Abstract
SUMMARY: Intracranial solitary fibrous tumors are rare, and intraventricular fibrous tumors are even more unusual. We report a case of solitary fibrous tumor in the region of trigone and body of the left lateral ventricle and discuss the clinical presentation, CT characteristics, and histopathologic features with 1-year follow-up. We speculate that the tumor arose from the perivascular connective tissue of the choroid plexus.
Solitary fibrous tumors (SFTs) were initially described by Klemperer and Rabin1 in 1931 as a focal pleural mass. Since then, SFTs have been detected in different extracranial sites, including the mediastinum, pericardium, nasopharyngeal sinuses, liver, thyroid, mesentery, urinary bladder, prostate, orbit, and nervous system. In the central nervous system, they usually present as dura-based masses; however, parenchymal lesions have been reported in the brain and spinal cord.2
Case Report
A 55-year-old woman presented with severe headache and vomiting for 10 days. She had experienced a single episode of generalized tonic-clonic seizures with a postictal weakness on the right side that had improved by the time she was admitted. There was no language dysfunction, and there were no features of dominant parietal or temporal lobe dysfunction. She had papilledema and her visual fields were normal. There were impaired parieto-cortical sensations in the left upper and lower limbs. A CT scan of the brain showed a well-circumscribed lobulated mass in the trigone and body of the left lateral ventricle. It was 5 cm in its largest diameter. There were few foci of calcification at the periphery of the lesion with areas of hyperattenuation and hypoattenuation on the nonenhanced CT scan (Fig 1A). The lesion showed intense but heterogenous enhancement with a central nonenhancing area (Fig 1B). The ventricles were minimally enlarged with mild edema in the adjacent parenchyma. Preoperative MR imaging was not available. Our primary preoperative considerations were an intraventricular meningioma or a glioma.
Fig 1.
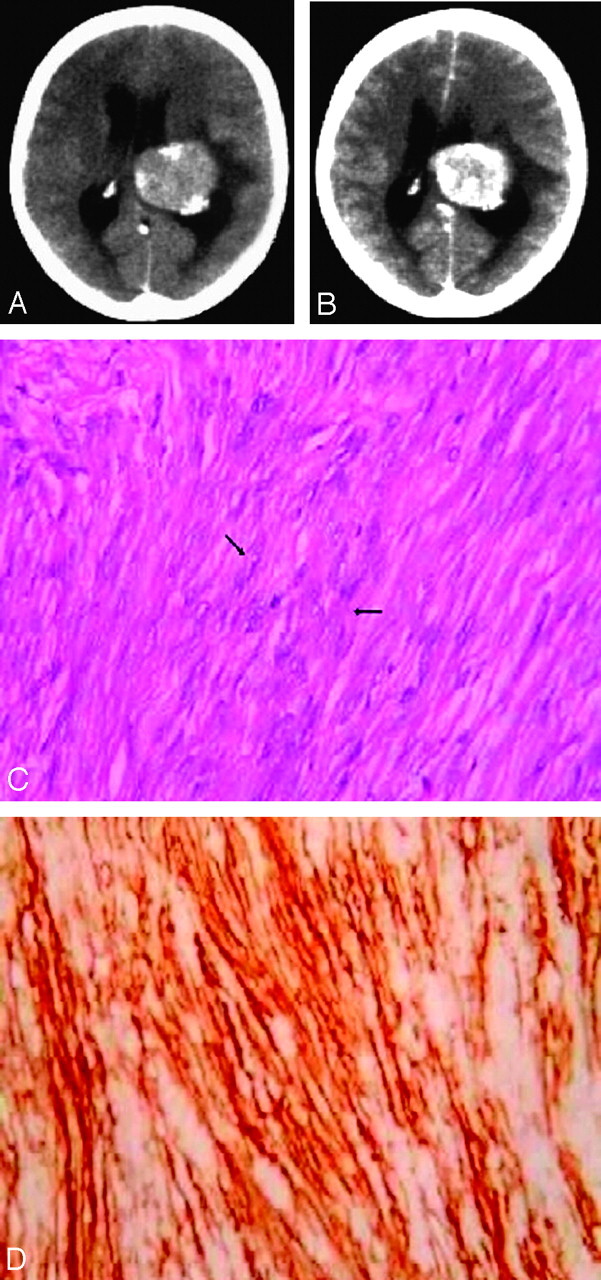
A 55-year-old woman presented with headache and vomiting with left lateral ventricular mass.
