Abstract
BACKGROUND AND PURPOSE: Epileptic syndromes or neurodevelopmental delay may be associated with congenital anomalies of the shape or the orientation of the hippocampus. Scarce data are available about quantitative hippocampal developmental changes during fetal life, in particular about the progressive rotational changes of the hippocampal infolding angle (HIA), which can be considered a hallmark of hippocampal development. We hypothesized that prenatal MR imaging could demonstrate the progressive rotation of the hippocampus, providing quantitative data by means of the HIA determination.
METHODS: We retrospectively selected 62 fetal MR imaging cases with normal brain at prenatal and postnatal imaging. The gestational age ranged from 20 to 37 weeks. The coronal section encompassing the pons was used to perform the measurement of HIA. HIA was defined as the angle between the line connecting the lateral margin of the cornu ammonis with the medial superior margin of the subiculum and the line passing through the midline structures.
RESULTS: A significant positive correlation was found between the HIA value and the gestational age. The HIA was generally below 70° before the gestational week 25 and above 70° after week 30.
CONCLUSION: Prenatal MR imaging allowed the progressive rotation of hippocampus to be detected during fetal life, providing normative data about HIA changes. These data could support further investigations to assess how fetal HIA anomalies might affect postnatal neurologic outcome.
Developmental alterations of the hippocampus may be associated with epileptic syndromes or neurodevelopmental delay. In particular, in patients carrying cortical malformations, the hippocampus may present anomalies of shape or orientation, such as malrotation or verticalization.1,2 These reports highlight how a detailed evaluation by magnetic resonance (MR) imaging of the hippocampal formation may help in better understanding complex cortical malformations associated with epileptic syndromes. However, a qualitative analysis may not always allow a congenital anomaly of the hippocampus to be detected, so quantitative methods may be required. The progressive change in the hippocampal infolding angle (HIA) during its rotation process can be considered a hallmark of hippocampal development. HIA measurement by MR imaging has proved to be useful in assessing the normal development of the hippocampus postnatally.3 Pathology studies have provided fundamental information about human hippocampal developmental changes, albeit mostly in a qualitative way and focusing on embryonal age or the very early fetal period.4 However, scarce data are available about quantitative hippocampal developmental changes, in particular regarding the rotation process, occurring after gestational week 20.
We hypothesized that the progressive rotation of the hippocampus, which occurs during its maturation, could be detected and quantified by means of the HIA determination using prenatal MR imaging. This approach might help to fill the gap between the pathology data from early gestation studies and the MR imaging data from postnatal life studies. Moreover, it might provide normal values of the HIA in the gestational age (GA) range typical of the current clinical prenatal MR imaging, forming the basis for a possible use of HIA measurement in clinical fetal imaging.
