Abstract
BACKGROUND AND PURPOSE: Our aim was to describe an expanded experience with endovascular mechanical embolectomy in a broad group of patients, including those not meeting entry criteria for the MERCI multicenter trials.
METHODS: We performed an analysis of all patients with ischemic stroke treated with the Merci Clot Retrieval Device at a single academic center outside of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trials.
RESULTS: Twenty-four patients were treated with the device. Nine were MERCI trial ineligible: 4 received intravenous (IV) tissue plasminogen activator (tPA), 1 received IV tPA and was younger than 18 years of age, and 4 had time-to-treatment of longer than 8 hours. Mean age was 64 years (range, 14–89 years; 42% women). Median National Institutes of Health Stroke Scale (NIHSS) score was 21 (range, 11–30). Median symptoms-to-procedure-start time was 303 minutes (range, 85–2385 minutes). Recanalization (Thrombolysis in Myocardial Infarction, 2–3) was achieved in 15/24 (63%). In device-only patients, recanalization occurred in 10/16. In patients who failed IV tPA undergoing rescue embolectomy, recanalization was achieved in 4/5. Three patients unresponsive to device therapy received rescue intra-arterial tPA/abciximab; recanalization was achieved in 2/3. Recanalization was achieved in 3/4 patients in whom treatment was started longer than 8 hours after symptom onset. Asymptomatic hemorrhage occurred in 38%; symptomatic hemorrhage, in 8%. Three device fractures occurred; none worsened clinical outcome. In-hospital mortality was 17%; 90-day mortality, 29%. Good 90-day functional outcome (modified Rankin Scale, ≤2) was achieved by 25% (6/24).
CONCLUSIONS: Endovascular mechanical embolectomy is an effective means of achieving revascularization in patients with acute ischemic stroke, including patients with late treatment start and intravenous tPA failure. Device-based therapy achieved recanalization in nearly two thirds of patients and good clinical outcomes in one fourth, with symptomatic hemorrhage in less than one tenth.
The modern era of recanalization therapy for acute ischemic stroke began in the mid-1990s with the publication of the pivotal National Institute of Neurologic Disorders and Stroke (NINDS) Tissue Plasminogen Activator (tPA) trials and subsequent US Food and Drug Administration (FDA) approval of intravenous (IV) tPA for the treatment of stroke. The NINDS–tPA trials showed that treatment within 3 hours from symptom onset benefited patients with stroke despite an increased risk of symptomatic hemorrhage.1,2 Yet, after nearly a decade of experience, only 1%–3% of patients with stroke in the United States currently receive intravenous tPA.3,4 Presentation beyond the narrow 3-hour therapeutic time window is the leading reason for treatment disqualification, indicating an urgent need to extend the time window for recanalization treatment.4,5
Endovascular mechanical interventions offer promise for the treatment of acute ischemic stroke for patients presenting beyond the 3-hour time window, as well as for patients presenting in <3 hours who are ineligible for tPA because of contraindications to systemic exposure to fibrinolytic agents and for patients who have failed to respond to IV tPA. Compared with intra-arterial fibrinolytic strategies, mechanical embolectomy offers the advantage of avoidance of fibrinolytic exposure and, therefore, potentially decreased rates of hemorrhagic transformation. The Merci Clot Retrieval Device system (Concentric Medical, Mountain View, Calif) was designed specifically for percutaneous thrombectomy in brain vasculature. The Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trials 1 and 2 demonstrated that endovascular mechanical embolectomy is technically efficacious in achieving recanalization in patients within 8 hours of stroke onset, among patients not previously exposed to fibrinolytic agents.6,7 On the basis of these findings, in August 2004, the FDA cleared the system for the indication of thrombus removal to restore blood flow in the cerebral vasculature in patients with ischemic stroke.8 The device was cleared without language explicitly specifying a restricted treatment-time window and for patients who failed IV tPA therapy, even though the MERCI trials had a maximal treatment time of 8 hours and did not include patients who were treated with IV tPA.
