Abstract
SUMMARY: Histologic patterns of cellular architecture often suggest a tissue diagnosis. One distinctive histologic pattern seen within some tumors of the nervous system is the palisade. The purpose of this report is to review the significance of palisades and pseudopalisades in the context of such tumors as schwannomas and glioblastomas.
Histologic patterns of cellular architecture often suggest a tissue diagnosis. Pathologists rely on these patterns much as radiologists rely on gray-scale patterns on images. Appreciation of these patterns can enhance the interactions of the neuroradiologist with the neuropathologist and can deepen the understanding of commonly occurring tumors. One distinctive histologic pattern seen within certain tumors of the nervous system is the palisade. The purpose of this report is to review the significance of palisades and pseudopalisades in the context of such tumors as schwannomas and glioblastomas.
What Are Palisades and Pseudopalisades?
A palisade is a strong fence or protective perimeter made of a row of wooden poles or stakes driven into the ground. Fortifications consisting of palisades made of stout logs were relatively common in the American frontier in the 18th and 19th centuries because stone and brick building materials were scarce (Fig 1). Pathologists examining the microscopic appearance of certain tumors noted arrangements of elongated nuclei stacked in neat rows and attached the descriptive term “palisades” on the basis of their resemblance to these fortifications. Nuclear palisades may be considered “primary” when they reflect a natural tendency of the nuclei to develop this distinctive pattern of growth or “secondary” when the alignment forms as a response to external influences such as necrosis.1 The latter have been termed “pseudopalisades” to distinguish them from primary palisades. Although not pathognomonic, palisades are most often seen in schwannomas, whereas pseudopalisades are typical of glioblastomas.1
Fig 1.
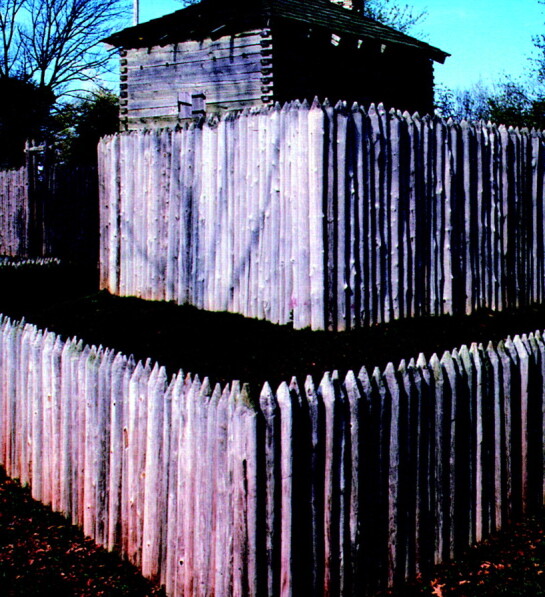
Photograph of the reconstructed 18th century Fort Massac in southern Illinois, demonstrating walls made of log palisades (courtesy of James P. Rowen).
Palisades and Schwannomas
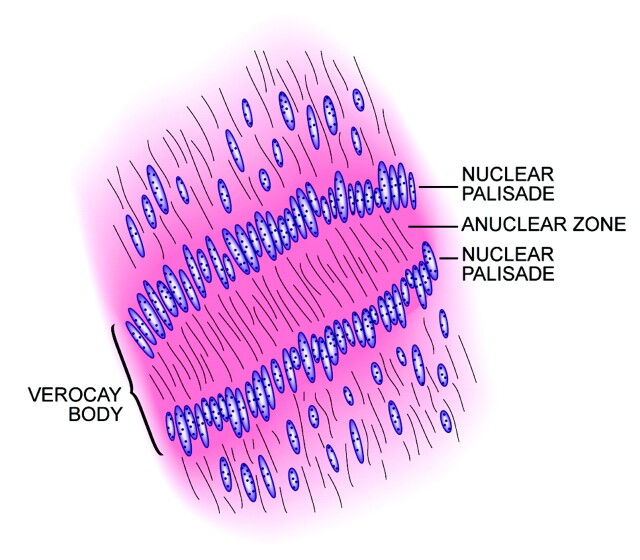
The peculiar alignment of nuclei into parallel rows was described in detail by the Uruguayan pathologist Jose Juan Verocay (1876–1927) in 1910, while working at the Institute of Pathology of the German University of Prague, Austria-Hungary, with Hans Chiari.2,3 Examining multiple nerve sheath tumors in a 31-year-old field worker who died of complications of neurofibromatosis type 2, Verocay noted a “peculiar arrangement of nuclei in transverse bands.”2 These bands of fusiform nuclei alternated with clear zones devoid of nuclei. Later investigators named these structures “Verocay bodies” and defined them as stacked arrangements of elongated palisading nuclei alternating with anuclear zones containing cell processes (Figs 2 and 3).4–7 These Verocay bodies are typically found in the more densely packed Antoni A regions, rather than in the loose or microcystic Antoni B areas.
