Abstract
SUMMARY: Inflammatory myofibroblastic tumor (IMT), Tolosa-Hunt syndrome (THS), and idiopathic hypertrophic pachymeningitis (IHP) seem to be part of a spectrum of disorders that have diverse locations but similar histologic and imaging findings. We report a case of a 50-year-old man presenting with multiple progressive cranial nerves palsies with leptomeningeal cranial nerve enhancement on MRI (II, V1–V3, and X), orbital and infraorbital masses, prominence within the left cavernous sinus, and diffuse dural enhancement. Biopsies of the orbital lesion and infraorbital nerve revealed IMT. The patient’s lesions, symptoms, and dural enhancement quickly improved with steroid administration and nearly resolved over multiple subsequent scans over the next few months. This case illustrates a rare case of pseudotumor mimicking a more aggressive appearance that would usually portend a case of malignancy. There is a potential association of IMT, THS, and IHP, which may have existed in a concomitant fashion in this patient. The case also describes the unique finding of enhancement of the cisternal segments of multiple cranial nerves (simulating leptomeningeal malignant involvement), which may be related to inflammatory perineural edema or ischemic neuropathy.
Inflammatory myofibroblastic tumor (IMT), Tolosa-Hunt syndrome (THS), and idiopathic hypertrophic pachymeningitis (IHP) seem to be part of a spectrum of disorders that have diverse imaging appearances but similar histologic findings. This case illustrates overlapping findings typically associated with each of these entities and illustrates that the entire scenario could portend a case of malignancy. The case also demonstrates that IMT should be entertained in the differential diagnosis of multiple enhancing cranial nerves (simulating malignant leptomeningeal involvement) at the skull base, though this is an unlikely possibility. This is the first case, to our knowledge, in which this particular imaging finding is described in association with IMT/THS/IHP, though the finding of multiple cranial nerve palsies is common in each of these entities.
Case Report
A 50-year-old man presented to the emergency department with progressively worsening vision in the left eye over the previous 9 days, as well as persistent left orbital pain and headache for the previous several months. The patient demonstrated multiple cranial neuropathies, with no significant previous history of malignancy or systemic disease. The cranial neuropathies included symptoms of vision loss (II), ptosis (III, IV, VI), decreased facial and jaw sensation over the left side (V1, V2, V3), and dysphonia/dysphagia/hoarseness, thought to represent left vocal cord paralysis (X).
Results of a head CT performed emergently (data not shown) were interpreted as normal. A contrast-enhanced MR imaging of the brain and orbits was performed the same evening, on a 1.5T MR scanner (Siemens, Erlangen, Germany) with a standard head coil. Parameters for the precontrast T1-weighted MR imaging sequences focused on the skull base and orbits included a 20-cm FOV, 256 × 256 matrix, 3-mm section thickness with 1-mm gap, and TR/TE of 650–690/7–17 ms, obtained in coronal and axial planes. T1-weighted examinations with similar parameters and with fat saturation were performed after administration of approximately 15–20 mL of intravenous gadolinium-based contrast (gadopentetate dimeglumine; Magnevist, Schering, Germany). Parameters for precontrast fat-saturated T2-weighted images were: 20-cm FOV, 512 × 256 matrix, 3-mm section thickness with 0.3-mm gap, and TR/TE of 4200/17 ms.
The initial MR examination demonstrated a 1.5–2.0 cm contrast-enhancing mass with irregular margins located inferior to the left inferior rectus muscle, and also demonstrated that this mass was confluent with a region of abnormal thickening and contrast enhancement of the superior wall of the left maxillary sinus. The center of this poorly defined mass appeared to be an enlarged and enhancing left infraorbital nerve (V2). Circumferential perineural contrast enhancement surrounding the left optic nerve (II) was also present, as was enhancement of V1 within the left orbital apex. Abnormal contrast enhancement and thickening was also noted within the Meckel cave, and there was enhancement extending through the skull base at the foramen ovale (V3). The cisternal segments of the bilateral trigeminal (V) and left vagus (X) nerves also appeared to have abnormal contrast enhancement. There was only mild prominence and widening of the left cavernous sinus compared with the opposite side. Diffuse pachymeningeal contrast enhancement was noted as well. This combination of multiple enhancing cranial nerves with an enlarged infraorbital foramen and possible paranasal sinus mass led to concern of perineural involvement by malignancy, including adenoid cystic carcinoma, mucoepidermoid carcinoma, melanoma, lymphoma, metastatic disease, or extension of squamous cell carcinoma (from an undetermined site) with pachymeningeal and leptomeningeal involvement. The radiologists involved added the possibility of pseudotumor and/or Tolosa-Hunt syndrome, given the infraorbital mass and the mild left cavernous sinus prominence; however, the primary concern at that point was to exclude malignancy, particularly given the suspected leptomeningeal findings.
