Abstract
BACKGROUND AND PURPOSE: Recanalization remains a major drawback in the endovascular treatment of intracranial aneurysms. Here, we report on our preliminary clinical experience with a new bioactive coil.
PATIENTS AND METHODS: In a prospective study, 25 aneurysms were treated in 24 patients. Endovascular occlusion of the aneurysm was performed by using a novel polyglycol acid–loaded coil (Cerecyte). Mean aneurysm volume was 114.7 mm3, with a mean dome height of 5.2 mm and a neck width of 2.8 mm. The primary goal of this study was to assess the safety of this new polymer-loaded coil in terms of periprocedural, technical, or angiographic complications. The secondary scope was to evaluate treatment efficacy regarding primary aneurysm occlusion, packing attenuation, and recanalization at follow-up angiography at 6 months.
RESULTS: There were no major technical or angiographic complications resulting in permanent morbidity. Complete initial occlusion of the aneurysm was accomplished in 17 patients, and a neck remnant was present in 8 patients. All aneurysms with complete initial occlusion remained stable at 6 months. Progression of a neck remnant without need for retreatment was noted in 2 patients, whereas 5 neck remnants turned to complete occlusion. Thus, complete occlusion rate at 6 months was 88%.
CONCLUSION: In this preliminary study the use of Cerecyte coils was safe, with an incidence of procedural complications comparable with that of bare platinum coils. Although most of the aneurysms in this study were small, the immediate and 6-month follow-up angiographic results are encouraging so that in our opinion, a larger clinical trial is warranted.
The International Subarachnoid Aneurysm Trial (ISAT) study1 has demonstrated a better outcome in patients treated by the endovascular approach compared with patients who underwent neurosurgical clipping. Nevertheless, incomplete primary aneurysm occlusion and aneurysm recurrence remain major drawbacks of interventional aneurysm treatment. The frequency of angiographic aneurysm recurrence following coil occlusion is reported to be between 17% and 33%.2,3 Even though the risk for recurrent hemorrhage after endovascular treatment seems to be low in patients treated by the endovascular approach,2,3 a reduction of the aneurysm recurrence rate is worthwhile. This, however, must not be associated with an increased complication rate. Several approaches to reduce the recanalization rate have been reported in the past, including bioactive4–9 or radioactive10 coil loading and hydrogel coverage of the coil.11 However, most of these studies were performed on animals, whereas human data are sparse.12–18 Recently, a new bioactive coil loaded with polyglycolic acid (PGA) has been approved for the endovascular occlusion of cerebral aneurysms (Cerecyte, Micrus Endovascular, San Jose, Calif), which may enhance the treatment of cerebral aneurysms by inducing a tissue response, potentially accelerating the healing of the aneurysm. The primary purpose of the present study was to evaluate procedural safety of this new bioactive coil in terms of periprocedural technical or angiographic complications. The secondary aim was to study treatment efficacy regarding primary aneurysm occlusion, packing attenuation, and recanalization at follow-up angiography 6 months after treatment.
Patients and Methods
The Cerecyte coil consists of a regular bare platinum coil with PGA running through the lumen of the primary platinum wind, which also provides stretch resistance when placing coils into the aneurysm (Fig 1). The PGA within Cerecyte is biodegraded by hydrolysis, which is the molecular breakdown of the polymer through contact with water molecules from the blood and other body fluids. Water from the blood passes through the openings of the platinum primary wind of Cerecyte, leading to hydrolysis of the PGA within the coil. The product line of Cerecyte coils (Micrus 10 System, Mircus Endovascular) includes MicroSphere coils (2–10 mm) as well as HeliPaq and UltiPaq coils. Twenty-four patients (mean age, 49 years; 18 women) with 25 intracranial aneurysms were included in this prospective study, which was approved by the local ethics committee. Patient inclusion was consecutive, with the exception of 4 patients who were part of another study in which the study protocol precluded the use of bioactive coils. The patient data are shown in the Table.