Fig 2.
Drawing of a Verocay body illustrating the parallel rows of fusiform nuclei (modified with permission from Springer-Verlag1).
Fig 3.
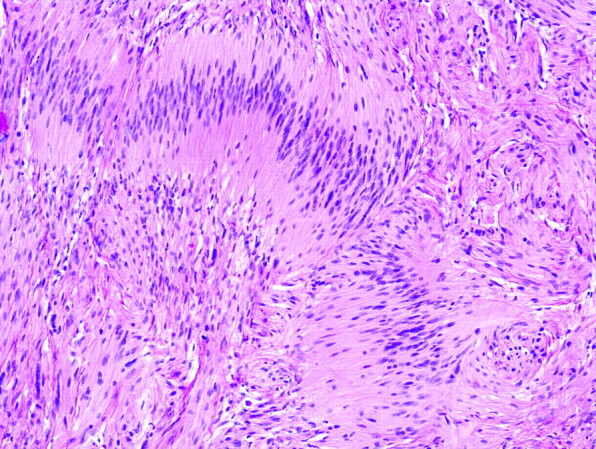
Photomicrograph of Verocay bodies in a schwannoma characterized by linear arrangements of elongated tumor nuclei (hematoxylin-eosin [H&E], original magnification ×400).
Verocay’s observations provided pathologists with a valuable clue in differentiating types of nerve sheath tumors microscopically. At the time of Verocay’s original paper, nerve sheath tumors were simply termed “neuromas,” a name introduced by Louis Odier (1748–1817) in 1803. Some early investigators postulated that these tumors developed from connective tissue, whereas others believed that they arose from nervous tissue. Von Recklinghausen (1833–1910) coined the term “neurofibroma” to unify, within a single concept, the seemingly disparate origins of multiple deep soft-tissue tumors and superficial cutaneous lesions seen in patients with phakomatoses. Nuclear palisading and Verocay bodies were recognized as especially prominent in a group of tumors that came to be known as schwannomas, but not in other varieties of nerve sheath tumors such as neurofibromas.
The mechanism for the formation of the characteristic pattern of palisades and Verocay bodies in schwannomas is not completely understood. Some investigators have identified large amounts of laminin associated with cells participating in Verocay bodies.8 Laminins are large cruciform glycoproteins that promote cell adhesion and that are normally found in the basement membranes of many types of cells such as Schwann cells.9–13 Cell adhesion is an important property of Schwann cells and facilitates myelination of axons and repair of injury.14 Presumably the overexpression of laminins in portions of schwannomas prompts the alignment of cells into a tight pattern of rows. Interestingly, schwannomas are composed of a relatively pure proliferation of Schwann cells, which are completely surrounded by a laminin-rich basement membrane. In contrast, neurofibromas are composed of a mixture of cell types, including fibroblasts, perineural cells, mast cells, and entrapped native neural elements, in addition to Schwann cells. The former cell types do not produce basement membrane, and as such, neurofibromas contain considerably less laminin than schwannomas. This composition may partially explain the relative paucity of Verocay bodies in neurofibromas compared with schwannomas.15,16 Also, lysophosphatidic acid (LPA), an extracellular phospholipid involved in extracellular signaling pathways that regulate Schwann cell adhesion and structure, when applied to Schwann cells in vitro, has been found to induce cluster formation.14 Conceivably, overexpression of LPA or other similar adhesion-signaling molecules in some schwannomas could have a role in the formation of Verocay bodies.
