Abstract
SUMMARY: Although anatomic variations are well known in the A1 segment of the anterior cerebral artery (ACA), anomalous origin of a cortical artery from the A1 segment is extremely rare. The only reported cortical branch to arise from the A1 segment is the fronto-orbital artery. We report a case of anomalous origin of the callosomarginal artery (CMA) in association with a saccular aneurysm from the A1 segment of the left ACA in a 35-year-old man who presented with intracerebral hemorrhage. To our knowledge, this is the first report of anomalous origin of the CMA from the A1 segment.
Anatomic variations are well known in the A1 segment of the anterior cerebral artery (ACA).1,2 These variations include hypoplasia, aplasia, duplication, fenestration, and infraoptic course.3,4 Normally the A1 segment gives rise to the medial lenticulostriate arteries and the recurrent artery of Heubner.1,5 Occasionally large branches such as an anomalous cortical branch or an accessory middle cerebral artery (MCA) may be seen arising from the A1 segment. The fronto-orbital artery (FOA) is the only cortical branch reported to arise anomalously from the A1 segment.1,3,4 We describe a case of anomalous origin of callosomarginal artery (CMA) from the A1 segment and the associated saccular aneurysm at its origin.
Case Report
A 35-year-old man underwent CT of head following an episode of severe headache. The scan showed a small intracerebral hematoma in the left basifrontal region with extension into the adjacent frontal horn. Cerebral angiography performed later showed a 13 × 5.6 mm saccular aneurysm arising from the left A1 segment (Fig 1A–F). The aneurysm was best demonstrated on angiograms with caudal angulation. In addition, there was a prominent cortical branch originating from the left A1 segment in close relationship with the proximal aspect of the neck of the aneurysm. This anomalous cortical branch coursed anteromedially first and then ascended anteriorly parallel to the postcommunicating segment of the left anterior cerebral artery (ACA). On tracing the artery further, we saw it continuing its parallel course superiorly and posteriorly before it ended at the level of coronal suture. The anomalous branch gave rise to multiple cortical branches (Fig 2). There was associated aplasia of the right A1 segment. The remainder of the cerebral circulation was normal. At surgery, the angiographic findings of the left A1 aneurysm and the anomalous cortical branch were verified, and the aneurysm was clipped successfully.
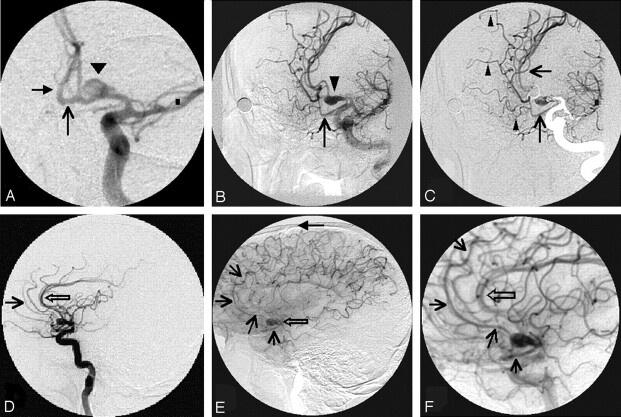
Fig 1.
A, Anteroposterior view with 15° caudal angulation of the left carotid arterial phase shows clearly the origin of the saccular aneurysm (arrowhead) from the left A1 segment. Note the filling of AComA (arrow) and right A2 segment (solid arrow). B, Left anterior oblique view of the left carotid arterial phase shows the medially directed saccular aneurysm (arrowhead) and the anomalous CMA (arrow). C, Same view as in Fig 1B with a mask from the late arterial frames shows the relationship of the saccular aneurysm and the anomalous CMA clearly. The superior course of the anomalous cortical artery (arrows) and the 3 branches (arrowheads) arising from it are seen. D, Lateral view of the left carotid arterial phase shows the pericallosal artery (open arrow). Note the absent CMA from the pericallosal artery. The superiorly coursing segment of the anomalous cortical artery can be visualized anteriorly (arrow). E, Lateral view of the left carotid late arterial phase shows the saccular aneurysm (open arrow) directed anteriorly. Note the anomalous cortical artery (arrows) initially coursing below the aneurysm, then ascending in front of it, and coursing all the way up to the level of coronal suture (solid arrow). F, Magnified lateral view of the left carotid late arterial phase clearly shows the parallel course of the anomalous CMA (arrow) to the pericallosal artery (open arrow).