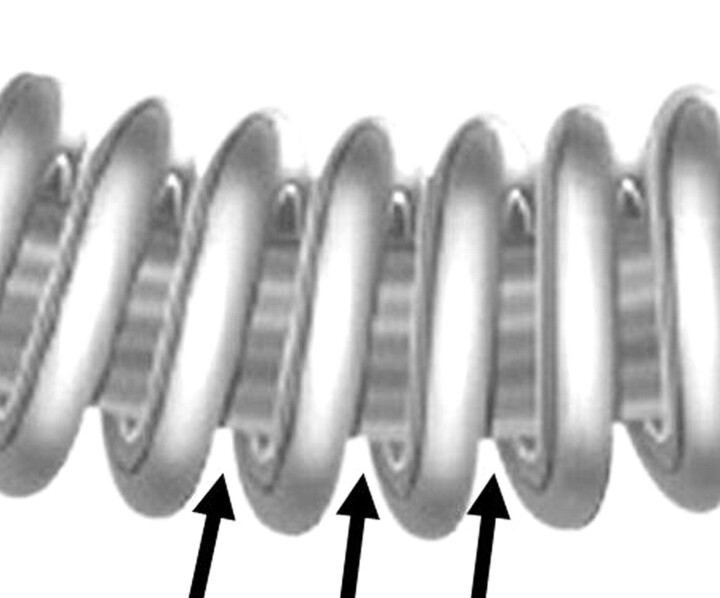
Fig 1.
Line drawing of a Cerecyte coil, consisting of a regular bare platinum coil with PGA running through the lumen of the primary platinum wind of the Cerecyte coil (arrows). This also provides stretch resistance when placing coils into the aneurysm. There is some space between the platinum primary winding of the coil for water to pass inside the loops, leading to hydrolysis of the PGA within the coil.
Patient data
| Patient Number | Sex | Age | Hunt & Hess Grade | Localization | Aneurysm Volume (mm3) | Neck Size (mm) | Dome size (mm) | Number of Cerecyte (bare) Coils | Percentage Aneurysm Occlusion (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 36 | 1 | PcomA | 120.5 | 2.5 | 5.4 | 7 | 30.7 |
| 2 | M | 36 | 1 | PcomA | 44.8 | 2.4 | 3.4 | 4 | 28.1 |
| 3 | F | 29 | 0 | AcomA | 11.8 | 1.5 | 2.2 | 3 | 30.5 |
| 4 | F | 73 | 3 | AcomA | 45.9 | 1.7 | 2.4 | 2 | 18.1 |
| 5 | F | 39 | 4 | AcomA | 41.4 | 1.3 | 2.8 | 4 | 22.2 |
| 6 | F | 71 | 0 | MCA | 185.0 | 2.2 | 4.8 | 5 | 31.3 |
| 7 | F | 60 | 0 | BA | 138.6 | 4.8 | 6.7 | 10 (4) | 24.6 |
| 8 | M | 44 | 1 | MCA | 50.8 | 2.1 | 2.6 | 3 | 20.3 |
| 9 | F | 79 | 1 | PcomA | 121.5 | 3.3 | 7.2 | 4 | 18.0 |
| AcomA | 74.7 | 2.5 | 3.4 | 9 | 39.1 | ||||
| 10 | M | 43 | 0 | AcomA | 360.1 | 4.9 | 10.1 | 5 (4) | 18.1 |
| 11 | F | 40 | 2 | AcomA | 79.4 | 1.9 | 3.4 | 5 | 18.4 |
| 12 | M | 40 | 1 | OA | 26.5 | 1.6 | 2.2 | 3 | 22.6 |
| 13 | F | 52 | 2 | BA | 128.8 | 3.2 | 7.1 | 4 | 21.6 |
| 14 | M | 55 | 0 | AcomA | 80.5 | 2.4 | 3.3 | 3 | 16.9 |
| 15 | F | 47 | 0 | BA | 108.9 | 3.9 | 8.2 | 8 | 25.9 |
| 16 | F | 39 | 0 | MCA | 65.8 | 2.8 | 4.1 | 5 (1) | 31.0 |
| 17 | F | 42 | 4 | PcomA | 81.4 | 2.2 | 5.2 | 5 | 26.9 |
| 18 | F | 53 | 2 | ICA | 140.4 | 2.8 | 4.1 | 6 | 16.9 |
| 19 | M | 59 | 1 | AcomA | 164.5 | 3.1 | 6.3 | 6 (1) | 18.1 |
| 20 | F | 54 | 1 | AcomA | 148.6 | 3.1 | 6.8 | 5 | 18.0 |
| 21 | F | 46 | 1 | AcomA | 159.6 | 3.9 | 8.8 | 7 | 24.7 |
| 22 | M | 43 | 0 | ICA | 321.7 | 4.7 | 11.3 | 12 | 19.5 |
| 23 | F | 45 | 1 | AcomA | 52.4 | 2.3 | 3.2 | 3 | 23.2 |
| 24 | F | 51 | 0 | AcomA | 113.7 | 2.4 | 5.2 | 6 | 24.8 |
Note:—ICA, internal carotid artery; PcomA, posterior communicating artery; AcomA, anterior communicating artery; MCA, middle cerebral artery; BA, basilar artery; OA, ophthalmic artery; aneurysm occlusion: 1, complete occlusion; 24, neck remnant; 3, residual aneurysm.