The finding of palisades and Verocay bodies within a tumor may suggest the diagnosis of schwannoma in the appropriate clinical and histologic setting, but their presence is by no means pathognomic. Other varieties of nerve sheath tumors, central nervous system (CNS) tumors, soft-tissue tumors, and even carcinomas may display palisades (Table).17–34 Perhaps most frustrating among these is the occasional fibrous meningioma with palisades, given that schwannoma is often the main differential diagnostic consideration in the spinal canal and cerebellopontine angle. The presence of psammoma bodies, epithelial membrane antigen immunoreactivity, and a lack of pericellular collagen IV deposition (ie, basement membrane) would substantiate the diagnosis of meningioma and rule out schwannoma in such a case. Conversely, not all varieties of schwannomas display palisades. For example, cellular schwannomas usually have few if any Verocay bodies.35 Also, palisades tend to be more common in spinal schwannomas and somewhat less common in schwannomas of the cranial nerves.36 Nevertheless, the demonstration of Verocay bodies within a nerve sheath tumor may significantly contribute to formulating the final diagnosis of schwannoma.
Lesions associated with palisades or Verocay bodies
| Category | Examples |
|---|---|
| Peripheral nerve sheath tumors | Schwannoma |
| Palisaded encapsulated neuroma | |
| Neurofibroma | |
| Malignant peripheral nerve sheath tumor (MPNST) | |
| Central nervous system tumors | Meningioma (mostly the fibrous variant) |
| Medulloblastoma | |
| Supratentorial primitive neuroectodermal tumor | |
| Pilocytic astrocytomas | |
| Oligodendroglioma | |
| Ependymoma | |
| Craniopharyngioma | |
| Soft tissue tumors | Spindle cell lipomas |
| Cutaneous fibrous histiocytoma | |
| Angioleiomyoma | |
| Cutaneous leiomyoma | |
| Cutaneous leiomyosarcoma | |
| Fibrous mesothelioma | |
| Dermatofibrosarcoma protuberans | |
| Myofibroblastoma | |
| Myofibroblastic dermatofibroma | |
| Epithelial neoplasms | Basal cell carcinoma |
| Basal cell adenoma | |
| Skin adnexal tumors | |
| Melanocytic tumors | Malignant melanoma |
| Giant congenital nevi | |
| Cutaneous malignant melanotic neurocristic tumor |
In addition to Verocay bodies in schwannomas, an exaggerated version containing many rows of aligned nuclei is often referred to as “rhythmic palisades” or a “spongioblastic pattern.” The latter is derived from the fact that this is the defining feature for a rare and highly controversial primitive pediatric brain tumor referred to as “polar spongioblastoma” or “primitive polar spongioblastoma” (Fig 4). First described by Russell and Cairns in 1947,37 it is defined by the presence of rhythmic palisades of compactly packed bipolar cells that were thought to resemble radial glia during fetal development. However, the identical pattern has since been encountered in a wide range of CNS neoplasms, including medulloblastoma, supratentorial primitive neuroectodermal tumor (“central neuroblastoma”), pilocytic astrocytoma, oligodendroglioma, and ependymoma.38 Therefore, most neuropathologists now consider it to represent a morphologic pattern, rather than a specific entity, and this is reflected in the 2000 World Health Organization revision, 39 in which the diagnosis of polar spongioblastoma was dropped. The mechanism for this otherwise spectacular and eye-catching growth pattern remains poorly understood.
Fig 4.
