Abstract
BACKGROUND AND PURPOSE:T2 hyperintensity of the middle cerebellar peduncle (MCP) is described in a number of diseases, including multiple system atrophy (MSA). We hypothesize that mild MCP hyperintensity on fluid-attenuated inversion recovery (FLAIR) imaging can be a normal finding. To our knowledge, a detailed study of the prevalence of this finding in various age groups with the FLAIR sequence has not been described.
METHODS:One hundred twenty-two patients underwent an axial FLAIR examination of the brain as part of either a hearing loss or tinnitus work-up (ie, to exclude an acoustic neuroma or a retrocochlear cause). Subjects aged 15–78 years were included to reflect an even spread through the decades and were divided into 6 age groups. A radiologist and an MR imaging fellow graded the examinations subjectively, blinded to age: 0 for normal or 1 for the presence of MCP hyperintensity if the increased signal intensity was greater than that of adjacent pons and cerebellar white matter. Spearman rank correlation test of MCP hyperintensity with age and analysis of variance (ANOVA) were performed.
RESULTS:Of 122 patients, we identified 17 with MCP FLAIR hyperintensity. None of these patients had a clinical condition that could cause MCP hyperintensity. MCP hyperintensity did not show a statistically significant correlation with age (r = 0.05, P = .62). Patients were divided into 6 age groups, and ANOVA showed no statistically significant difference in the incidence of MCP hyperintensity between different age groups (P = .95). However, results were highly reproducible with excellent interobserver correlation (r = 0.97, P < .001).
CONCLUSIONS:Mild MCP FLAIR hyperintensity can occur normally, and this finding shows no relationship with age.
Multiple system atrophy (MSA) is a sporadic progressive adult-onset neurodegenerative disorder associated with varying degrees of parkinsonism and autonomic, pyramidal, and cerebellar dysfunction.1 Hyperintensity of the middle cerebellar peduncle (MCP) on T2-weighted images has been suggested to be more frequent in patients with MSA,1,2 especially the cerebellar dominant type of multiple system atrophy (MSA-c).3–6 It is reported to be a useful radiologic sign to differentiate between subtypes of MSA5,7,8 and between MSA and Parkinson disease (PD) or other atypical parkinsonian disorders,2–4,6 which often have overlapping clinical features.
The control groups in the previous literature assessing T2 hyperintensity of the MCP in MSA have often been relatively small.2,3 We hypothesized that mild fluid-attenuated inversion recovery (FLAIR) MCP hyperintensity can be a normal finding in all age groups. The aim of this study was to estimate the prevalence of this finding in various age groups on FLAIR imaging.
Methods
Subjects
All patients who underwent an axial FLAIR examination of the brain as part of either a hearing loss or tinnitus work-up (ie, to exclude an acoustic neuroma or a retrocochlear cause) from April 2000 to November 2004 were selected, following hospital ethics committee approval. All patients were reviewed for any evidence of concomitant neurologic disease, and such patients were excluded. Patients were divided into 6 age groups: younger than 30, 31–40, 41–50, 51–60, 61–70, and 71–80 years of age. There were significantly more patients in the over 50 age groups than in the younger than 30 group, which had only 20 patients. Therefore, a similar number of patients were randomly selected from each age group to reflect an even spread through the decades from 15 to 78 years of age. A total of 122 patients with no provided clinical evidence of MSA were included in this study.
MR Imaging
MR examinations were performed on a 1.5T whole-body unit (Signa LX platform, GE Healthcare, Milwaukee, Wis) by using a quadrature head coil. Axial fast FLAIR MR imaging was performed as follows: TR = 9000 ms, TE = 140 ms, TI = 2200 ms, FOV = 240 mm, matrix = 256 × 192, NEX = 1, section thickness = 5 mm, intersection gap = 2 mm, scan time = 3 minutes 36 seconds with 20 sections covering the whole brain. T2-weighted spin-echo or fast spin-echo sequences were not part of the protocol.
