the best section displaying the fimbria. In those cases in which the hippocampal sulcus was too shallow to be measured, we considered it to equal 0 mm. In the other cases, we took the measurement either vertically or obliquely, according to the relative position between the fimbria and the subiculum (Fig 1). Hippocampal cavities were defined as sharply demarcated cystic structures (isointense with CSF) localized at the apex of the hippocampal fold. The greatest dimension of each of the hippocampal cavities was determined on coronal T1-weighted images. We also used a visual rating scale to evaluate MTA10 on coronal T1-weighted images (possible range of scores for each side, 0–4).
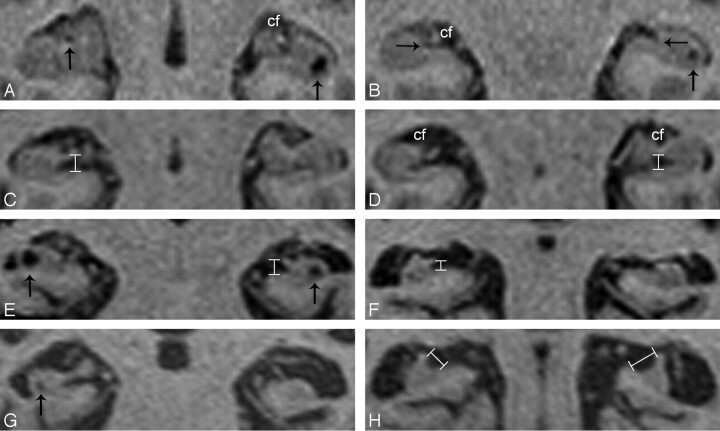
Fig 1.
Magnified coronal high-resolution T1-weighted images of the hippocampal region.
A and B, Coronal T1-weighted image of a 68-year-old nondemented control showing discrete enlargement of the choroidal fissures (cf), suggesting medial temporal lobe atrophy (MTA) grade 1, and hippocampal cavities bilaterally (vertical arrows). Note that both hippocampal sulci are not enlarged (horizontal arrows).
C and D, Coronal T1-weighted image of a 54-year-old patient with Alzheimer disease showing enlargement of the hippocampal sulcus, measured between the fimbria and the subiculum (vertical measurement overlays), and enlargement of the choroidal fissures (cf) (MTA grade 1).
E and F, Coronal T1-weighted image of a 76-year-old patient with Alzheimer disease showing enlargement of the hippocampal sulcus (vertical measurement overlays), moderate-to-severe MTA (grade 3) and hippocampal cavities bilaterally (vertical arrows).
G and H, Coronal T1-weighted image of a 93-year-old patient with Alzheimer disease showing severe MTA (grade 4) and a small hippocampal cavity on the right side (vertical arrow). Note that the fimbria appears laterally displaced; this displacement contributes to an increase of the fimbriosubicular distance (oblique measurement overlays).
Focal abnormalities measuring <1 mm were not included. Cystlike structures localized medially in the hippocampus and appearing to be the hippocampal sulcus continuation in contiguous sections were considered part of the sulcus, not hippocampal cavities. Care was taken to avoid the inclusion of pulsation artifacts, recognizable by linear patterns of signal-intensity banding attributable to phase misregistration.11
Statistical Analysis
Statistical analysis was performed by means of SPSS 11.0 (SPSS for Windows, Chicago, Ill) and MedCalc (MedCalc Software, Mariakerke, Belgium). We used the Fisher exact test to compare categorical variables and the Mann-Whitney U test to compare scores between independent groups of patients. For comparisons of discrete and continuous variables, we used the independent-samples Student t test, because the distribution of data was approximately normal. Correlations were tested by using the Spearman rank correlation coefficient (rs). Statistical significance was considered when P values were <.05.
To assess interobserver agreement, we used the kappa (κ) coefficient for nominal variables, the weighted κ coefficient for ordinal and discrete variables, and the intraclass correlation coefficient (ICC) for continuous variables. Strength of agreement was interpreted according to adapted guidelines proposed by Landis and Koch.12
We also calculated the statistical power of the study on the basis of the standardized difference for the most relevant variable.
Results
Table 1 summarizes the clinical and radiologic characteristics of the patients and controls. All patients had Alzheimer disease of mild-to-moderate severity.