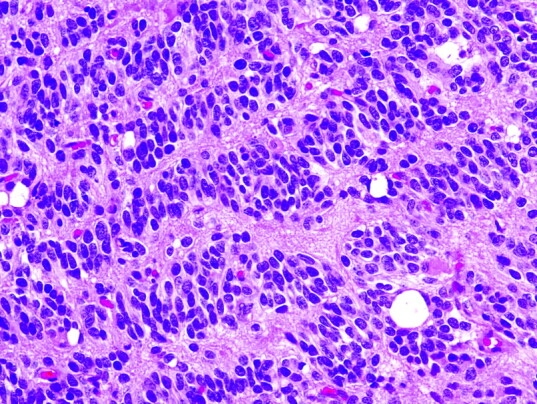
Spongioblastic tumor showing rhythmic palisades (linear waves of tumor nuclei) or spongioblastic pattern. This feature is now considered a relatively nonspecific pattern, and other regions of this tumor showed classic histologic features of anaplastic oligodendroglioma (H&E, original magnification ×400).
Pseudopalisades and Glioblastomas
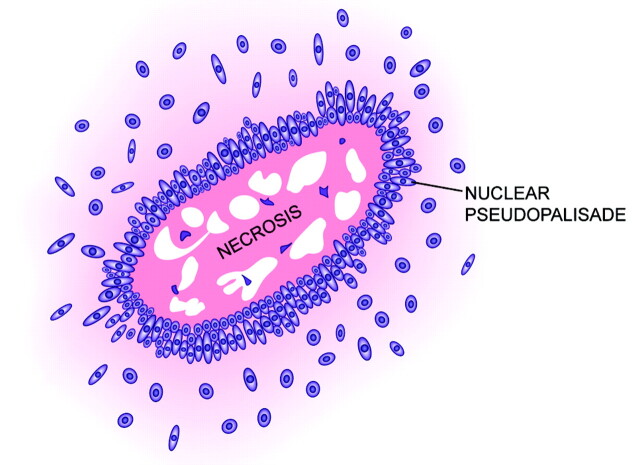
In contrast to the neat nuclear arrangement of the palisades found in schwannomas, the nuclei in pseudopalisades, though aligned, tend to be less well organized (Fig 5). Rather than reflecting a primary organizational behavior of cells of a given tumor type, pseudopalisades are thought to represent a reaction to external factors occurring within the tumor bed. Notably, pseudopalisades are associated with necrosis and are nearly constant features of glioblastoma. In fact, pseudopalisades have been incorporated into the pathologic definition of this aggressive brain tumor, distinguishing it from lower grades of astrocytomas (Fig 6).40,41
Fig 5.
Drawing of pseudopalisading, illustrating the garlandlike array of nuclei surrounding a region of necrosis (modified with permission from Springer-Verlag1).
Fig 6.
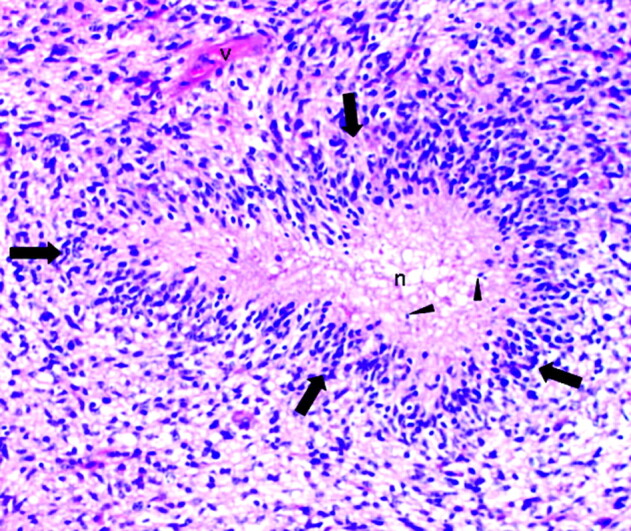
Pseudopalisading necrosis in a glioblastoma characterized by a garlandlike arrangement of hypercellular tumor nuclei (arrows) lining up around irregular foci of tumor necrosis (n) containing pyknotic nuclei (arrowheads). Note tumor vessel (v) (H&E; original magnification ×200).