Patients presented with acute subarachnoid hemorrhage in 15 cases, and there were 9 incidental findings. Interventions were performed on a biplane angiography unit with 3D capability (Axiom Artis BA, Siemens, Erlangen, Germany). Volume calculation of the aneurysm was performed on a workstation with a 3D software tool (Leonardo, Siemens) at a fixed window setting. After manual segmentation of the aneurysm, volume is calculated automatically by the program. Mean aneurysm volume was 114.7 mm3 (range, 11.8–360.1 mm3; SD, 82.7 mm3). Mean size of the dome was 5.2 mm (SD, 2.5 mm) and of the neck, 2.8 mm (SD, 1.0 mm) with 12 aneurysms revealing a dome-to-neck ratio of <2. All procedures were performed with the patient under general anesthesia by using a transfemoral approach. Under systemic heparinization (50 IU/kg of body weight for 15 minutes followed by 25 IU/kg of body weight per hour), a guiding catheter (Envoy, Cordis, Miami Lakes, Fla) was placed in the internal carotid or vertebral artery, and the aneurysm was probed by using a microcatheter (Prowler, Cordis) and a microwire (Glidewire GT Hydrophilic, Terumo, Tokyo, Japan). Subsequently, the aneurysm was occluded with Cerecyte coils as densely as possible. The standard approach included placement of 1 or two 3D coils to obtain a basket, followed by subsequent filling with Standard and UltraSoft coils (Boston Scientific Corp, Natick, Mass). Overall, 134 Cerecyte coils (mean, 5.4 coils) were used. If a particular coil size or type was not available, bare coils of the same manufacturer were used (overall, 10 coils in 4 patients). Coil volume was calculated by a spreadsheet supplied by the manufacturer, providing volume per coil millimeter of every coil type. Packing attenuation was calculated as the ratio of coil volume and aneurysm volume × 100.19
In 2 patients, an intracranial stent (Neuroform, Boston Scientific) was used. The balloon remodeling technique was used in 2 patients. Follow-up conventional angiography was performed 6 months after initial treatment of the aneurysm. The protocol included 4 standard planes, 3D angiography, and at least 2 subsequent magnified views according to the best view of the aneurysm neck on 3D images. For all initial and follow-up angiograms, aneurysm occlusion was determined according to published criteria.20 Technical and angiographic complications and clinical outcome were recorded for every patient.
Results
Initial treatment and follow-up results are shown in the Table. At the beginning of this case series, Cerecyte coils differed from bare coils in terms of increased friction in the microcatheter and delayed coil detachment in 2 patients. This resulted in an asymptomatic prolapse of a proximal coil loop into the parent vessel without clinical sequelae in 1 patient. In November 2004, after 5 aneurysms had been treated, the design of the Cerecyte coil was modified and improved. Thereafter, increased friction or delayed detachment did not occur, and the handling of Cerecyte coils was identical to that of the bare Micrus platinum coils (Micrus Endovascular).