As an FDA-approved device, the Merci Clot Retrieval Device system is now beginning to be widely applied in a broad ischemic stroke population, including not only in patients who meet the entry criteria of the MERCI multicenter trials but also in patients who have failed to recanalize after IV tPA and patients presenting beyond 8 hours of onset. We analyzed our experience with endovascular mechanical embolectomy with the Merci Retriever in a similar wide range of patients.
Materials and Methods
Study Design
Data in a prospectively maintained interventional data base at a Comprehensive Stroke Center were analyzed. The inclusion criterion for this analysis was that the patient received embolectomy in the cerebral arteries with the Merci Clot Retrieval Device system. The study exclusion criterion was that the patient was enrolled in the MERCI 1 or MERCI 2 clinical trial. (Patients enrolled in the MERCI trials have previously been reported in the trial publications.)
Patients were treated either 1) after enrollment in an FDA-approved investigator-initiated single-center clinical trial with the aim of gathering additional data on the Merci Clot Retrieval Device system after the completion of the MERCI trials or 2) on an “off-label” basis, because the patients did not qualify for an active clinical trial and their attending neurology and interventional neuroradiology physicians felt that in their individual clinical situation, mechanical embolectomy offered the best chance of a good clinical outcome. The inclusion/exclusion criteria for the formal investigator-initiated trial conducted under an Investigational Device Exemption were nearly identical to the MERCI criteria,6,7 except that treatment was permitted in patients who had received IV tPA but failed to recanalize. Clinicians generally followed MERCI Trial entry criteria when treating patients off-label, except for pursuing treatment in IV tPA nonrecanalized patients and in patients beyond 8 hours after last known well time if multimodal MR or CT imaging suggested a relatively small established core infarct (less than one third of the middle cerebral artery field) and relatively large persisting salvageable penumbra (≥ 20% of tissue experiencing reduced cerebral blood flow of sufficient amplitude to threaten tissue integrity)9,10 Patients were excluded from treatment with the device if the they did not have a large mismatch between the core infarct and salvageable penumbra. Analysis and reporting of results was approved by the UCLA Institutional Review Board.
Demographic, clinical, and laboratory data were recorded for each patient. Outcome measures included arterial recanalization (Thrombolysis in Myocardial Infarction [TIMI] grade, 2 or 3), presence of symptomatic and asymptomatic hemorrhage, 90-day modified Rankin Scale score (mRS), in-hospital mortality, and 90-day mortality.
The Merci Retrieval System
The Merci Retrieval System (Concentric Medical) consists of the Merci Clot Retrieval Device, the Merci Balloon Guide Catheter, and the Merci Microcatheter. The Balloon Guide Catheter is 9F with a large 2.1-mm lumen and a balloon located at its distal tip. The Merci Concentric Retrieval Device is a tapered wire of memory-shaped nitinol with 5 helical loops of decreasing diameter (from 2.8 to 1.1 mm at it distal end). The Merci device is advanced through the microcatheter in its straight configuration and resumes its preimposed helical shape once it is delivered into the occluded intracranial artery to ensnare the thrombus.
Procedure
The Balloon Guide Catheter was placed in the common or internal carotid artery for anterior circulation occlusion or in the subclavian artery for posterior circulation occlusion. Using standard cerebral catheterization techniques, we guided the microcatheter into the occluded vessel and passed beyond the thrombus. A selective angiogram was obtained distal to the thrombus to evaluate the size and tortuosity of the distal arteries, where the Merci device was to be deployed. The Merci device was then advanced through the microcatheter, and 2–3 helical loops were deployed beyond the thrombus. The Merci device was then retracted at the contact of the thrombus, and the proximal loops were then deployed within the thrombus. The balloon of the guide catheter was inflated to block or reduce orthograde blood flow in the feeding vessel during removal of the thrombus, and 5 clockwise rotations were applied to the Merci device to further ensnare the thrombus. The Merci device with the ensnared thrombus and the microcatheter were withdrawn together into the Balloon Guide Catheter lumen. Continuous aspiration was applied to the Balloon Guide Catheter to promote complete evacuation of the thrombus. Once a device pass was completed, the balloon of the Balloon Guide Catheter was deflated to re-establish flow. If the occlusion persisted, then the procedure could be repeated, up to a total of no more than 6 passes of the Merci retriever. If mechanical embolectomy was unable to restore flow, then additional treatment was left to the discretion of the treating team.