The relationship between necrosis and pseudopalisading nuclei remained unclear for many years. Pathologists had long noted that the development of necrosis within astrocytomas signaled a transition to an accelerated form of aggressive behavior. Mechanisms for this necrosis and cell death include apoptosis and coagulative necrosis.42,43 Apoptosis is a form of genetically programmed cell death, usually mediated through specific tumor necrosis factors (TNF) such as the proteins FasL and Fas.42,44 The dying cells are then cleared without an associated inflammatory response.43 Coagulative necrosis, more common than apoptosis, consists of the simultaneous death of many contiguous cells due to an insult such as hypoxia or ischemia and results in a geographic zone of congealed protein, pyknotic nuclei, and cell fragments. Possible mechanisms for coagulative necrosis include infarction due to tumor metabolism outstripping nutrient supply, decreased blood supply due to collapsing fragile vessels caused by increased extracellular pressure from edema, vessel occlusion due to frank tumor invasion, and thrombosis due to the elaboration of procoagulation factors.42 Regardless of the mechanism of cell death, pathologists have also noted that attenuated clusters of nuclei surround the areas of necrosis in a characteristic garlandlike arrangement of rows that became known as pseudopalisades. Although high levels of protein Fas have been found within the pseudopalisades of some glioblastomas indicating a link with apoptosis, this observation does not fully explain the association of pseudopalisades with coagulative necrosis or the typical pattern of alignment.42
Other observations concerning the pseudopalisading nuclei were also intriguing. Despite the crowded alignment of the nuclei suggesting accelerated growth and cell division, the cells within the pseudopalisades were actually less mitotically active than adjacent astrocytoma nuclei. The increased numbers of nuclei also suggested an influx of inflammatory cells; however, this hypothesis was later refuted when the nuclei were actually shown to be entirely astrocytic.45 Finally, these nuclei were not just large numbers of hardy surviving nuclei from necrotic areas but were actually shown to display increased apoptosis.42,46 Thus investigators concluded that the nuclei forming the pseudopalisades seemed to be migrating tumor cells.
Another noteworthy observation was the high expression of hypoxia-inducible factor 1α (HIF 1α) within pseudopalisades. HIF 1α is triggered by cellular hypoxia and, in turn, stimulates production of vascular endothelial growth factor (VEGF). VEGF is a potent glycoprotein that promotes endothelial proliferation and angiogenesis.41,45,47–50 Tumors typically are unable to achieve diameters greater than 1–2 mm without development of new nutrient-providing vessels.51 As tumors grow beyond this critical diameter, rising levels of hypoxia upregulate VEGF expression.40 The resulting angiogenesis would then promote further tumor growth. HIF 1α also mediates the production of proteases, enzymes that dissolve proteins and, therefore, facilitate invasiveness. Thus, it is possible that the tumor cells comprising pseudopalisades migrated away from their blood supply, became hypoxic, and were stimulating new vascular growth. However, this explanation would seem to predict a random appearance of the nuclei and does not fully explain the well-ordered garlandlike appearance that surrounds the regions of necrosis. Alternatively, a vascular catastrophe, such as occlusion of a feeding vessel, could have created a hypoxic field and stimulated the outward migration of cells. Supporting this latter theory is the observation of thrombosed vessels in the center of over 50% of pseudopalisades.45
Several mechanisms for vascular occlusion have been proposed. For example, high levels of angiopoetin-2 (ang 2) have been measured in high-grade gliomas, and ang 2 may cause vascular injury, leading to thrombosis and subsequent hypoxia. Secondly, leaky neovessels could allow plasma coagulation factors to enter the extravascular spaces and become activated. Other tissue factors could also cause coagulation.42,45
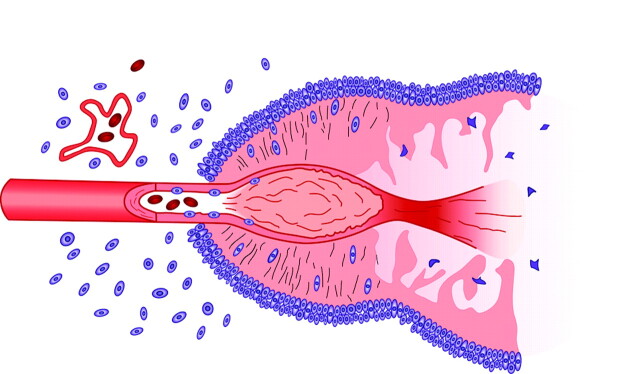
Brat et al42 have proposed a compelling argument for the formation of pseudopalisades by using the emerging information on the role of hypoxia and vessel occlusion in glioblastomas. According to these investigators, the glioblastomas that either arise de novo or develop from preexisting astrocytomas grow sufficiently to stimulate vascular proliferation. Expression of ang 2 mediates endothelial damage, which, in turn, initiates vascular occlusion and hypoxia. Cells unable to survive the decreased oxygen tensions succumb and form the nidus of coagulation necrosis. Other cells, however, begin migrating to the periphery of the hypoxic field in moving waves (pseudopalisades). The migrating hypoxic cells secrete VEGF, proteases, and other factors, which cause microvascular proliferation and enhanced invasiveness in regions ringing the hypoxic field. These latter effects prompt further outward expansion of the glioblastoma cells and result in enhanced aggressiveness (Fig 7).