In patients treated with Cerecyte coils, there were 2 asymptomatic vessel branch occlusions. There was no neurologic deterioration after the procedure, and clinical outcome assessed at 6 months was excellent (no deficit) in 18 patients and good (mild motor, sensory, or language deficit that does not interfere with daily life) in 6 patients. There was no patient with a fair or poor (severe deficit, coma, death) outcome. Primary complete aneurysm occlusion was achieved in 17 patients (68%), a neck remnant was left in 8 aneurysms (32%), and there was no residual aneurysm. Mean percentage of aneurysm coil occlusion initially was 23.6% (SD, 5.8%; range, 18.8–39.1%). Follow-up angiography 6 months after treatment revealed stable complete occlusion in all aneurysms with complete initial occlusion (Fig 2). Of the 8 initial neck remnants, 5 turned to complete occlusion (Fig 3), 1 remained stable, and 2 neck remnants slightly progressed without need for retreatment.
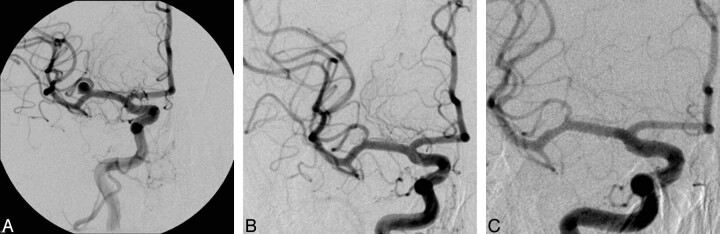
Fig 2.
Incidental left-sided middle cerebral artery aneurysm (A) with complete initial angiographic occlusion (B). On follow-up angiography at 6 months, there is persistent occlusion of the aneurysm (C).
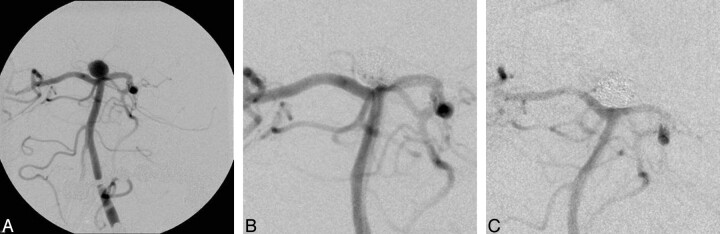
Fig 3.
Basilar tip aneurysm (A) with a clinical presentation of a subarachnoid hemorrhage, grade II. After treatment, packing attenuation is assessed as moderate, and there is residual filling of contrast medium at the neck initially (B). On follow-up angiography 6 months later, the aneurysm is now completely occluded (C).
Thus, after 6 months, complete angiographic occlusion was achieved in 88%, and a neck remnant was present in 12% of the aneurysms. There was no secondary vessel narrowing at the site of the aneurysm at 6 months’ follow-up. One patient developed hypoaesthesia of the right hand 6 days after complete occlusion of a large terminal ICA aneurysm (Fig 4A, -B). T2-weighted MR imaging revealed a hyperintensity surrounding the coiled aneurysm with slight mass effect (Fig 4C). On apparent diffusion coefficient (ADC) maps, this area revealed increased ADC values indicating vasogenic edema (Fig 4D). Complete septic work-up failed to identify an organism in this patient. Both symptoms and edema completely resolved after 5 days.