The next morning, CSF evaluation revealed no hemorrhage, bacteria, or pleocytosis. Subsequent CT scans of the chest and abdomen were negative for abnormal nodules, masses, lymphadenopathy, or any associated malignant process elsewhere in the body. Clinical and serum evaluation demonstrated elevated erythrocyte sedimentation rate (51 mm/h; reference range, <20 mm/h) and elevated C-reactive protein (3.3 mg/dL; reference range, <0.8 mg/dL), both nonspecific indicators of inflammation. No evidence was found during the course of clinical and laboratory evaluation to suggest sarcoidosis, sepsis, elevated antinuclear antibodies, or other significant hematologic, rheumatologic, or electrolyte abnormalities. The extensive work-up revealed no evidence of tuberculosis in the lungs or CSF. Dynamic barium examination revealed moderate to severe asymmetric left-sided palate weakness during swallowing, indicating unilateral palsy of X.
The patient underwent biopsy of the infraorbital mass 4 days later. During the procedure, targeted at the general area of the orbital floor mass, the surgeons located the infraorbital nerve, which appeared grossly thickened; this nerve was also biopsied. Biopsy results of both locations disclosed nonspecific chronic inflammatory reaction, consisting primarily of isolated lymphoid aggregates within fibrous tissue. A few infiltrating lymphocytes and fibroblasts showing mild reactive atypia were also present. There was no evidence of granulomatous inflammation or neoplasia. Immunophenotyping of the lymphoid cells was negative for lymphoma. Upon further discussion with 2 separate pathologists, both thought this to represent an inflammatory myofibroblastic tumor (ie, pseudotumor of the orbit).
A contrast-enhanced CT of the neck performed 5 days later (data not shown) was noncontributory, other than demonstrating mild thickening of the lateral wall of the left maxillary sinus and scattered, nonenlarged lymph nodes within the jugulodigastric chains.
The patient soon began a regimen of oral prednisone, after which there was rapid improvement of the symptoms of cranial neuropathy. Repeat MR imaging was performed 5 weeks later, with scan parameters similar to the initial examination. This examination demonstrated significant improvement, with nearly resolved left optic nerve and infraorbital nerve enhancement, as well as resolution of the diffuse dural enhancement (Fig 1, I, J). The orbital floor mass was also moderately decreased in size. Multiple subsequent MR imaging scans performed monthly for the next several months demonstrated progressive decrease in size of the orbital floor mass, and the patient’s clinical symptoms continued to improve. Six months after initiation of therapy, the patient suffered from mild left eye pain, persistent decrease in left visual acuity (though improved compared with the initial findings), and mild persistent hoarseness. The patient was still on oral prednisone at this time. It is noteworthy that during this course of treatment, the prednisone was dramatically reduced at 1 point, and the patient’s symptoms promptly worsened; subsequent resumption of a dose similar to the initial dose again led to improvement in the patient’s clinical symptoms.
Fig 1.