Methods
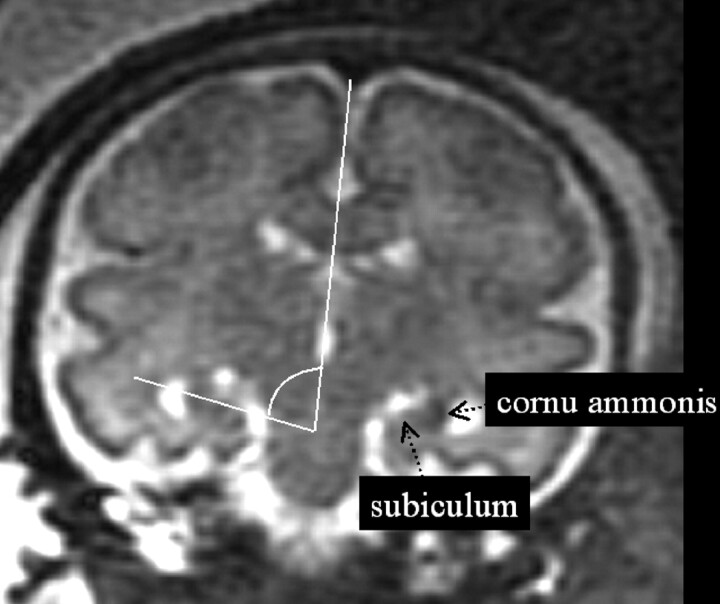
Among approximately 672 fetal MR imaging studies performed at our institution between August 2000 and August 2005, we retrospectively selected 62 fetal cases with the following characteristics: studies performed for suspected body lesions or because brain malformations had been detected in previous pregnancies, normal brain resulting from prenatal MR imaging report. All cases had to have shown normal brain at postnatal sonography or MR imaging examination. In all cases, a normal neurodevelopmental evaluation at postnatal age ranging from a minimum of 3 months to a maximum of 42 months could be collected from different sources: clinical records from child neurologists at our institution or others and written or oral reports from family pediatricians. The GA ranged from 20 to 37 weeks; the mean GA was 26.61 ± 5.07 (SD) weeks. There were 35 male and 27 female fetuses with the same mean GA for both groups (26.57 ± 5.22 weeks for male fetuses and 26.66 ± 4.98 weeks for female fetuses). All imaging studies were performed with the same 1.5T scanner (Horizon, EchoSpeed; General Electric, Milwaukee, Wis), by using a surface abdominal coil. All mothers signed the fetal MR imaging consent form in use at our institution. No fetal or maternal sedation was required. The scanning technique was based on single-shot, fast spin-echo, T2-weighted, 4-mm thick contiguous sections (TR/TE = 3000/180 ms, FOV = 340 mm, matrix = 320 × 320). Coronal sections were positioned orthogonally to axial ones, which had been acquired parallel to the subcallosal plane. The coronal section encompassing the pons was used to perform the measurement of HIA. This single section was chosen at pons level because it represented a spatial compromise with respect to the 2 coronal thinner sections selected in the cited postnatal HIA study,3 because the dual level thinner section postnatal protocol could not be exactly reproduced in prenatal imaging. The crossing lines identifying the HIA were traced by a pediatric neuroradiologist using the measurement tool of the main console of the scanner. HIA was defined as the angle between the line connecting the lateral margin of the cornu ammonis with the medial superior margin of the subiculum and the line passing through the midline structures (Fig 1).
Fig 1.
The method used to assess the hippocampal infolding angle (HIA) is shown on the coronal T2-weighted single-shot, fast spin-echo section encompassing the pons.
Results
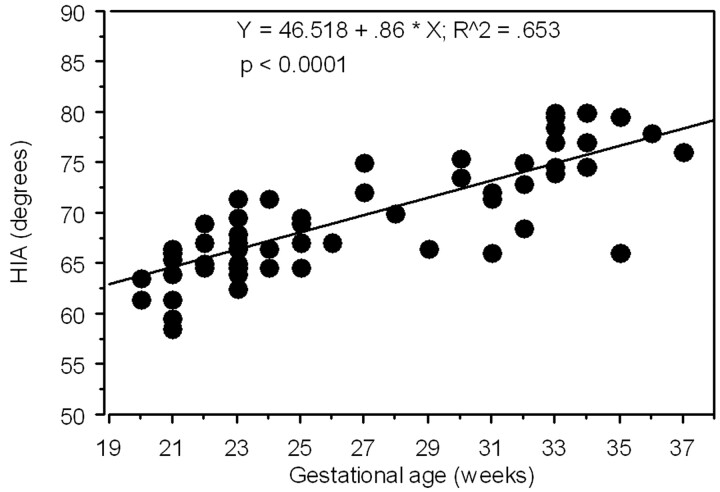
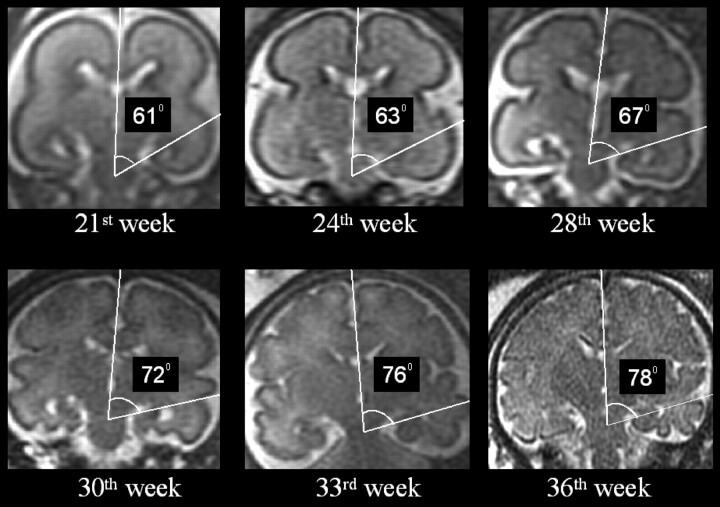
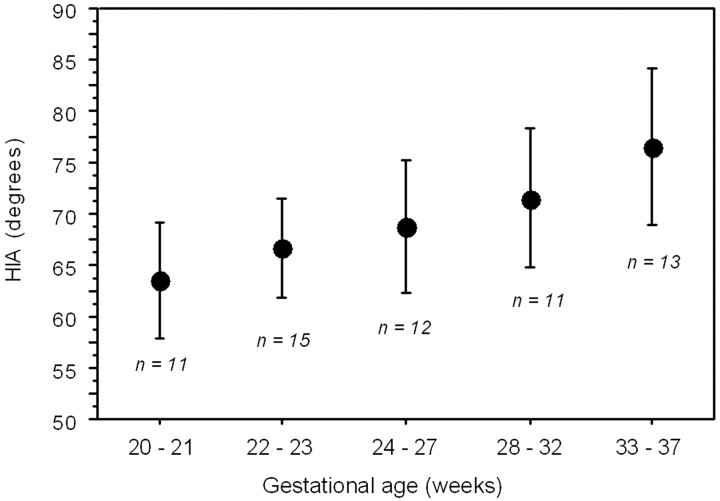