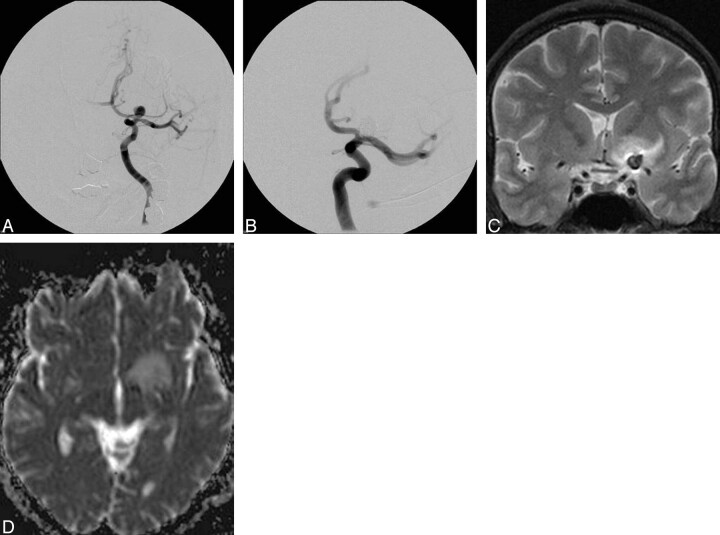
Fig 4.
Anteroposterior angiogram of an incidental carotid T aneurysm (A), which was completely occluded with Cerecyte coils exclusively (B). Six days after treatment, the patient presented with a hypoaesthesia of the right hand. T2-weighted MR image (C) reveals perianeurismal edema with slight mass effect, which was bright on ADC maps (D), indicating vasogenic edema. Symptoms and MR imaging findings completely resolved within 5 days.
Discussion
Recanalization after coil occlusion of aneurysms represents a major drawback of the endovascular approach and has been reported in up to 17% and 33%.2,3 Nevertheless, the long-term risk for rebleeding in coil-occluded aneurysms is considerably low, from 0.2%3 to 0.32%21 per patient-year according to the data of the ISAT study.1 Therefore, any attempt to reduce recanalization must not be associated with a higher periprocedural complication rate, which might vitiate the benefit of the endovascular therapy compared with neurosurgery. Here, we present a single-center experience with a new bioactive coil, which is supposed to enhance healing of the aneurysm and thus reduce recanalization. One drawback of the present study is a relatively small aneurysm volume (mean, 114.7 mm3). Smaller aneurysms are less likely to recanalize. Therefore, the preliminary results are unknown for large and wide-necked aneurysms, and we await a larger clinical experience for these more-challenging intracranial aneurysms. In a recent article on small aneurysms treated with bare Guglielmi coils, an overall recurrence rate of 26% was found, with 10% needing retreatment.22 In these patients, a substantially higher initial coil-packing attenuation was achieved.22
The lower packing attenuation and still high persistent occlusion rate in the present study using Cerecyte coils may indicate a benefit of this new bioactive coil compared with bare coils. Initial complete occlusion results are in the range of published data.2,20,23 Also, the packing attenuation was similar to that of other previous studies.24,25 Angiographic complications with transient vessel-branch occlusion were seen in 2 patients (8%), 1 of which was caused by vasospasm, the other presumably by thromboembolism. This is in the lower range of published data on periprocedural vessel occlusion26 and was not associated with clinical deterioration. There was no periprocedural morbidity or mortality, and outcome after 6 months was considerably favorable; this outcome may also be caused by the good initial clinical condition of this consecutive group of patients (median Hunt and Hess scale, grade I). In 1 patient with a large aneurysm, transient perianeurysmal edema evolved several days after treatment without evidence of an organism in the blood or CSF. A similar finding has been reported in other loaded coils,27 probably caused by an exuberant inflammatory response to the bioactive material. Especially in large aneurysms, this potential complication should be considered as a possible trade-off for a possibly lower recanalization rate.
Follow-up angiography displayed persistent occlusion in all aneurysms with complete initial occlusion and 3 neck remnants. In 5 cases, complete secondary occlusion of an initial neck remnant was present. Because the Raymond scale does not account for within-grade changes of neck remnants or residual aneurysms, we have added a group, worsening of neck remnants without need for retreatment, in 2 patients. The complete angiographic occlusion rate at 6 months was 88%, which is higher than that in recent studies.2,3,15,17,18,22 The data of a preliminary clinical study on the first bioactive coil on the market (Matrix, Boston Scientific) suggested a low complete initial occlusion rate of the aneurysm (15%), which was associated with a relatively high rate of early rebleeding of 7%.17,28 Secondary aneurysm occlusion or regression was reported in 49%, and a stable angiographic result, according to the Raymond scale was reported in 36%. Applying the Raymond scale20 exclusively implies that residual aneurysms cannot further recanalize and remain stable despite further aneurysm recanalization.