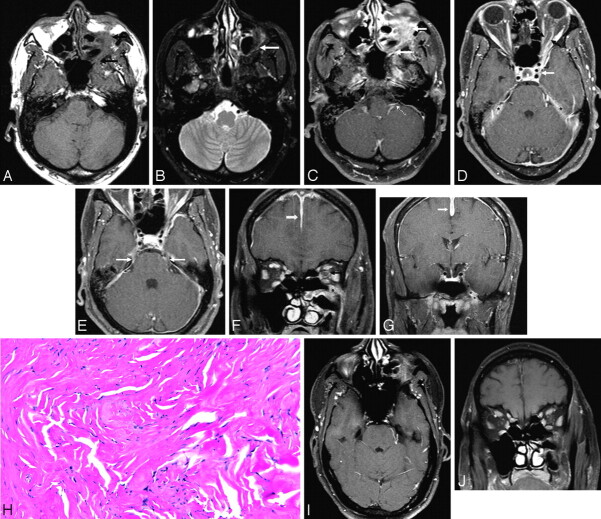
Brain MR imaging of a 50-year-old man who presented to the emergency department with multiple cranial neuropathies (left II, III, V, VI, X), the most acute and prominent being visual symptoms. Axial noncontrast T1-weighted image shows left infraorbital mass with loss of the normal fat plane and replacement by soft tissue intensity that extends along the inferior orbital fissure to the infraorbital foramen and can be seen to involve the pterygopalatine fossa and foramen rotundum (A, black arrows), mildly hypointense on fat-saturated T2-weighted images (B, white arrow). There is abnormal contrast enhancement in those locations on fat-saturated postcontrast images (C, white arrows), along the expected course of both V1 and V2, and left cisternal segment of X (C, white dotted arrow). Abnormal contrast enhancement surrounds the left optic nerve (D, black arrows), and mildly expands the left cavernous sinus (D, white arrows), with abnormal pachymeningeal enhancement as well (D, asterisks). Abnormal enhancement is also along the cisternal segment of V bilaterally (E, white arrows). Coronal postcontrast fat-saturated T1-weighted images demonstrate the mass centered on the infraorbital foramen (F, asterisk), the optic nerve sheath involvement (F, black arrow), and erosion of the left maxillary sinus roof. Diffuse pachymeningeal thickening and abnormal contrast enhancement is also present along the falx cerebri (F and G, white arrows). Meckel cave and the left foramen ovale were also diffusely involved (G, asterisks). Subsequent biopsy of the infraorbital mass (H, 20× magnification) demonstrated mild reactive atypia of fibroblasts, with a nonspecific chronic inflammatory reaction consisting primarily of isolated lymphoid aggregates within fibrous tissue. A few infiltrating lymphocytes and fibroblasts showing mild reactive atypia are also present. The whole picture was indicative of inflammatory myofibroblastic tumor (pseudotumor). The patient’s symptoms markedly improved immediately after steroid therapy, and follow-up imaging was performed numerous times over several months, demonstrating progressive improvement per imaging and improved clinical cranial neuropathies. Axial (I) and coronal (J) contrast-enhanced fat-saturated T1-weighted images performed 2 months after steroid therapy initiation demonstrate marked improvement, with only mild residual infraorbital mass, resolved enhancement of II, cisternal segments of V, X, and resolved pachymeningeal enhancement.
Discussion
IMT, also known as “plasma cell granuloma,” “myoblastoma,” or “inflammatory pseudotumor,” is a lesion characterized by proliferation of myofibroblastic spindle cells with mixed inflammatory infiltrates of plasma cells, lymphocytes, eosinophils, and histocytes; the constituent inflammatory cells are mature and polyclonal.1 Three dominant histologic patterns have been described, and these patterns can mimic nodular fasciitis, desmoid fibromatosis, or fibrous histiocytoma.2 Histopathologic discrimination of these entities may require careful attention not only to the histopathologic findings but also to clinical course and to additional diagnostic tools, particularly immunocytochemistry. These lesions were once thought to be reactive in nature but have come to be considered a true soft tissue neoplasm with potential for recurrence and multifocality3; however, the exact cause and pathogenesis of this spectrum of inflammatory disease is largely unknown.4
IMTs have a number of appearances that may overlap, most often including orbital involvement when located in the head and neck area. Other possible areas of involvement include the larynx, paranasal sinuses, parapharyngeal space, infratemporal fossa, bony structures, and the soft tissues of the neck and esophagus.4–6 Locations outside of the head and neck include most often the lung and/or pleura, though examples have been described in the abdomen, mediastinum, skin, and soft tissues among many other sites.2 The occurrence of these findings at various sites throughout the body may be grouped under the general designation of multifocal fibrosclerosis.7
Orbital IMT usually comprises a mass that often presents as focal enlargement of extraocular musculature but can also present elsewhere within the orbit (inside or outside the cone). Extraorbital extension of orbital IMTs may occur, and these may contiguously involve adjacent structures such as the cavernous sinus, conal structures, maxillary sinus, and anterior or middle cranial fossae.8
The most typical imaging presentation of IMT on MR is as a soft-tissue mass, isointense to hypointense relative to the brain on both T1- and T2-weighted images,5,6,9 with homogeneous enhancement on postcontrast series.5,10 The relative hypointensity of these lesions on T2-weighted images, as seen in this case, is probably attributable to the decreased free water content and associated lack of mobile protons related to a high degree of fibrosis,5 and also to an elevated cellularity or high nucleus-to-cytoplasm ratio.4
There is some speculation in the literature that IMT, THS, and IHP may be various presentations of a spectrum of inflammatory disorders that have diverse locations but share similar histologic, clinical, and imaging findings.8,11–13 THS is the term usually applied to retro-orbital pseudotumor involvement, predominantly of the cavernous sinus,9,12 with MR imaging findings similar to IMT; these may be related processes.12 Isolated cases of THS have been described to have concurrent pachymeningitis in other portions of the cranial vault.14 Conversely, IHP (usually presenting with diffuse dural thickening) with simultaneous orbital mass has been described.13
Holodny et al15 describe a case of a tumefactive fibroinflammatory lesion of the skull base and upper neck that apparently progressed to involve the opposite cavernous sinus, temporal lobe, and tentorium. Similar findings were also described by Cho et al16 in a patient with multiple cranial neuropathies and uveitis due to an erosive skull base fibroinflammatory lesion with diffuse dural enhancement. Some of the diffuse and contiguous imaging findings seen in those cases were present in our case as well.