Fig 7.
Schematic representation of the formation of a pseudopalisade. Growth of the glioblastoma stimulates neo-angiogenesis. Expression of ang 2 causes endothelial damage, which, in turn, produces vascular occlusion and hypoxia. Cells unable to survive the hypoxia succumb and form the nidus of coagulation necrosis. Other cells, however, migrate to the periphery of the hypoxic field in waves forming pseudopalisades. The migrating hypoxic cells secrete VEGF, proteases, and other factors that cause further microvascular proliferation and enhanced invasiveness in regions ringing the hypoxic field. These latter effects prompt further aggressive outward expansion of the glioblastoma cells (modified with permission from Brat et al42).
Conclusions
In summary, the neat stacking of parallel rows of elongated nuclei represents distinctive histologic patterns known as palisades. Primary palisades are found in schwannomas as well as in many other tumors and are useful clues in pathologic identification. Pseudopalisades are somewhat less well organized and represent cells migrating from hypoxic centers of necrosis in glioblastomas. The finding of pseudopalisading necrosis signifies aggressive tumor behavior.
References
- 1.Zülch KJ. Histology of brain tumors. In: Brain Tumors: Their Biology and Pathology. Berlin, Germany: Springer-Verlag;1986. :118–34
- 2.Verocay J. Zur kenntnis der “neurofibrome.” Beitr Pathol Anat 1910;48:1–69 [Google Scholar]
- 3.Enstable-Puig JF, de Estable-Puig RF, Haymaker W. Jose Verocay: pioneer Uruguayan neuropathologist [in Spanish]. Arch Fund Roux Ocefa 1970;4:135–38 [PubMed] [Google Scholar]
- 4.Burger PC, Scheithauer BW, Vogel FS. The brain: tumors. In: Surgical Pathology of the Nervous System and Its Coverings. New York: Churchill Livingstone;2002. :160–378
- 5.Citow JS. Wollman RL, Macdonald RL, et al. Oncology. In: Neuropathology and Neuroradiology: A Review. New York: Thieme;2001. :46–108
- 6.Harkin JC, Reed RJ. Solitary benign nerve sheath tumors. In: Atlas of Tumor Pathology: Tumors of the Peripheral Nervous System. Washington, D.C: Armed Forces Institute of Pathology;1968. :29–65
- 7.Ellison D, Love S, Chimelli L, et al. Peripheral nerve sheath neoplasms. In: Neuropathology: A reference Text of CNS Pathology. Edinburgh, Scotland: Mosby;2004. :695–702
- 8.Reibel J, Wewer U, Albrechtsen R. The pattern of distribution of laminin in neurogenic tumors, granular cell tumors, and nevi of the oral mucosa. Acta Pathol Microbiol Immunol Scand [A] 1985;93:41–47 [DOI] [PubMed] [Google Scholar]
- 9.Roberts DD, Wewer UM, Liotta LA, et al. Laminin-dependent and laminin-independent adhesion of human melanoma cells to sulfatides. Cancer Res 1988;48:3367–73 [PubMed] [Google Scholar]
- 10.Beck K, Hunter I, Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J 1990;4:148–60 [DOI] [PubMed] [Google Scholar]
- 11.Petruzzelli L, Takami M, Humes HD. Structure and function of cell adhesion molecules. Am J Med 1999;106:467–76 [DOI] [PubMed] [Google Scholar]
- 12.Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol 2003;15:621–32 [DOI] [PubMed] [Google Scholar]