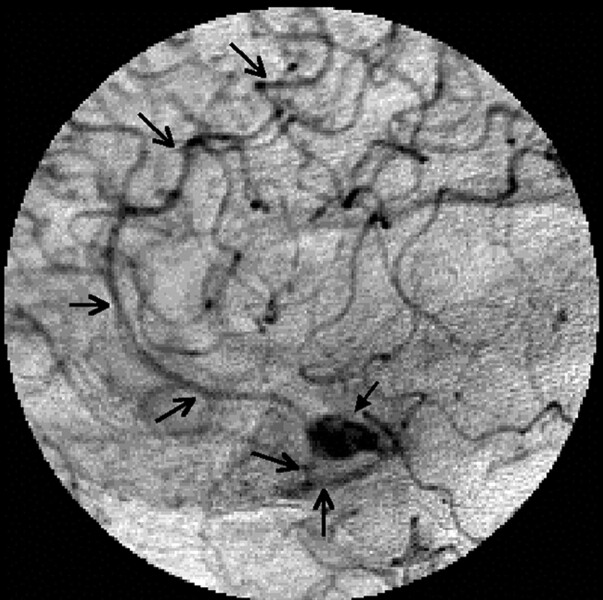
Fig 2.
Magnified lateral view of the left carotid late arterial phase clearly traces the entire course of the anomalous cortical artery and branches arising from it (arrows). Note the anteriorly directed saccular aneurysm (solid arrow).
Discussion
One of the 2 terminal branches of the internal carotid artery (ICA), the ACA courses medially and superiorly above the optic chiasm to enter the interhemispheric fissure. Here it communicates with its counterpart through the anterior communicating artery (AComA).5 ACA is divided into 5 segments, namely A1–A5. The A1 segment is also called the precommunicating segment. Anatomic variations are well known in the caliber, course, and branches of ACA.1 The most common variation observed in the A1 segment is hypoplasia, which may be observed in up to 15% of cases.1 Aplasia of the A1 segment is very rare, with a reported incidence of up to 1.1%.1 Other rare anomalies involving the A1 segment include fenestration, duplication, and an infraoptic course. Mäuer et al6 have even reported a case of infraoptic ACA perforating the optic nerve and continuing distally. An anomalous origin of a cortical branch from the A1 segment is extremely uncommon. The FOA is the only cortical branch reported to have arisen from the A1 segment.1 This artery, also called the orbitofrontal artery, normally originates as the first cortical branch of the postcommunicating segment and supplies the gyrus rectus, medial olfactory gyrus, olfactory bulb, olfactory tract, and anterior part of superior frontal gyrus.5 Occasionally, it may arise in common origin with the frontopolar artery, the second branch of the postcommunicating segment.2 Rarely FOA may arise from the A1 segment.1,3,4 The incidence of FOA arising from A1 segment is reported to be 4%.7
The CMA is defined as the artery that courses in or near the cingulate sulcus and gives origin to 2 or more cortical branches.5 This artery runs a course parallel to the pericallosal artery. Rhoton5 considers it the largest branch of the pericallosal artery because it shows large variation in its origin from the postcommunicating segment. However, others consider it as 1 of the 2 terminal branches of ACA, along with the pericallosal artery.1 The CMA is present in 80% of hemispheres.5 It gives origin to the 3 internal frontal arteries, even though the most consistent branch to originate from it is the middle internal frontal artery. These internal frontal arteries supply the superior, middle, and posterior segments of the superior frontal gyrus. The posterior internal frontal artery, at times, may give rise to the artery of the paracentral lobule.5 The CMA shows considerable variation in its origin and may arise anywhere from the A2 to the A4 segment but never from the A1 segment. Krayenbuhl and Yasargil1 state that up to 72% of CMAs arise near the genu of corpus callosum. In 19% of cases, the CMA arises from the A2 segment close to the AComA and in 9% of cases, from a strong frontopolar artery.1
For several reasons, we consider the anomalous artery originating from left A1 in our patient as the CMA. First, it satisfies the definition of Rhoton5 for the CMA. Second, it has a course parallel to the pericallosal artery. Third, the usual CMA was absent from the left ACA in our patient. Fourth, the template to identify the cortical branches of the ACA clearly indicated that the distal branch from the anomalous artery in our patient was either the middle internal frontal or posterior internal artery. Our literature search revealed that other than the frontopolar artery, no named cortical branch has been reported to arise from A1 segment.1,3,4 To our knowledge, ours is the first case of an anomalous origin of CMA from the A1 segment.