Table 1:
Characteristics of patients with Alzheimer disease (AD) (n = 21) and nondemented elderly control subjects (n = 15) including age, mini-mental state examination (MMSE), hippocampal sulcus (HS) width, number and size of hippocampal cavities (HC), and medial temporal lobe atrophy (MTA) score
| Characteristic | Mean (SD) |
||
|---|---|---|---|
| AD Patients | Control Subjects | P Value | |
| Age | 69.3 (10.9) | 68.9 (8.0) | .91 |
| MMSE score* | 18.8† (4.5) | 27.9† (1.9) | <.0001 |
| HS width‡ (mm), observer 1 | 2.9 (1.0) | 1.4 (0.6) | <.0001 |
| HS width‡ (mm), observer 2 | 2.8 (1.1) | 1.4 (0.7) | <.0001 |
| HS width‡ (mm), interobserver average | 2.8 (0.9) | 1.4 (0.6) | <.0001 |
| Number of HC, observer 1 | 2.6 (3.2) | 3.6 (2.8) | .32 |
| Number of HC, observer 2 | 1.9 (2.4) | 3.6 (2.9) | .06 |
| Number of HC, interobserver average | 2.2 (2.7) | 3.6 (2.8) | .15 |
| Mean size of HC (mm), observer 1 | 1.7 (1.0) | 2.0 (1.1) | .29 |
| Mean size of HC (mm), observer 2 | 1.3 (1.1) | 2.0 (0.8) | .06 |
| Mean size of HC (mm), interobserver average | 1.5 (0.9) | 2.0 (0.9) | .10 |
| MTA score‡§, observer 1 | 2.2† (1.0) | 1.1† (0.7) | <.01 |
| MTA score‡§, observer 2 | 1.7† (1.1) | 0.6† (0.7) | <.01 |
| MTA score‡§, interobserver average | 2.0† (1.1) | 0.8† (0.6) | <.01 |
Note:—
Lower values indicate greater severity.
Please note that means of scores are presented, whereas we used the Mann-Whitney U test to compare differences between groups.
Values are left/right averages.
Higher values indicate greater severity.
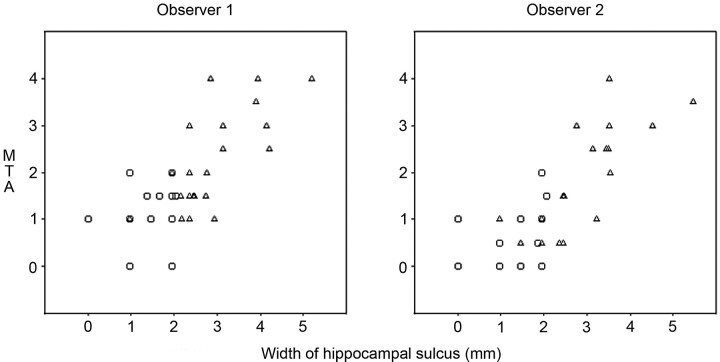
Both observers found the maximal hippocampal sulcus width significantly larger in patients with Alzheimer disease than in controls (P < .0001). The interobserver averaged fimbriosubicular distance in patients with Alzheimer disease was 2.84 mm (SD = 0.94), approximately twice that of the corresponding distance in nondemented subjects (1.41 mm, SD = 0.58) (Fig 1). Both observers found higher MTA scores significantly associated with Alzheimer disease (P < .01), as well as a significant correlation between the fimbriosubicular distance and MTA (observer 1, rs = 0.71; observer 2, rs = 0.74; P < .0001) (Fig 2). Table 2 shows the correspondence between fimbriosubicular measurements and visual rating grades of MTA.
Fig 2.
Scatterplots displaying the averaged left/right hippocampal sulcus width (fimbriosubicular distance) plotted against the medial temporal atrophy score. Triangles represent patients with Alzheimer disease, and circles correspond to control subjects.
Table 2:
Interobserver averaged hippocampal sulcus (HS) width (fimbriosubicular distance) according to grade of medial temporal lobe atrophy (MTA) in patients with Alzheimer disease (AD) (n = 21) and nondemented elderly control subjects (n = 15)
| MTA score | Mean HS Width in mm (SD) |
P Value | |
|---|---|---|---|
| AD Patients | Control Subjects | ||
| 0 (n = 3) | 1.4 (0.5) | ||
| 1 (n = 18) | 2.1 (0.5) | 1.3 (0.7) | P < 0.05 |
| 2 (n = 6) | 2.5 (0.1) | 1.7 (0.4) | P < 0.05 |
| 3 (n = 6) | 3.5 (0.4) | ||
| 4 (n = 3) | 4.1 (1.1) | ||
One of the observers found hippocampal cavities in 14 (66.7%) of the 21 patients, and in 14 (93.3%) of the 15 controls (P = .10). The other found hippocampal cavities in 16 (76.2%) patients, and in 13 (86.7%) controls (P = .67). None of the observers found significant differences between patients with Alzheimer disease and nondemented subjects with respect to number or size of hippocampal cavities, nor did they find a significant correlation between number or size of hippocampal cavities and MTA.