- 13.Miettinen M, Foidart JM, Ekblom P. Immunohistochemical demonstration of laminin, the major glycoprotein of basement membranes, as an aid in the diagnosis of soft tissue tumors. Am J Clin Pathol 1983;79:306–11 [DOI] [PubMed] [Google Scholar]
- 14.Weiner JA, Fukushima N, Contos JJ, et al. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J Neurosci 2001;21:7069–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erlandson RA, Woodruff JM. Peripheral nerve sheath tumors: an electron microscopic study of 43 cases. Cancer 1982;49:273–87 [DOI] [PubMed] [Google Scholar]
- 16.Chen SY, Miller AS. Neurofibroma and schwannoma of the oral cavity: a clinical and ultrastructural study. Oral Surg Oral Med Oral Pathol 1979;47:522–28 [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita S, Hirai A, Izaki S, et al. Leiomyosarcoma of the skin with generalized metastases: electron microscopic and immunohistochemical study. J Dermatol 1991;18:654–60 [DOI] [PubMed] [Google Scholar]
- 18.Reed RJ, Fine RM, Meltzer HD. Palisaded, encapsulated neuromas of the skin. Arch Dermatol 1972;106:865–70 [PubMed] [Google Scholar]
- 19.Zamecnik M, Michal M. Solitary fibrous tumor (fibrous mesothelioma): report of 2 cases in an extraserous location [in Slovak]. Cesk Patol 1998;34:58–62 [PubMed] [Google Scholar]
- 20.Schwob VS, Santa Cruz DJ. Palisading cutaneous fibrous histiocytoma. J Cutan Pathol 1986;13:403–07 [DOI] [PubMed] [Google Scholar]
- 21.Allen PW. Subcutaneous spindle cell lipoma. In: Tumors and Proliferations of Adipose Tissue: A Clinickopathologic Approach. New York: Masson;1981. :20–26
- 22.Akasaka T, Imamura Y, Mori Y, et al. Trichoblastoma with rippled-pattern. J Dermatol 1997;24:174–78 [DOI] [PubMed] [Google Scholar]
- 23.San Juan J, Monteagudo C, Navarro P, et al. Basal cell carcinoma with prominent central palisading of epithelial cells mimicking schwannoma. J Cutan Pathol 1999;26:528–32 [DOI] [PubMed] [Google Scholar]
- 24.Graham BS, Barr RJ. Rippled-pattern sebaceous trichoblastoma. J Cutan Pathol 2000;27:455–59 [DOI] [PubMed] [Google Scholar]
- 25.Lespi PJ, Smit R. Verocay body–prominent cutaneous leiomyoma. Am J Dermatopathol 1999;21:110–11 [DOI] [PubMed] [Google Scholar]
- 26.Baugh W, Quigley MM, Barrett TL. Palisaded angioleiomyoma. J Cutan Pathol 2000;27:526–28 [DOI] [PubMed] [Google Scholar]
- 27.Zelger BG, Steiner H, Kutzner H, et al. Verocay body–prominent cutaneous schwannoma. Am J Dermatopathol 1997;19:242–49 [DOI] [PubMed] [Google Scholar]
- 28.Dabski C, Reiman HM Jr, Muller SA. Neurofibrosarcoma of skin and subcutaneous tissues. Mayo Clin Proc 1990;65:164–72 [DOI] [PubMed] [Google Scholar]
- 29.Ahn SK, Chang SN, Lee SH, et al. Verocay bodies in neurofibroma. Int J Dermatol 1994;33:886–87 [DOI] [PubMed] [Google Scholar]
- 30.Radulescu D, Sarbu M. Giant congenital nevi: a clinical and histopathological study. Rom J Morphol Embryol 1994;40:19–22 [PubMed] [Google Scholar]
- 31.Misago N, Narisawa Y. Rippled-pattern sebaceoma. Am J Dermatopathol 2001;23:437–43 [DOI] [PubMed] [Google Scholar]
- 32.Llatjos R, Fernandez-Figueras MT, Diaz-Cascajo C, et al. Palisading and Verocay body–prominent dermatofibrosarcoma protuberans: a report of three cases. Histopathology 2000;37:452–55 [DOI] [PubMed] [Google Scholar]