All patients underwent CT immediately postprocedure. Most patients underwent MR imaging 3–12 hours postprocedure. Patients who received IV or intra-arterial lytic agents also underwent CT or MR imaging 24 hours after lytic therapy. In addition, CT or MR imaging was performed whenever a patient experienced clinical worsening. The occurrence of asymptomatic hemorrhage was defined as any non-subarachnoid blood on the 24-hour CT scan or MR image without a decline of 4 points or more in the NIHSS score. Symptomatic hemorrhage was defined as an increase in the NIHSS of 4 points or more associated with any intracranial hemorrhage.11 Intracerebral hemorrhages were also classified radiologically according to the Berger scale.12
Statistical Analysis
All comparisons were made by using the 2-tailed Fisher exact test. All analyses were performed by using SPSS for Windows, Version 12.0 (SPSS, Chicago, Ill).
Results
Between August 11, 2002, and June 24, 2004, a total of 24 patients were treated outside of the MERCI trials at our center. Of these, 11 were treated in the single-center formal clinical trial and 13 on a compassionate care off-label basis. Baseline patient characteristics for the cohort are presented in Table 1. Of the cohort, 9 patients had presenting characteristics that would have excluded them from the MERCI trials: 4 due to prior treatment with IV tPA, 1 due to both prior treatment with IV tPA and age younger than 18 years, and 4 due to time-to-treatment initiation ≥8 hours. Fifteen patients would have qualified for the MERCI trials.
Table 1:
Baseline patient characteristics
| Patient | Age | Sex | NIHSS Pretreatment | Target Vessel | Reason for Exclusion from MERCI |
|---|---|---|---|---|---|
| 1 | 32 | M | 17 | Basilar artery | Treatment >8 hours |
| 2 | 53 | M | 26 | Left carotid T | Study unavailable |
| 3 | 58 | M | 11 | Right carotid L | Treatment >8 hours |
| 4 | 58 | F | 21 | Left middle cerebral artery | Study unavailable |
| 5 | 48 | M | 21 | Left carotid L | Study unavailable |
| 6 | 73 | M | 19 | Left carotid L | Treatment >8 hours |
| 7 | 72 | F | 13 | Right middle cerebral artery | Study unavailable |
| 8 | 73 | F | 18 | Left carotid L | Study unavailable |
| 9 | 53 | F | 22 | Left middle cerebral artery | Study unavailable |
| 10 | 23 | M | 22 | Left middle cerebral artery | Study unavailable |
| 11 | 86 | F | 12 | Right middle cerebral artery | IV tPA |
| 12 | 89 | F | 27 | Left middle cerebral artery | Treatment >8 hours |
| 13 | 68 | M | 13 | Right middle cerebral artery | Study unavailable |
| 14 | 39 | M | 15 | Left middle cerebral artery | IV tPA |
| 15 | 72 | M | 22 | Left middle cerebral artery | Study unavailable |
| 16 | 83 | M | 30 | Left carotid L | IV tPA |
| 17 | 32 | F | 17 | Right middle cerebral artery | IV tPA |
| 18 | 73 | F | 17 | Right middle cerebral artery | Study unavailable |
| 19 | 69 | M | 25 | Right carotid T | Study unavailable |
| 20 | 77 | M | 25 | Left middle cerebral artery | Study unavailable |
| 21 | 71 | M | 25 | Left carotid L | Study unavailable |
| 22 | 14 | M | 11 | Right middle cerebral artery | IV tPA and <18 years |
| 23 | 87 | F | 28 | Left carotid T | Study unavailable |
| 24 | 87 | F | 27 | Left middle cerebral artery | Study unavailable |
Note:—Carotid T indicates distal carotid artery, ipsilateral anterior cerebral artery, and ipsilateral middle cerebral artery; carotid L, distal carotid artery and ipsilateral middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; M, male; F, female; tPA, tissue plasminogen activator.