The interobserver reliability analysis revealed a very good agreement in the assessment of the number of hippocampal cavities (weighted κ = 0.90) and hippocampal sulcus width (ICC = 0.85), good agreement in the assessment of MTA (weighted κ = 0.80), and moderate agreement in the judgment of presence (κ = 0.58) and size of hippocampal cavities (ICC = 0.56).
On the basis of a standardized difference of 1.34 for the hippocampal sulcus width, the statistical power of the study was approximately 0.90 (P = .01).
Discussion
Our results show that enlargement of the hippocampal sulcus, assessed by a linear measurement between the fimbria and the subiculum, is significantly associated with Alzheimer disease and that hippocampal sulcus enlargement is significantly correlated with higher visual rating scores of MTA. The early involvement of both the entorhinal cortex and the subiculum by Alzheimer pathology1 resulting in disruption of the perforant pathway,13,14 which conveys most of the input connections from the neocortex to the hippocampus and partially transverses the obliterated hippocampal fissure,15 explains, in part, the tissue loss leading to hippocampal sulcus enlargement. In addition, it is also conceivable that the consequent loss of hippocampal efferences may well cause atrophy of the fimbria. Finally, in certain patients with severe hippocampal atrophy, the fimbria appears to be laterally displaced; therefore, an additional increase of the fimbriosubicular distance occurs (Fig 1H).
MTA can be assessed by using visual rating scales, linear measurements of temporal lobe structures, and volumetry of the hippocampus.2 Previous studies using CT axial thin sections parallel to the long axis of the temporal lobe found enlargement of the choroidal/hippocampal fissure complex in Alzheimer disease, assessed either by visual rating of the medial hippocampal lucency or by using a quantitative stereologic approach.16–18 Other studies found linear measurements of the temporal horn width reliable for the detection of Alzheimer disease.19,20 We used a linear measurement between the fimbria and the subiculum on magnified coronal high-resolution T1-weighted images to estimate the maximal hippocampal sulcus width. Because both the fimbria and the subiculum correspond to hippocampal structures, this measurement may reflect purely hippocampal atrophy better than the choroidal fissure width or the temporal horn width, both surrounded superiorly and laterally by white matter of the temporal lobe. We also used a visual rating scale for MTA10 based on subjective evaluation of the choroidal fissure width, the temporal horn width, and the hippocampal height. Because this scale does not currently consider the hippocampal sulcus width, its inclusion as an item to rate MTA may perhaps have diagnostic significance for the detection of patients with Alzheimer disease. In fact, we found the fimbriosubicular distance significantly larger in patients with Alzheimer disease than in controls with similar MTA scores (Table 2).
Hippocampal cavities are usually defined as cystic remnants of the primitive hippocampal fissure,6,7 though some authors believe they also represent enlarged perivascular (Virchow-Robin) spaces.6,21 In any case, their presence, increased number, or enlargement could reflect focal hippocampal atrophy, and one would expect them to be more frequent or larger in patients with Alzheimer disease than in healthy elderly subjects. Actually, enlarged Virchow-Robin spaces reflecting focal brain atrophy around perforating arteries in the striatum and centrum semiovale22,23 are associated with white matter lesions and cognitive impairment.24 Furthermore, enlarged Virchow-Robin spaces were recently found to be a sensitive indicator of cerebral microvascular disease.25 Nevertheless, we did not find a significant difference between patients with Alzheimer disease and nondemented subjects with respect to occurrence, number, or size of hippocampal cavities. We also did not find a significant correlation between number or size of hippocampal cavities and MTA. However, hippocampal cavities are less commonly seen in younger than in elderly subjects,6,7,26 and their number and size were found to be higher in nondemented subjects carrying apolipoprotein E genotypes conferring risk for the development of degenerative and vascular brain pathology.26 One explanation for the apparent disagreement between these findings and our results is perhaps the existence of a cancellation effect between MTA and hippocampal cavities, either because some hippocampal cavities may disappear by merging with the hippocampal sulcus as it enlarges or because small hippocampi cannot harbor too many cavities.
On the basis of the standardized difference for the hippocampal sulcus width, the statistical power of this study is high, but the relatively small sample size still represents a limitation to detect (as significant) other less-marked differences. Another limitation is the fact that some of the performed measurements were very small and, therefore, possibly influenced by the existence of partial volume effects. To avoid this problem, we used magnified coronal high-resolution T1-weighted images displayed without pixel interpolation, which enabled us to identify the exact number of pixels for each individual measurement. Nevertheless, we recognize that both a larger sample size and the use of MR imaging sequences acquired at higher field strengths (enabling more spatial resolution) would be important to confirm our findings.