- 33.Pearson JP, Weiss SW, Headington JT. Cutaneous malignant melanotic neurocristic tumors arising in neurocristic hamartomas: a melanocytic tumor morphologically and biologically distinct from common melanoma. Am J Surg Pathol 1996;20:665–77 [DOI] [PubMed] [Google Scholar]
- 34.Sexton M, Maize JC. Malignant melanoma simulating Schwannian differentiation. Am J Dermatopathol 1985;7(suppl):171–76 [DOI] [PubMed] [Google Scholar]
- 35.Casadei GP, Scheithauer BW, Hirose T, et al. Cellular schwannoma: a clinicopathologic, DNA flow cytometric, and proliferation marker study of 70 patients. Cancer 1995;75:1109–19 [DOI] [PubMed] [Google Scholar]
- 36.Burger PC, Scheithauer BW. Tumors of the peripheral nerve sheath. In: Atlas of Tumor Pathology: Tumors of the Central Nervous System. Washington, DC: Armed Forces Institute of Pathology;1994. :333–43
- 37.Russell DS, Cairns H. Polar spongioblastomas [in Spanish]. Arch Histol Norm Patol 1947;3:423–41 [Google Scholar]
- 38.Schiffer D, Cravioto H, Giordana MT, et al. Is polar spongioblastoma a tumor entity? J Neurosurg 1993;78:587–91 [DOI] [PubMed] [Google Scholar]
- 39.Kleihues P, Cavenee W, eds. World Health Organization Classification of tumours: Pathology and Genetics—Tumours of the Nervous System. Lyon, France: IARC Press;2000
- 40.Brat DJ, Mapstone TB. Malignant glioma physiology: cellular response to hypoxia and its role in tumor progression. Ann Intern Med 2003;138:659–68 [DOI] [PubMed] [Google Scholar]
- 41.Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992;359:845–48 [DOI] [PubMed] [Google Scholar]
- 42.Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest 2004;84:397–405 [DOI] [PubMed] [Google Scholar]
- 43.Wharton SB, McNelis U, Bell HS, et al. Expression of poly(ADP-ribose) polymerase and distribution of poly(ADP-ribosyl)ation in glioblastoma and in glioma multicellular tumor spheroid model. Neuropathol Appl Neurobiol 2000;26:528–35 [DOI] [PubMed] [Google Scholar]
- 44.Seilhean D, DeGirolami U, Gray F. Basic pathology of the central nervous system. In: Gray F, DeGirolami U, Poirer J, eds. Escourolle and Poirer’s Manual of Basic Neuropathology. Philadelphia: Butterworth Heinemann;2004. :1–20
- 45.Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res 2004;64:920–27 [DOI] [PubMed] [Google Scholar]
- 46.Brat DJ, Castellano-Sanchez A, Hunter SB, et al. Pseudopalisading cells in glioblastoma are hypoxic and could represent a rapidly migrating population. Paper presented at: Society for Neuro-Oncology Eighth Annual Meeting; November 13–16,2003; Keystone, Colo
- 47.Shweiki D, Itin A, Soffer D, et al.. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992;359:843–45 [DOI] [PubMed] [Google Scholar]
- 48.Brat DJ, Castellano-Sanchez A, Kaur B, et al. Genetic and biologic progression in astrocytomas and their relation to angiogenic dysregulation. Adv Anat Pathol 2002;9:24–36 [DOI] [PubMed] [Google Scholar]
- 49.Takano S, Yoshii Y, Kondo S, et al. Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res 1996;56:2185–90 [PubMed] [Google Scholar]
- 50.Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Genet Dev 2001;11:293–99 [DOI] [PubMed] [Google Scholar]
- 51.Folkman J, What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990;82:4–6 [DOI] [PubMed] [Google Scholar]