The mean age for the cohort was 64 years (range, 14–89 years), and 42% were women. Pretreatment median NIHSS score was 21 (range, 11–30). Among the patients pretreated with IV tPA, median pretreatment NIHSS was 17 (range, 11–30). Among patients treated beyond 8 hours, median pretreatment NIHSS was 18 (range, 11–27). Target clot sites were the internal carotid artery,9 middle cerebral artery,13 and basilar artery.1 Median time from last known well time to procedure start was 303 minutes (range, 85–2385 minutes). Among the 5 patients treated after IV tPA, the first pass of the Merci device occurred at a median of 177 minutes (range, 85–405 minutes) after the start of IV fibrinolysis. In the 4 patients treated beyond 8 hours, the first device pass occurred at 480, 550, 1365, and 2385 minutes after last known well time.
For the entire cohort, at the end of the mechanical embolectomy procedure, partial recanalization (TIMI, 2) was achieved in 29% (7/24), and complete recanalization, in 25% (6/24) with an average of 3 passes with the device. Three patients received rescue intra–arterial pharmacologic therapy, including intra-arterial tPA in 2 and intra-arterial abciximab in 1, after failing to recanalize with only mechanical clot retrieval. Two of the 3 patients who received chemical therapy after mechanical intervention had partial recanalization of their target vessels. Accordingly, at the end of all endovascular therapies, 9 patients had achieved partial and 6 patients, complete recanalization. Of the patients treated solely with the Merci device, without pretreatment IV or posttreatment intra-arterial lytic or platelet disaggregating agents, recanalization occurred in 63% (10/16). Among the 5 patients receiving rescue mechanical embolectomy with the Merci device after failing to recanalize with IV tPA, partial recanalization was achieved in zero and complete in 3. Recanalization was achieved in 75% (3/4) of patients in whom mechanical embolectomy treatment was started more than 8 hours from symptom onset. Recanalization by target vessel location was the following: internal carotid artery, 33%; middle cerebral artery, 60%; and basilar artery, 100%.
Fracture of the coil and detachment of the tip of the Merci device occurred in 3 patients. Interviews with the investigators indicated that the fractures occurred from over-torquing the device. The training program and instructions for use were revised, and design modifications were made to increase the strength of the device. In 2, the detached tip was successfully captured and removed from the vasculature by deployment of an additional Merci device. None of the 3 patients achieved recanalization of the target vessel. There was no involvement of additional vascular territories or clinical worsening associated with detachment of the device or with the failure to retrieve the Merci device tip. There were 2 cases of procedure-related subarachnoid hemorrhage, and no cases of procedure-related ischemia in a new vascular territory.
Across the cohort, asymptomatic hemorrhage occurred in 38% (9/24) (Table 2). Asymptomatic hemorrhages occurred in 2/3 (67%) patients treated with the Merci device followed by rescue chemical thrombolysis, 2/5 (40%) patients treated with IV fibrinolysis followed by rescue with the Merci device, and 5/16 (31%) of patients treated with the Merci device alone. Symptomatic hemorrhage occurred in 2 patients (8.3%), both of whom received mechanical embolectomy with the Merci device as rescue treatment after failure to recanalize with IV tPA. Parenchymal hematomas type 2 occurred in 2 patients, both of whom received rescue mechanical embolectomy after failure to recanalize with IV tPA. No patient experienced ischemia in a previously uninvolved vascular territory or vascular dissection as a consequence of the embolectomy procedure.