Data Collection
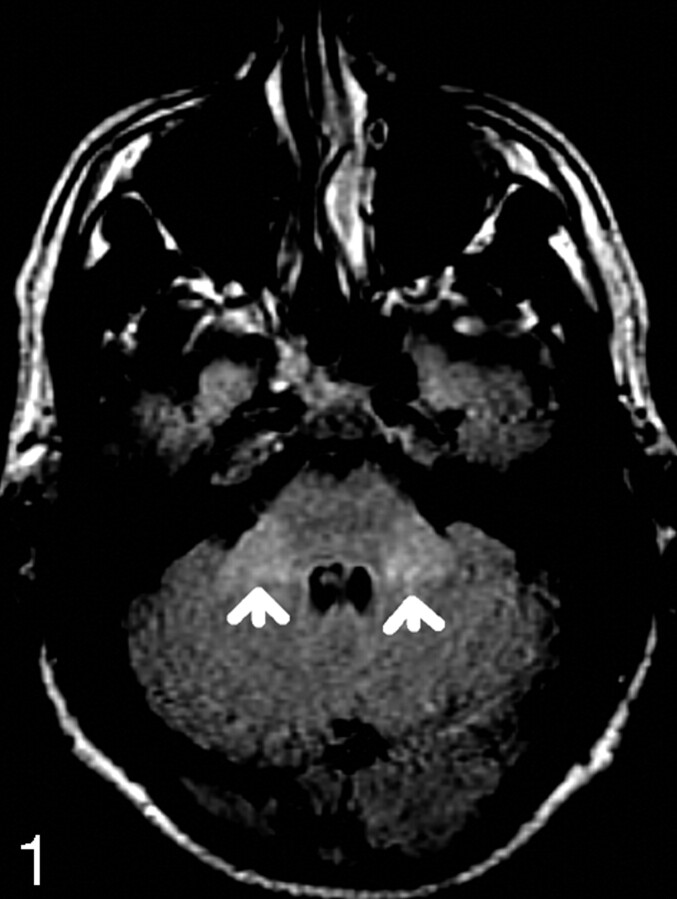
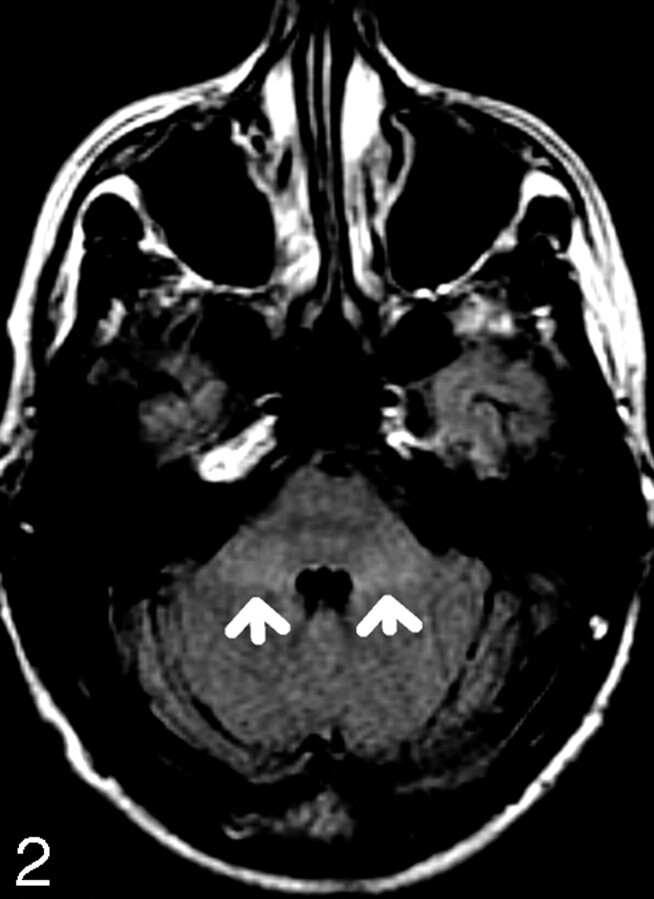
The MR images were independently reviewed by 2 readers, a radiologist with 10 years of MR imaging reporting experience (reader A) and a MR fellow (reader B). Both were blinded to the patients’ ages and sex. The signal intensity of the MCP was graded subjectively as either normal (designated 0) or increased (designated 1) (Figs 1 and 2). Each study was considered positive if the signal intensity extending across the width of MCP was greater than that of adjacent pons and cerebellar white matter. The data were recorded according to the age groups in a Microsoft Excel spreadsheet.
Fig 1.
72-year-old patient with MCP hyperintensity (arrows).
Fig 2.
21-year-old patient with MCP hyperintensity (arrows).
Scans with positive MCP hyperintensity were reviewed, and we ensured that this was not the result of CSF pulsation artifacts. All patients with MCP hyperintensity were screened by medical record review and/or phone interview to ensure that they did not have any disease that produced MCP T2 hyperintensity. A wide range of diseases were considered in the screening process, including neurodegenerative, metabolic, cerebrovascular, inflammatory, and demyelinating diseases and neoplasms.
Statistical Analysis
The data were analyzed independently for each reader. Correlation of prevalence of hyperintensity of the MCPs on FLAIR images with increasing age was assessed by computing the Spearman correlation coefficient and analysis of variance (ANOVA). P values below 0.05 were considered to be significant. Interobserver variability was assessed by computing the Pearson correlation coefficient.