The main clinical features of all 3 of these disorders include headache and cranial polyneuropathy, usually leading to visual symptoms.11,14,17 Regarding treatment, they typically respond to steroid therapy.8,14,17 However, these disorders may recur as the steroid therapy is tapered, as in the case described here.
This case is unique in that the classic imaging findings of each of these 3 fibroinflammatory disorders were present in this patient at the same time: dural thickening (typically seen with IHP), cavernous sinus prominence (THS), and orbital mass (IMT). In addition, to our knowledge, there are no cases of IMT in the recent literature presenting with perineural enhancement of multiple cranial nerves; many of the enhancing cranial nerves in this case appeared as leptomeningeal involvement. A previous case report of pseudotumor describes a mass of the geniculate portion of the facial nerve, believed after histologic examination to originate from the nerve sheath, but does not note abnormal leptomeningeal enhancement.18 The etiology of the contrast enhancement of the cisternal vagus and trigeminal nerves in the case described here is uncertain; this enhancement could presumably be related to contiguous involvement of the adjacent dura, with subsequent inflammation, perineural edema, and enhancement of those nerves. The possibility of infiltration into the brain and leptomeninges is supported by pathologic features of IMTs.1,19 Some portion of the enhancement in this case may be related to fibrous encasement produced by the hypertrophic dura and associated ischemic damage; ischemic neuropathy may present with enhancement due, at least in part, to compromise of the blood-brain barrier. This has been described in ischemic optic neuropathy.20,21
The histologic findings in the present case, as well as the improvement in the patient’s clinical symptoms seen after the initiation of steroid therapy, correlate well with the clinical and radiologic findings of idiopathic fibro-inflammatory disorder (IMT-IHP-THS). Also of particular interest in this case was the circumferential enhancement of the optic nerve, presumably from inflammatory involvement of the dural sheath (either by tumor deposition/spread or by direct inflammation) simulating meningioma.
The differential diagnosis for the radiologic findings described here is extensive. Meningioma, lymphoma, sarcoidosis, Wegener disease, syphilis, vasculitis, intraorbital tumors, perineural spread of head and neck malignancies, tuberculosis, and fungal infections are among the possibilities. The diffuse and contiguous perineural involvement (particularly of II and V1–V3) simulated malignant spread through the cavernous sinus, with simultaneous pachymeningitis and potential leptomeningeal carcinomatosis. This was promptly excluded as a likely cause because results of multiple lumbar punctures and the subsequent extensive work-up for malignancy were negative. Intracranial hypotension from lumbar puncture can be excluded as an etiology, because these findings were present on initial examination, preceding the initial CSF examination.
The simultaneous findings of orbital mass, perineural enhancement, and enhancing dural thickening support the theory that IHP, IMT, and THS are somehow related and may coexist in a given patient. The presence of perineural leptomeningeal enhancement is a novel finding in this spectrum of disease, though cranial neuropathy is a frequent clinical manifestation; this enhancement could be related to direct infiltration of inflammatory tissue into the nerve or related to ischemic neuropathy.