Table 2:
Patient treatment variables and outcomes
| Patient | Target Vessel | Time to Treatment (min) | Additional Intervention | Posttreatment TIMI | Hemorrhage | Day 90 mRS |
|---|---|---|---|---|---|---|
| 1 | Basilar artery | 2385 | None | II | None | 5 |
| 2 | Left carotid T | 85 | None | III | None | 3 |
| 3 | Right carotid L | 550 | None | 0 | Asymptomatic SAH | 4 |
| 4 | Left middle cerebral artery | 285 | IA tPA | I | Asymptomatic ICH | 2 |
| 5 | Left carotid L | 255 | None | I | None | 4 |
| 6 | Left carotid L | 1365 | None | III | None | 6 |
| 7 | Right middle cerebral artery | 320 | IA Abciximab | II | None | 0 |
| 8 | Left carotid L | 310 | None | 0 | None | 5 |
| 9 | Left middle cerebral artery | 320 | None | II | None | 1 |
| 10 | Left middle cerebral artery | 225 | None | 0 | None | 3 |
| 11 | Right middle cerebral artery | 190 | IA tPA | II | Asymptomatic ICH | 2 |
| 12 | Left middle cerebral artery | 480 | None | III | Asymptomatic ICH | 6 |
| 13 | Right middle cerebral artery | 330 | None | II | None | 0 |
| 14 | Left middle cerebral artery | 220 | (IV tPA) | I | Asymptomatic ICH | 3 |
| 15 | Left middle cerebral artery | 415 | None | II | Asymptomatic SAH | 6 |
| 16 | Left carotid L | 320 | (IV tPA) | III | Symptomatic ICH | 6 |
| 17 | Right middle cerebral artery | 355 | (IV tPA) | III | Asymptomatic ICH | 0 |
| 18 | Right middle cerebral artery | 390 | None | II | None | 4 |
| 19 | Right carotid T | 330 | None | II | Asymptomatic ICH | 4 |
| 20 | Left middle cerebral artery | 290 | (IV tPA) | I | None | 4 |
| 21 | Left carotid L | 135 | None | 0 | None | 6 |
| 22 | Right middle cerebral artery | 120 | (IV tPA) | II | Symptomatic ICH | 3 |
| 23 | Left carotid T | 160 | None | II | Asymptomatic SAH | 6 |
| 24 | Left middle cerebral artery | 295 | None | 0 | None | 6 |
Note:—TIMI indicates Thrombolysis in Myocardial Infarction; mRS, modified Rankin scale; carotid T, distal carotid artery, ipsilateral anterior cerebral artery, and ipsilateral middle cerebral artery; carotid L, distal carotid artery and ipsilateral middle cerebral artery; IA, intra-arterial; tPA, tissue plasminogen activator; SAH, subarachnoid hemorrhage; ICM, intracerebral hemorrhage.
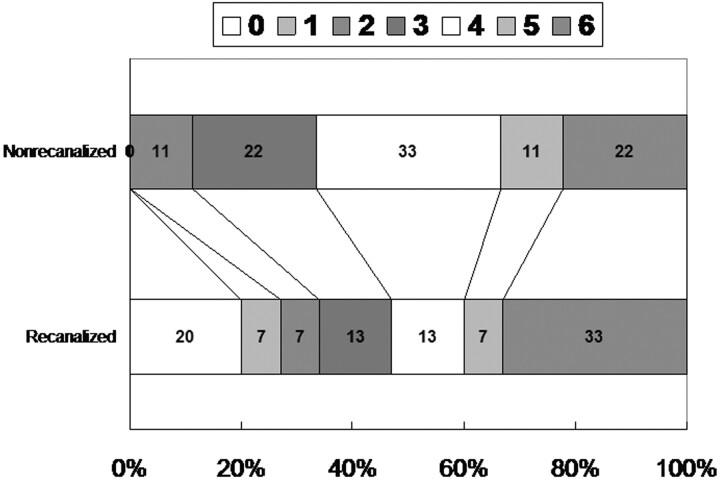
Overall, good day 90 functional outcome (mRS, 0–2) was achieved by 6 patients (25%). Figure 1 shows the Rankin outcomes for patients experiencing and not experiencing recanalization at the conclusion of mechanical and chemical endovascular interventions. Good functional outcome (mRS, 0–2) was achieved in 5 recanalized patients and 1 nonrecanalized patient (33% versus 11%; P = .351). Four patients who received mechanical embolectomy plus an IV or intra-arterial chemical recanalization therapy achieved favorable day 90 outcome, whereas only 1 patient who received only mechanical embolectomy did (50% versus 12%; P = .129).