Results
Of the 122 patients, we identified 17 with MCP hyperintensity. None of these patients had clinical conditions that caused MCP hyperintensity. Its prevalence is estimated to be 14%. The frequency of this sign is similar across all age groups with no statistically significant correlation of MCP hyperintensity with age (r = 0.05, P = .62), and ANOVA showed no statistically significant difference in incidence of MCP hyperintensity between age groups (P = .95). However, the Pearson correlation coefficient showed that the detection of MCP hyperintensity was highly reproducible with excellent interobserver agreement (r = 0.97, P < .001).
Discussion
MSA is a sporadic progressive adult-onset neurodegenerative disorder associated with varying degrees of parkinsonism and autonomic, pyramidal, and cerebellar dysfunction.1 The true worldwide prevalence of this disease is not known, given the difficulty in differentiating MSA from other disorders. The prevalence is approximately 4.4 per 100,000 persons in the United Kingdom9 and approximately 2.3 per 100,000 and 4.9 per 100,000 in the Faroe Islands and Italy, respectively.10,11 A prevalence as high as 252 per 100,000 has been reported in a German door-to-door study of people aged 65 years or older.12
Two major motor presentations can be distinguished clinically. Parkinsonian features predominate in 80% of patients (MSA-p subtype), whereas cerebellar ataxia predominates in the remaining 20% of patients (MSA-c subtype).13 A definite diagnosis requires pathologic confirmation that demonstrates cell loss and gliosis as well as glial cytoplasmic inclusions in the affected structures.2 The diagnosis is often difficult, especially in the early stages. Distinguishing MSA from idiopathic PD and other atypical parkinsonian syndromes clinically carries a high rate of misdiagnosis because they share overlapping clinical features.14
Conventional MR imaging may often disclose characteristic abnormality in the striatum, brain stem, and cerebellum in patients with MSA,1,4,7,15 and it has been suggested that these findings may help to differentiate between subtypes of MSA,5,7,8 and between MSA and PD and other atypical parkinsonian disorders.2–4,6 Putaminal atrophy, T2 hypointensity, and a T2 hyperintense rim in the outer margin of the putamen have been reported in patients with MSA.1 Putaminal atrophy and the T2 hyperintense rim were shown to be more prominent in MSA-p than in PD and were highly specific for MSA-p.5 Infratentorial signs, including atrophy and T2 hyperintensity of the pons, MCP, and the cerebellum, have been observed in both subtypes of MSA.5 The pontine cruciform hyperintensity, the “hot cross bun” sign, and MCP hyperintensity are thought to represent degeneration and correspond to the lack of histologic staining of myelin of the transverse pontocerebellar fibers.4,5 In particular, T2 hyperintensity of the pons and MCP have been reported to be more frequent in patients with MSA,1,2 especially the cerebellar variant (MSA-c),3–6 and are believed to have significant correlation with cerebellar dysfunction.2,5,6
Lee et al5 described a 100% specificity of the MCP hyperintensity on T2-weighted spin-echo images for favoring MSA over PD for both MSA-c and MSA-p, but the sensitivity for MSA-p was poor (22%), whereas the sensitivity for MSA-c was fair (88%). However, MCP hyperintensity is not specific for MSA because bilateral MCP hyperintensity has been observed in a number of conditions, including metabolic diseases such as Wilson disease and alcoholic liver cirrhosis, neoplasms, cerebrovascular diseases, and inflammatory and demyelinating diseases such as multiple sclerosis and acute disseminated encephalomyelitis.16 The diagnostic utility of MCP T2 hyperintensity for MSA increases when considered in combination with other infratentorial abnormalities, including atrophy2,7 and pontine cruciform hyperintensity.1,4,5 T2-weighted FLAIR imaging may also be a useful diagnostic tool for MSA,17 because it is shown to be more sensitive for the detection of MCP hyperintensity.5 However, the prevalence of MCP T2 hyperintensity among healthy patients on the T2-weighted FLAIR sequence has not been further studied in a large cohort.
With the number of our control patients larger than that in any previous study,2,3 our data suggest the prevalence of mild FLAIR MCP hyperintensity is approximately 14% in individuals referred for MR imaging to exclude a retrocochlear cause for tinnitus or hearing loss. The prevalence of this sign remained similar for all age groups, with no statistically significant correlation of the MCP hyperintensity with age. Therefore, we propose that mild MCP hyperintensity on FLAIR images, as demonstrated in the accompanying figures, can be a normal finding in all age groups and should not be interpreted in isolation as being pathologic. Perhaps this reflects a relative variation of myelin attenuation within the normal population. Such findings should be interpreted in combination with other MR imaging abnormalities and the clinical symptoms before suggesting a diagnosis of MSA.