The data of this trial have recently been challenged by others because of the initial results after treatment.28 In the Matrix coil, the bioactive material (PGA) is on the surface of the coil. Especially in the first generation of these coils, this may have been the reason for increased friction in the catheter and, possibly, also a lower initial occlusion rate.15,23 Only recently, the first 2 peer-reviewed clinical case series on the experience with the Matrix coil have been published.15,18 In the first study,15 angiographic follow-up was available in 21 patients. Of these, 11 had been treated solely with Matrix coils. As in our study, periprocedural safety was comparable to bare platinum coils, but handling of Matrix coils was hampered by increased friction and compartmentalization. Recanalization at 6-month follow-up was present in 3 of 21 patients (14%), requiring retreatment in 2 patients. Moreover, there were 5 cases of residual neck not requiring retreatment. There is no mention of regression of initial neck remnants or residual aneurysms at follow-up angiography at 6 months. In a second trial,18 complete angiographic occlusion initially and at 6 months was accomplished in 68% of all aneurysms. There were 3 major recanalizations (12%) with a need for retreatment. Most of these patients had been treated with Matrix coils exclusively. Again, these authors attributed their results to the stiffness of the Matrix coils, preventing an attenuated packing.
As an important issue, the Cerecyte coil does not differ in terms of stiffness or handling from the bare platinum coil. Even though the overall recanalization and complete occlusion rate are lower in the present study, any statistical comparison is invalid because of the small sample size of these studies and the lack of a control group. As a most important result, our data indicate that the use of Cerecyte coils is feasible and safe. A high complete angiographic occlusion rate at 6 months (88%) warrants a larger controlled clinical trial.
Conclusion
These preliminary data suggest that this new PGA–loaded coil is not associated with an increased complication rate. The handling of the coil and the primary occlusion rate are comparable to those of the bare platinum coils. The low recanalization rate is promising and warrants a larger randomized controlled trial.
Acknowledgments
We would like to thank Viola Steffens and Sonja Bendszus for collecting the patient data. The authors have neither financial interest in the results of this study nor affiliation with the producer of the device. Martin Bendszus holds a professorship donated by the Schering GmbH to the University of Würzburg but has no financial interest.
References
- 1.Molyneux AJ, Kerr RSC, Stratton I, et al, and the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1267–74 [DOI] [PubMed] [Google Scholar]
- 2.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrence after selective endovascular treatment of aneurysms with detachable platinum coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 3.Molyneux AJ, Kerr RS, Yu LM, et al, and the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809–17 [DOI] [PubMed] [Google Scholar]
- 4.Murayama Y, Suzuki Y, Vinuela F, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part I. In vitro study. AJNR Am J Neuroradiol 1999;20:1986–91 [PMC free article] [PubMed] [Google Scholar]
- 5.Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–37 [DOI] [PubMed] [Google Scholar]
- 6.Murayama Y, Vinuela F, Suzuki Y, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery 1997;40:1233–43 [DOI] [PubMed] [Google Scholar]
- 7.Abrahams JM, Forman MS, Grady MS, et al. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. AJNR Am J Neuroradiol 2001;22:1410–17 [PMC free article] [PubMed] [Google Scholar]
- 8.Kallmes DF, Fujiwara NH, Yuen D, et al. A collagen-based coil for embolization of saccular aneurysms in a New Zealand White rabbit model. AJNR Am J Neuroradiol 2003;24:591–96 [PMC free article] [PubMed] [Google Scholar]