A, Axial nonenhanced CT image of the brain at the level of the atrium and body of the lateral ventricles shows a lobulated left ventricular mass with peripherally situated foci of calcification. There are focal areas of hyperattenuation and hypoattenuation in the lesion.
B, Axial contrast-enhanced CT image of the brain at the level of the body of the lateral ventricles reveals a lobulated left ventricular mass with intense but heterogenous enhancement with a central nonenhancing area.
C, Photomicrograph illustrates fascicular arrangement of spindle-shaped tumor cells (arrows) with attenuated intercellular collagen (hematoxylin-eosin, 400×).
D, Photomicrograph illustrates CD34 immunopositivity in the tumor, which is compatible with a diagnosis of solitary fibrous tumor (avidin peroxidase, 400×).
A left temporoparietal craniotomy was done and, using sonography guidance,3 a transcortical track was made to the tumor through the middle temporal gyrus. The tumor was well circumscribed, firm and fibrous, and not amenable to removal with the suction. The tumor was moderately vascular and was attached to the choroid plexus. The ultrasonic surgical aspirator was particularly useful in debulking the tumor. On histopathologic examination (Fig 1C, -D), the tumor was composed of interlacing fascicles of spindle-shaped cells set in a rich collagenous stroma. There was no evidence of nuclear atypia or anaplasia. Immunohistochemistry revealed that the tumor cells were positive for vimentin, CD34, and bcl-2 and were negative for S100 protein and epithelial membrane antigen (EMA). The MIB-1 labeling index was <2%.
In the immediate postoperative period, the patient developed a Wernicke aphasia with impaired comprehension and neologisms. The visual fields were normal. The contrast-enhanced CT of the head performed on postoperative day 7 demonstrated no residual tumor. Her language functions, though impaired at discharge, were improving. The postgadolinium T1-weighted MR imaging obtained after 1 year showed no tumor recurrence, and her comprehension had improved to normal.
Discussion
SFTs of the central nervous system are rare neoplasms but have been well characterized in the pathology literature. They usually present as dura-based masses and resemble meningiomas clinically and radiologically. These tumors can occur at any age and in most locations, regardless of proximity to the meninges, suggesting that the cells of origin are not meningothelial but the mesenchyme of the cerebral vasculature.4 The rarity of the SFT is attributed to the relative paucity of connective tissue within the central nervous system5; however, parenchymal locations have been described in the brain and spinal cord.2
On nonenhanced CT scan, SFT appears as a relatively well circumscribed, partially calcified heterogenous mass.4 It usually demonstrates variable degrees of enhancement with intravenous contrast administration. On MR imaging, the SFT is isointense with normal brain parenchyma on T1-weighted images, hyperintense on T2-weighted images, and shows intense and homogeneous enhancement after intravenous administration of gadolinium.5 Sometimes the lesion can be isointense to brain parenchyma on both T1- and T2-weighted images.6 Patchy or focal areas of hypointensity and hyperintensity on T1- and T2-weighted imaging is a notable finding.4 Rarely, T1-weighted hyperintensity is noted in a trapped ventricle as a result of elevated protein in the fluid.5 MR spectroscopy shows high peaks of lipid and lactate, but normal choline and creatine peaks with unaltered ratio.4 Cerebral angiography reveals a capillary blush in the mass with elevation of the adjacent vascular structures as a result of mass effect.6