No statistically significant difference was noted between the right and the left HIA (paired t test). Therefore, the mean value between the right and the left measurement of the HIA was used to assess the correlation with the GA by linear regression analysis. There was no statistically significant difference regarding the mean HIA value between male and female fetuses (Mann-Whitney test, P = .2115). A significant positive correlation was found between the HIA value and the GA (Fig 2). The HIA was generally below 70° before gestational week 25 and above 70° after week 30, with variable intermediate values for the gestational ages in between (Fig 3). The average value of HIA was considered by clustering the fetuses in 5 groups by GA to provide a graphic representation (Fig 4) that might be used as normal reference in clinical prenatal MR imaging. As shown in Fig 4, the number of younger fetuses (before the gestational week 24) in our cohort was prevalent, so younger fetuses could be divided in 2 clusters.
Fig 2.
Graph illustrating the significantly positive correlation between the HIA (average of values relative of both hemispheres) and the gestational age (GA).
Fig 3.
Coronal T2-weighted single-shot, fast spin-echo sections depicting the change of HIA value in 6 representative cases of different GA.
Fig 4.
Graph illustrating the average HIA value in the 5 GA groups into which the 62-fetus population was divided. The number (n) of fetuses for each group is reported. Bars indicate the 2 SD range.
Discussion
This work has shown that prenatal MR imaging is able to detect the progressive rotation of the human hippocampus occurring with brain maturation, a phenomenon that had not been assessed previously week-by-week during the late second and third trimester of gestation. Prenatal MR imaging has provided quantitative normative data about changes of the HIA, which is an important key feature of the hippocampal development.
Because in vitro morphometric measurements on pathology specimens of the brain can be affected by distortion of the parenchyma as a result of dehydration and fixation procedure, in vivo MR imaging provides the unique opportunity of assessing HIA changes during fetal brain maturation. Quantitative data on hippocampal development from pathology studies are scarce; in particular, quantitative in vitro data on HIA changes during the late second and third trimester of fetal life are substantially lacking. During the development of the fetal brain, the main components of the hippocampus (ie, cornu ammonis and dentate gyrus) progressively grow, change shape, and relate to each other, generally resembling (gestational week 17–18) the C-shaped adult hippocampus.4 Starting from this period, the hippocampus is fully recognizable, as is the HIA. From here on, the hippocampal macroscopic development is mainly based on its infolding within the medial temporal lobe, which makes it rotate from an almost vertical to a horizontal position.
Previous work by Kier et al4 assessed the hippocampal development comparing results of pathologic examinations and ex vivo MR imaging data in a cohort of fetuses aborted at the GA of 13–24 weeks. Their approach took advantage of the high resolution of the ex vivo MR imaging, and it showed the changes over time of all the components of the hippocampal formation in detail, albeit in qualitative terms and with no specific quantification of HIA changes. Most of the prenatal MR imaging studies in clinical settings are performed from gestational week 20 to term (ie, in the age range we focused on in our protocol).