Our study has limitations. In particular, we relied on the provided clinical details, medical records, and phone interviews to exclude any patients with MSA or other diseases potentially resulting in this finding from our study. Therefore, the accuracy of our findings depends on the accuracy of the provided data. However, the low prevalence of this disease9–12 suggests that these limitations are unlikely to significantly alter the results.
Conclusion
According to the previous literature, mild MCP T2 hyperintensity is a potentially specific and useful sign for diagnosing MSA, especially the cerebellar variant. However, the significance of mild MCP hyperintensity on FLAIR images should be interpreted with caution because it appears to be a relatively frequent finding with a similar incidence in all age groups. The presence of such T2-weighted FLAIR mild MCP hyperintensity, therefore, warrants appropriate review of other MR imaging and clinical findings to support the diagnosis of MSA-c and should not necessarily be considered abnormal.
References
- 1.Seppi K, Schocke MF, Wenning GK, et al. How to diagnose MSA early: the role of magnetic resonance imaging. J Neural Transm 2005;112:1625–34 [DOI] [PubMed] [Google Scholar]
- 2.Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 1998;65:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrag A, Good CD, Miszkiel K, et al. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 2000;55:1239–40 [DOI] [PubMed] [Google Scholar]
- 4.Savoiardo M. Differential diagnosis of Parkinson’s disease and atypical parkinsonian disorders by magnetic resonance imaging. Neurol Sci 2003;24:S35–S37 [DOI] [PubMed] [Google Scholar]
- 5.Lee EA, Cho HI, Kim SS, et al. Comparison of magnetic resonance imaging in subtypes of multiple system atrophy. Parkinsonism Relat Disord 2004;10:363–68 [DOI] [PubMed] [Google Scholar]
- 6.Burk K, Buhring U, Schulz JB, et al. Clinical and magnetic resonance imaging characteristics of sporadic cerebellar ataxia. Arch Neurol 2005;62:981–85 [DOI] [PubMed] [Google Scholar]
- 7.Naka H, Ohshita T, Murata Y, et al. Characteristic MRI findings in multiple system atrophy: comparison of the three subtypes. Neuroradiology 2002;44:204–09 [DOI] [PubMed] [Google Scholar]
- 8.Yagishita T, Kojima S, Hirayama K. MRI study of degenerative process in multiple system atrophy [in Japanese]. Rinsho Shinkeigaku 1995;35:126–31 [PubMed] [Google Scholar]
- 9.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 1999;354:1771–75 [DOI] [PubMed] [Google Scholar]
- 10.Wermuth L, Joensen P, Bunger N, et al. High prevalence of Parkinson’s disease in the Faroe Islands. Neurology 1997;49:426–32 [DOI] [PubMed] [Google Scholar]
- 11.Chio A, Magnani C, Schiffer D. Prevalence of Parkinson’s disease in Northwestern Italy: comparison of tracer methodology and clinical ascertainment of cases. Mov Disord 1998;13:400–05 [DOI] [PubMed] [Google Scholar]
- 12.Trenkwalder C, Schwarz J, Gebhard J, et al. Starnberg trial on epidemiology of parkinsonism and hypertension in the elderly: prevalence of Parkinson’s disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995;52:1017–22 [DOI] [PubMed] [Google Scholar]
- 13.Wenning GK, Tison F, Seppi K, et al, and the Multiple System Atrophy Study Group. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord 2004;19:1391–402 [DOI] [PubMed] [Google Scholar]
- 14.Litvan I, Goetz CG, Jankovic J, et al. What is the accuracy of the clinical diagnosis of multiple system atrophy? A clinicopathologic study. Arch Neurol 1997;54:937–44 [DOI] [PubMed] [Google Scholar]
- 15.Schott JM, Simon JE, Fox NC, et al. Delineating the sites and progression of in vivo atrophy in multiple system atrophy using fluid-registered MRI. Mov Disord 2003;18:955–58 [DOI] [PubMed] [Google Scholar]
- 16.Okamoto K, Tokiguchi S, Furusawa K, et al. MR features of diseases involving bilateral middle cerebellar peduncles. AJNR Am J Neuroradiol 2003;24:1946–54 [PMC free article] [PubMed] [Google Scholar]
- 17.Limberg N, Jeavons S, Robertson T, et al. Pyramidal tract imaging in multiple-system atrophy. Mov Disord 2005;20:1527–28 [DOI] [PubMed] [Google Scholar]