The imaging differential diagnoses of extraventricular SFT include fibroblastic meningioma, meningeal hemangiopericytoma, neurofibroma, and schwannoma.6 Intraventricular SFTs may resemble meningioma, choroid plexus papilloma, ependymoma, and subependymal giant cell astrocytoma.5 On histologic exam, SFT can be similar to fibrous meningioma or hemangiopericytoma. On immunohistochemistry, SFTs show strong positivity for CD34, bcl-2, and vimentin and negativity for EMA and S-100.2 Fibrous meningioma characteristically express EMA and S-100 protein; CD34 reactivity is patchy and weak.7 Hemangiopericytoma show weak positivity for CD34, rarely for EMA, and is negative for S-100.7
At surgery, we found that the mass was adherent to the choroid plexus in the atrium of the left lateral ventricle with no attachment to the ependyma. This suggests that the cell of origin could be from the perivascular connective tissue of the choroid plexus. Because they are usually well circumscribed, they are amenable to a total resection.2 Until more is known about this entity, surgical removal is the preferred method of treatment.4 At a median follow-up of 40 months, no metastases or tumor-related mortalities were noted in those tumors that were completely excised.2 Recurrences after excision of SFTs are rare; however, the long-term biologic behavior is not known. Miyashita et al8 described a recurrent intracranial SFT with CSF dissemination in a patient who had a high MIB-1 count, indicating an aggressive tumor. Most SFTs are indolent in nature but extracranial metastasis to soft tissues and lungs has been described from a recurrent meningeal SFT.9 Nevertheless, if the proliferative index is low, small residual tumors can probably be followed up with surveillance imaging,4 reserving radiation therapy for those tumors with a high proliferative index or large residues in relatively inaccessible sites.
References
- 1.Klemperer P, Rabin CB. Primary neoplasms of the pleura: a report of five cases. Arch Pathol 1931;11:385–412 [DOI] [PubMed] [Google Scholar]
- 2.Tihan T, Viglione M, Rosenblum MK, et al. Solitary fibrous tumours in the central nervous system. A clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch Pathol Lab Med 2003;127:432–39 [DOI] [PubMed] [Google Scholar]
- 3.Abraham RG, Kumar NK, Chacko AG. A minimally invasive approach to deep-seated brain lesions using balloon dilatation and ultrasound guidance. Minim Invasive Neurosurg 2003;46:138–41 [DOI] [PubMed] [Google Scholar]
- 4.Kim KA, Gonzalez I, McComb JG, et al. Unusual presentations of cerebral solitary fibrous tumors: report of four cases. Neurosurgery 2004;54:1004–09 [DOI] [PubMed] [Google Scholar]
- 5.Wright DH, Naul LG, Hise JH, et al. Intraventricular fibroma: MR and pathologic comparison. AJNR Am J Neuroradiol 1993;14:491–92 [PMC free article] [PubMed] [Google Scholar]
- 6.Kocak A, Cayli SR, Sarac K, et al. Intraventricular solitary fibrous tumor: an unusual tumor with radiological, ultrastructural, and immunohistochemical evaluation: case report. Neurosurgery 2004;54:213–16 [DOI] [PubMed] [Google Scholar]
- 7.Perry A, Scheithauer BW, Nascimento AG. The immunophenotypic spectrum of meningeal hemangiopericytoma: a comparison with fibrous meningioma and solitary fibrous tumor of the meninges. Am J Surg Pathol 1997;21:1354–60 [DOI] [PubMed] [Google Scholar]
- 8.Miyashita K, Hayashi Y, Fujisawa H, et al. Recurrent intracranial solitary fibrous tumor with cerebrospinal fluid dissemination. Case report. J Neurosurg 2004;101:1045–48 [DOI] [PubMed] [Google Scholar]
- 9.Ng HK, Choi PC, Wong CW, et al. Metastatic solitary fibrous tumor of the meninges. Case report. J Neurosurg 2000;93:490–93 [DOI] [PubMed] [Google Scholar]