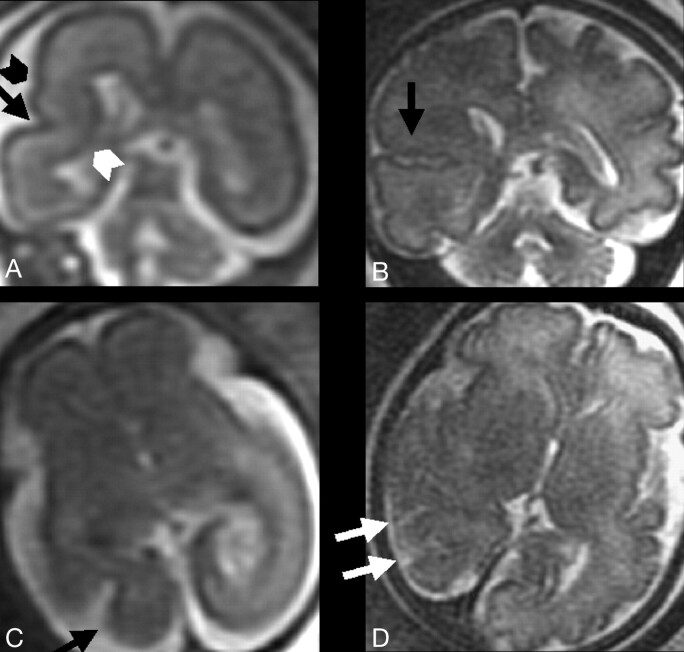
The HIA might be a useful parameter to be taken into account in clinical fetal MR imaging, helping to assess whether the mesial temporal lobe is developing normally. We have recently started occasionally measuring the HIA in the most unusual or difficult fetal MR imaging cases. We have not investigated those cases presenting dramatic and well-known anomalies (ie, alobar holoprosencephaly, complete agenesis of corpus callosum, extensive destructive lesions, etc); rather, we have evaluated the HIA in a few fetuses showing mostly focal cortical anomalies. At present, we cannot provide statistical data on this small group of abnormal cases. Nevertheless, we think it could be of some interest and a stimulus for further more extensive work to briefly discuss the clinical example reported in Figs 5 and 6. At gestational weeks 21 and 23, a fetal MR imaging study was performed in a 28-year-old primigravida because a reduction in the volume of the right temporal-occipital lobe had been noted at sonography. Two abnormal cortical sulci in the right temporal-occipital lobe were seen invaginating toward the atrium of the lateral ventricle (Fig 5). At gestational week 32, a third MR imaging study revealed 3 or 4 abnormally invaginated sulci. Cortical sulcation in the anterior part of right cerebral hemisphere appeared to be normal. To better assess the extent of such an unusual anomaly, the HIA was subsequently calculated (Fig 6), and results of the right one were clearly below the 2 SD value reported for the respective GA groups in Fig 4. This observation increased the accuracy of the prenatal MR imaging diagnosis in this case, suggesting also that the anterior and mesial part of the right temporal lobe had undergone developmental anomalies. Postnatal MR imaging confirmed the findings. From anecdotal reports, we obviously cannot infer what might be the clinical neurologic implication of the prenatal detection of some degree of hippocampal malrotation. We can only mention that postnatal data have related epilepsy and neurodevelopmental delay to hippocampal malrotation,1,2 whether associated with other cortical malformations or not. Very recently, hippocampal conformational anomalies have been detected in approximately 10% of normal adult subjects,5 though malrotational changes themselves were only a fraction of such anomalies. Therefore, an extensive study of an adequate number of fetuses with abnormal HIA and undergoing sufficiently long postnatal clinical follow-up would be needed to establish the clinical and prognostic value of prenatal HIA alterations.
Fig 5.
T2-weighted single-shot, fast spin-echo MR images from the fetal case reported in the “Discussion” section.
A and B, Two coronal sections from studies in gestational weeks 23 and 32, respectively, depicting at right temporal-occipital level the abnormal invagination of a cortical sulcus (black arrow). An additional abnormal smaller sulcus is visible (black arrowhead). The normal migrating cells band is focally thinned (white arrowhead). Note the integrity of ventricular wall, excluding the presence of a schizencephalic cleft.
C and D, Two axial sections from studies in gestational weeks 21 and 32, respectively, showing the early abnormal cortical sulcus (black arrow), followed later on by the development of multiple abnormal sulci (white arrows).
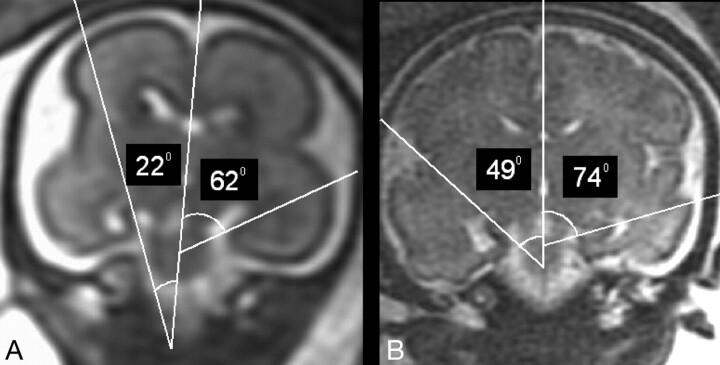
Fig 6.