Aneurysms arising from the A1 segment are extremely uncommon, with a reported incidence of 1%–2%.8 Most of these aneurysms arise from the junction of ACA with a perforator.8–11 Associated anomalies with an A1 segment aneurysm are well known. Fenestration is the most common anomaly to predispose to aneurysm formation of the A1 segment.8 Anomalous origin of a cortical branch from the A1 segment also predisposes to the formation of saccular aneurysms because of alteration in the hemodynamics.3,8,11 Aneurysms have also been reported at the origin of the accessory MCA from the A1 segment.8,12 In our patient, we observed the aneurysm arising from the distal aspect of the junction between the CMA and the left A1 segment, which was confirmed at surgery. The altered hemodynamics at this location must have been responsible for the formation of the aneurysm in our case. Most of the A1 segment aneurysms reported in the literature were small. Wakabayashi et al11 considered smaller size as one of the characteristic features of the A1 aneurysm. However, our patient had a large aneurysm, probably due to the augmentation of the hemodynamic stress from aplasia of the right A1 segment.
Suzuki et al8 and Hino et al10 have mentioned a high incidence of the multiplicity of aneurysms in association with the A1 segment aneurysm, even though we did not observe any additional aneurysms in our patient. While performing angiography for saccular aneurysms arising from the A1 segment, one should use caudal angulation or basal views to demonstrate the precise origin of the aneurysm and its neck.10 3D rotational angiography is likely to show this relationship better.10
To our knowledge, this is the first report in the literature of an anomalous origin of the CMA from the A1 segment and an associated saccular aneurysm at its origin. When an A1 aneurysm is found, one should carefully scrutinize the angiograms to identify anomalies. Further basal views should be used to delineate the aneurysm and associated anomalies better. When an anomalous cortical branch is identified, every effort should be made to trace its full course to aid surgery or endovascular treatment.
References
- 1.Krayenbuhl HA, Yasargil MG. Cerebral Angiography, 2nd ed, English ed. New York: Thieme Medical Publishers;1968. :20–85
- 2.Newton TH, Potts DG. Radiology of the Skull and Brain: Angiography. St. Louis: Mosby;1974. :1414
- 3.Hong SK. Ruptured proximal anterior cerebral artery (A1) aneurysm located at an anomalous branching of the fronto-orbital artery: a case report. J Korean Med Sci 1997;12:576–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee ER, Eastwood JD. An unusual variant of the fronto-orbital artery. AJNR Am J Neuroradiol 2000;21:939–40 [PMC free article] [PubMed] [Google Scholar]
- 5.Rhoton AL. The supratentorial arteries. Neurosurgery 2002;51(suppl 1):53–120 [PubMed] [Google Scholar]
- 6.Mäuer J, Mäuer E, Perneczky A. Surgically verified variations in the A1 segment of the anterior cerebral artery: report of 2 cases. J Neurosurg 1991;75:950–53 [DOI] [PubMed] [Google Scholar]
- 7.Marinkovic S, Milisavljevic M, Kovacevic M. Anatomical bases for surgical approach to the initial segment of the anterior cerebral artery: microanatomy of Heubner’s artery and perforating branches of the anterior cerebral artery. Surg Radiol Anat 1986;8:7–18 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki M, Onuma T, Sakurai Y, et al. Aneurysms arising from the proximal (A1) segment of the anterior cerebral artery: a study of 38 cases. J Neurosurg 1992;76:455–58 [DOI] [PubMed] [Google Scholar]
- 9.Handa J, Nakasu Y, Matsuda M, et al. Aneurysm of proximal segment of anterior cerebral artery. Surg Neurol 1984;22:486–90 [DOI] [PubMed] [Google Scholar]
- 10.Hino A, Fujimoto M, Iwamoto Y, et al. Surgery of proximal anterior cerebral artery aneurysms. Acta Neurochir (Wien) 2002;144:1291–96 [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi T, Tamaki N, Yamashita H, et al. Angiographic classification of horizontal segment of the anterior cerebral artery. Surg Neurol 1985;24:31–34 [DOI] [PubMed] [Google Scholar]
- 12.Sugita S, Yuge T, Miyagi J, et al. Giant aneurysm at the origin of the accessory middle cerebral artery. Surg Neurol 1995;44:128–30 [DOI] [PubMed] [Google Scholar]