References
- 1.Jeon YK, Chang KH, Suh YL, et al. Inflammatory myofibroblastic tumor of the central nervous system: clinicopathologic analysis of 10 cases. J Neuropathol Exp Neurol 2005;64:254–59 [DOI] [PubMed] [Google Scholar]
- 2.Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859–72 [DOI] [PubMed] [Google Scholar]
- 3.Brooks JSJ. Disorders of soft tissue. In: Mills SE, Carter D, Greenson JK, Oberman HA, et al, eds. Sternberg’s Diagnostic Surgical Pathology, 4th ed. Philadelphia: Lippincott Williams & Wilkins;2004:137–222 [Google Scholar]
- 4.Gasparotti R, Zanetti D, Bolzoni A, et al. Inflammatory myofibroblastic tumor of the temporal bone. AJNR Am J Neuroradiol 2003;24:2092–96 [PMC free article] [PubMed] [Google Scholar]
- 5.Han MH, Chi JG, Kim MS, et al. Fibrosing inflammatory pseudotumors involving the skull base: MR and CT manifestations with histopathologic comparison. AJNR Am J Neuroradiol 1996;17:515–21 [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama K, Inoue Y, Aiba T, et al. Unusual CT and MR findings of inflammatory pseudotumor in the parapharyngeal space: case report. AJNR Am J Neuroradiol 2001;22:1394–97 [PMC free article] [PubMed] [Google Scholar]
- 7.Berger JR, Snodgrass S, Glaser J, et al. Multifocal fibrosclerosis with hypertrophic intracranial pachymeningitis. Neurology 1989;39:1345–49 [DOI] [PubMed] [Google Scholar]
- 8.Mahr MA, Salomao DR, Garrity JA. Inflammatory orbital pseudotumor with extension beyond the orbit. Am J Ophthalmol 2004;138:396–400 [DOI] [PubMed] [Google Scholar]
- 9.Yousem DM, Atlas SW, Grossman RI, et al. MR imaging of Tolosa-Hunt syndrome. AJNR Am J Neuroradiol 1989;10:1181–84 [PMC free article] [PubMed] [Google Scholar]
- 10.Hardman JA, Halpin SF, Mars S, et al. MRI of idiopathic orbital inflammatory syndrome using fat saturation and Gd-DTPA. Neuroradiology 1995;37:475–78 [DOI] [PubMed] [Google Scholar]
- 11.Bosch J, Ortega-Aznar A, Tintore M, et al. [Hypertrophic pachymeningitis. A review of the histories of two cases and pathological relationship with the Tolosa-Hunt syndrome and the orbital pseudotumor]. Rev Neurol 2000;31:946–51 [PubMed] [Google Scholar]
- 12.Wasmeier C, Pfadenhauer K, Rosler A. Idiopathic inflammatory pseudotumor of the orbit and Tolosa-Hunt syndrome–are they the same disease? J Neurol 2002;249:1237–41 [DOI] [PubMed] [Google Scholar]
- 13.Wild T, Strotzer M, Volk M, et al. Idiopathic hypertrophic cranial pachymeningitis associated with an orbital pseudotumor. Eur Radiol 1999;9:1401–03 [DOI] [PubMed] [Google Scholar]
- 14.Miwa H, Koshimura I, Mizuno Y. Recurrent cranial neuropathy as a clinical presentation of idiopathic inflammation of the dura mater: a possible relationship to Tolosa-Hunt syndrome and cranial pachymeningitis. J Neurol Sci 1998;154:101–05 [DOI] [PubMed] [Google Scholar]
- 15.Holodny AI, Kirsch CF, Hameed M, et al. Tumefactive fibroinflammatory lesion of the neck with progressive invasion of the meninges, skull base, orbit, and brain. AJNR Am J Neuroradiol 2001;22:876–79 [PMC free article] [PubMed] [Google Scholar]
- 16.Cho AH, Lee BH, Kwak KW, et al. Inflammatory pseudotumor of temporal bone with pachymeningitis, cranial neuropathies, and uveitis. Eur Neurol 2004;51:238–40 [DOI] [PubMed] [Google Scholar]
- 17.Kupersmith MJ, Martin V, Heller G, et al. Idiopathic hypertrophic pachymeningitis. Neurology 2004;62:686–94 [DOI] [PubMed] [Google Scholar]
- 18.Yanagihara N, Segoe M, Gyo K, et al. Inflammatory pseudotumor of the facial nerve as a cause of recurrent facial palsy: case report. Am J Otol 1991;12:199–202 [PubMed] [Google Scholar]
- 19.Nishizaki T, Iwamoto F, Uesugi S, et al. Idiopathic cranial pachymeningoencephalitis focally affecting the parietal dura mater and adjacent brain parenchyma: case report. Neurosurgery 1997;40:840–43 [DOI] [PubMed] [Google Scholar]
- 20.Lee AG, Eggenberger ER, Kaufman DI, et al. Optic nerve enhancement on magnetic resonance imaging in arteritic ischemic optic neuropathy. J Neuroophthalmol 1999;19:235–37 [PubMed] [Google Scholar]
- 21.Vaphiades MS. Optic nerve enhancement in hypotensive ischemic optic neuropathy. J Neuroophthalmol 2004;24:235–36 [DOI] [PubMed] [Google Scholar]