Conclusion
Enlargement of the hippocampal sulcus, assessed by the fimbriosubicular distance on coronal T1-weighted imaging, is associated with MTA in patients with Alzheimer disease and may serve as a measure to rate MTA severity. By contrast, hippocampal cavities were not found to be significantly associated with MTA or Alzheimer disease and do not seem to have pathologic value.
References
- 1.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59 [DOI] [PubMed] [Google Scholar]
- 2.Bastos Leite AJ, Scheltens P, Barkhof F. Pathological aging of the brain: an overview. Top Magn Reson Imaging 2004;15:369–89 [DOI] [PubMed] [Google Scholar]
- 3.Humphrey T. The development of the human hippocampal fissure. J Anat 1967;101:655–76 [PMC free article] [PubMed] [Google Scholar]
- 4.Naidich TP, Daniels DL, Haughton VM, et al. Hippocampal formation and related structures of the limbic lobe: anatomic-MR correlation. Part I. Surface features and coronal sections. Radiology 1987;162:747–54 [DOI] [PubMed] [Google Scholar]
- 5.Duvernoy HM. The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Berlin, Germany: Springer-Verlag;2004
- 6.Sasaki M, Sone M, Ehara S, et al. Hippocampal sulcus remnant: potential cause of change in signal intensity in the hippocampus. Radiology 1993;188:743–46 [DOI] [PubMed] [Google Scholar]
- 7.Yoneoka Y, Kwee IL, Fujii Y, et al. Criteria for normalcy of cavities observed within the adult hippocampus: high-resolution magnetic resonance imaging study on a 3.0-T system. J Neuroimaging 2002;12:231–35 [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44 [DOI] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98 [DOI] [PubMed] [Google Scholar]
- 10.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 1992;55:967–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karis JP. Magnetic resonance imaging artifacts: a practical approach. In: Orrison WWJ, ed. Neuroimaging. Philadelphia: WB Saunders Company;2000. :507–13
- 12.Altman DG. Practical statistics for medical research. London, UK: Chapman & Hall;1991
- 13.Hyman BT, Van Horsen GW, Damasio AR, et al. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 1984;225:1168–70 [DOI] [PubMed] [Google Scholar]
- 14.Hyman BT, Van Hoesen GW, Kromer LJ, et al. Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann Neurol 1986;20:472–81 [DOI] [PubMed] [Google Scholar]
- 15.Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol 1934;46:113–77 [Google Scholar]
- 16.de Leon MJ, George AE, Stylopoulos LA, et al. Early marker for Alzheimer’s disease: the atrophic hippocampus. Lancet 1989;2:672–73 [DOI] [PubMed] [Google Scholar]
- 17.George AE, de Leon MJ, Stylopoulos LA, et al. CT diagnostic features of Alzheimer disease: importance of the choroidal/hippocampal fissure complex. AJNR Am J Neuroradiol 1990;11:101–07 [PMC free article] [PubMed] [Google Scholar]
- 18.de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol 1993;14:897–906 [PMC free article] [PubMed] [Google Scholar]
- 19.Frisoni GB, Beltramello A, Weiss C, et al. Linear measures of atrophy in mild Alzheimer disease. AJNR Am J Neuroradiol 1996;17:913–23 [PMC free article] [PubMed] [Google Scholar]
- 20.Frisoni GB, Geroldi C, Beltramello A, et al. Radial width of the temporal horn: a sensitive measure in Alzheimer disease. AJNR Am J Neuroradiol 2002;23:35–47 [PMC free article] [PubMed] [Google Scholar]
- 21.Bastos AC, Andermann F, Melancon D, et al. Late-onset temporal lobe epilepsy and dilatation of the hippocampal sulcus by an enlarged Virchow-Robin space. Neurology 1998;50:784–87 [DOI] [PubMed] [Google Scholar]
- 22.Awad IA, Johnson PC, Spetzler RF, et al. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke 1986;17:1090–97 [DOI] [PubMed] [Google Scholar]
- 23.Barkhof F. Enlarged Virchow-Robin spaces: do they matter? J Neurol Neurosurg Psychiatry 2004;75:1516–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maclullich AM, Wardlaw JM, Ferguson KJ, et al. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry 2004;75:1519–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patankar TF, Mitra D, Varma A, et al. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol 2005;26:1512–20 [PMC free article] [PubMed] [Google Scholar]
- 26.Barboriak DP, Doraiswamy PM, Krishnan KR, et al. Hippocampal sulcal cavities on MRI: relationship to age and apolipoprotein E genotype. Neurology 2000;54:2150–53 [DOI] [PubMed] [Google Scholar]