- 9.Marx WE, Cloft HJ, Helm GA, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intra-aneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol 2001;22:323–33 [PMC free article] [PubMed] [Google Scholar]
- 10.Raymond J, Leblanc P, Desfaits, et al. In situ beta radiation to prevent recanalization after coil embolization of cerebral aneurysms. Stroke 2002;33:421–27 [DOI] [PubMed] [Google Scholar]
- 11.Yoshino Y, Niimi Y, Song JK, et al. Endovascular treatment of intracranial aneurysms: comparative evaluation in a terminal bifurcation aneurysm model in dogs. J Neurosurg 2004;101:996–1003 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez NR, Patel AB, Murayama Y, et al. Angiographic evidence of aneurysm neck healing following endovascular treatment with bioactive coils. AJNR Am J Neuroradiol 2005;26:912–14 [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur AS, Wilson SA, Dixit S, et al. Hydrogel-coated coils for the treatment of cerebral aneurysms: preliminary results. Neurosurg Focus 2005;18:E1. [DOI] [PubMed] [Google Scholar]
- 14.Cloft HJ, Kallmes DF. Aneurysm packing with HydroCoil Embolic System versus platinum coils: initial clinical experience. AJNR Am J Neuroradiol 2004;25:60–62 [PMC free article] [PubMed] [Google Scholar]
- 15.Linfante I, Akkawi NM, Perlow A, et al. Polyglycolide/polylactide-coated platinum coils for patients with ruptured and unruptured cerebral aneurysms: a single-center experience. Stroke 2005;36:1948–53. Epub 2005 Jul 28 [DOI] [PubMed] [Google Scholar]
- 16.Raymond J, Roy D, Leblanc P, et al.. Endovascular treatment of intracranial aneurysms with radioactive coils: initial clinical experience. Stroke 2003;34:2801–06. Epub 2003 Nov 6 [DOI] [PubMed] [Google Scholar]
- 17.Matrix Newsletter. Fremont, Calif: Boston Scientific;2004
- 18.Taschner CA, Leclerc X, Rachdi H, et al. Matrix detachable coils for the endovascular treatment of intracranial aneurysms. Stroke 2005;36:2176–80 [DOI] [PubMed] [Google Scholar]
- 19.Slob MJ, Rooij W, Sluzewski M. Coil thickness and packing of cerebral aneurysms: a comparative study of two types of coils. AJNR Am J Neuroradiol 2005;26:901–03 [PMC free article] [PubMed] [Google Scholar]
- 20.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001;32:1998–2004 [DOI] [PubMed] [Google Scholar]
- 21.Sluzewski M, van Rooij WJ, Beute GN, et al.. Late rebleeding of ruptured intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol 26:2542–49 [PMC free article] [PubMed] [Google Scholar]
- 22.Kai Y, Hamada J, Morioka M, et al. Evaluation of the stability of small ruptured aneurysms with a small neck after embolization with Guglielmi detachable coils: correlation between coil packing ratio and coil compaction. Neurosurgery 2005;56:785–92 [DOI] [PubMed] [Google Scholar]
- 23.Niemann D, Aviv R, Cowsill C, et al. Anatomically conformable, three-dimensional, detachable platinum microcoil system for the treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2004;25:813–18 [PMC free article] [PubMed] [Google Scholar]
- 24.Sluzewski M, van Rooij WJ, Slob MJ, et al. Relation between aneurysm volume, packing, and compaction in 145 cerebral aneurysms treated with coils. Radiology 2004;231:653–58 [DOI] [PubMed] [Google Scholar]
- 25.Slob MJ, Sluzewski M, van Rooij WJ. The relation between packing and reopening in coiled intracranial aneurysms: a prospective study. Neuroradiology 2005;47:942–45 [DOI] [PubMed] [Google Scholar]
- 26.Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1541–47 [PMC free article] [PubMed] [Google Scholar]
- 27.Meyers PM, Lavine SD, Fitzsimmons BF, et al. Chemical meningitis after cerebral aneurysm treatment using two second-generation aneurysm coils: report of two cases. Neurosurgery 2004;55:1222. [DOI] [PubMed] [Google Scholar]
- 28.Sluzewski M, van Rooij WJ. Questionable interpretation of results of ACTIVE Study on Matrix coils by Boston Scientific. AJNR Am J Neuroradiol 2005;26:2163–64 [PMC free article] [PubMed] [Google Scholar]