Same case as Fig 5.
A and B, Two coronal T2-weighted single-shot, fast spin-echo sections from studies in gestational weeks 23 and 32, respectively, showing the abnormal value of the right HIA in comparison with the normal contralateral one.
The values of HIA (between 75° and 80°), which we measured close to term, are similar to the values of HIA recently reported in children by Okada et al.3 In their study, the HIA changed very slowly from the first 2 years of life toward the adult value, increasing by approximately 5° in the first 2 decades. In our study, the HIA value increased by approximately 15° in the 17 weeks of the intrauterine observation period. The faster changes rate of the HIA we found, with respect to postnatal period, might be due to the more rapid brain volume increase during fetal life and, even more likely, to the faster gyration and sulcation process; the latter starting from gestational age 23 or 24 weeks.6 The process of cortical gyration is definitely faster during prenatal life than during the postnatal period.
Okada et al3 found that the mean HIA value of the right side was significantly larger than that of the left side. In our fetal series, we did not find significant differences between the right and left side. We cannot provide a definite explanation for this discrepancy; however, a technical factor may have played an important role. The selected coronal section in some fetuses was very probably affected by a slight degree of head rotation, because the fetal head might have moved slightly right after scanning plane determination on the image acquisition console. Therefore, the possibility of highlighting minor differences in HIA in our prenatal protocol was limited, at least with respect to what was achieved by Okada et al.3 Nevertheless, the possibility that a difference between right and left mean HIA value would really develop only along with postnatal changes should be considered. Finally, the presence in the Okada et al3 series of epilepsy patients, who might have carried some minor degree of hippocampal anomaly, could have affected the comparison between right and left HIA mean values.
We found no statistically significant difference in the mean HIA value between male and female fetuses; however, we cannot derive any definite conclusion from this observation, because of the possibility that the number of subjects was not large enough for such subgroup statistical analysis. One of the main methodologic limitations of our study is related to possible variability associated with some shifting of the selected coronal plane in the frontal-occipital direction, which may have affected the consistency of the HIA measurement.
Conclusion
Prenatal MR imaging was able to provide quantitative normative data about hippocampal rotational process during fetal life by means of HIA determination. Our data show how the hippocampus rotates during fetal life with a higher rate compared with postnatal life and how it reaches, at term of gestation, values consistent with the ones reported in children; therefore, these data fill the gap existing between embryonal and postnatal findings about the developmental rotation process of the hippocampus. Our observations could support further investigations to assess how fetal HIA anomalies might affect postnatal neurologic outcome. The knowledge of the normal HIA range for each GA group might help in elucidating some of the more complicated cases in clinical fetal MR imaging. Moreover, it could be interesting to explore if HIA measurement in prenatal MR imaging might be used as a marker for dating clastic insults, which might have interfered at some point with the developmental rotation process of the hippocampus.
References
- 1.Sato N, Hatakeyama S, Shimizu N, et al. MR evaluation of the hippocampus in patients with congenital malformations of the brain. AJNR Am J Neuroradiol 2001;22:389–93 [PMC free article] [PubMed] [Google Scholar]
- 2.Baulac M, De Grissac N, Hasboun D, et al. Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol 1998;44:223–33 [DOI] [PubMed] [Google Scholar]
- 3.Okada Y, Toshinori K, Iwai K, et al. Evaluation of hippocampal infolding using magnetic resonance imaging. NeuroReport 2003;14:1405–09 [DOI] [PubMed] [Google Scholar]
- 4.Kier EL, Kim JH, Fulbright RK, et al. Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am J Neuroradiol 1997;18:525–32 [PMC free article] [PubMed] [Google Scholar]
- 5.Bernasconi N, Kinay D, Andermann F, et al. Analysis of shape and positioning of the hippocampal formation: an MRI study in patients with partial epilepsy and healthy controls. Brain 2005;128:2442–52 [DOI] [PubMed] [Google Scholar]
- 6.Garel C, Chantrel E, Elmaleh M, et al. Fetal MRI: normal gestational landmarks for cerebral biometry, gyration and myelination. Childs Nerv Syst 2003;19:422–25 [DOI] [PubMed] [Google Scholar]