Fig 1.
Day 90 mRS scores in the recanalized and nonrecanalized patients.
In-hospital mortality rate was 17%, and day 90 mortality rate was 29%. Day 90 mortality did not differ among recanalized patients (5/15) versus nonrecanalized patients (2/9) (P = .669).
Discussion
In addition to 15 patients who would have qualified for the MERCI trial, this series reports patients treated beyond the 8-hour MERCI study window and treated with fibrinolytic and platelet disaggregating therapies preceding treatment with mechanical embolectomy.
Overall the experience with mechanical embolectomy was comparable to that in the multicenter MERCI trials. Patients in this series achieved recanalization rates similar to, if not better than, those reported in the MERCI trials (63% versus 48%, P = .27). As in the MERCI trials, patient neurologic deficits before treatment were very severe (median NIHSS, 21; mean NIHSS, 20.2 versus MERCI, 20.1); nonetheless, nondisabled functional outcomes at 3 months were achieved in 24%, a rate comparable to that in MERCI (28%). The symptomatic hemorrhage rate was 0% in patients treated with the Merci device alone (versus 8% in MERCI, P = .59) and 0% in patients treated with rescue intra-arterial lytics or platelet disaggregates after failed mechanical embolectomy (versus 8% in MERCI, P > .5). Subarachnoid hemorrhage due to vessel rupture was assessed with a postprocedure diagnostic angiogram and a postprocedure head CT scan. Unlike in MERCI, no patient experienced vessel dissection or ischemia in a previously uninvolved territory. Ninety-day mortality was lower in this series than in the MERCI trial (29% versus 43.5%, P = .25).
Many patients present beyond the 8-hour time window that was studied in the MERCI trials. Positron-emission tomography and multimodal MR imaging studies have shown that a substantial proportion of patients will still exhibit regions of penumbral tissue up to 24 hours or longer after symptom onset.13–15 There are currently no approved therapies for treatment of these patients. Because of the concern that these patients may have an elevated risk of hemorrhagic transformation, mechanical embolectomy without exposure to intra-arterial fibrinolytics is an attractive option in the late interventional setting. In this series, 4 patients were treated beyond the 8-hour time window. No symptomatic bleeding occurred in these patients; asymptomatic bleeding occurred in 2/4. Recanalization was achieved in 3/4. However, no patient achieved a nondisabled final functional outcome. Given the late treatment start, we believe that the poor functional outcome in these patients is likely due to progression of infarction despite recanalization, reperfusion injury, or a combination of both.16 It is not yet clear if the potential benefit of embolectomy in acute stroke beyond 8 hours from the time of onset outweighs the risks.
Although treatment with IV tPA was an exclusion criterion in the MERCI trials, the FDA cleared the Merci Concentric retriever device for use in patients with ischemic stroke who had failed to recanalize with IV tPA, in addition to patients who are not IV tPA candidates. The experience in this series suggests both hope and caution when considering mechanical embolectomy after IV tPA. Recanalization rates in failed IV tPA patients were good (80%), and nondisabled functional outcomes were achieved in 20%. However, all cases of symptomatic hemorrhage in this series occurred in patients who received IV tPA before treatment with the Merci device. Combined therapy with IV tPA and the Merci device is likely to carry a greater risk of hemorrhagic transformation than treatment with either technique alone.
Conclusions
Our findings confirm the results of the MERCI trials. The Merci device is technically effective in clearing cerebral thrombi and restoring flow in the neurovasculature, both in patients meeting MERCI trial entry criteria and also in patients pretreated with IV tPA and patients presenting more than 8 hours after last known well time. Recanalization with the Merci device in this series was not associated with improved clinical outcome. The inability to detect an association between recanalization and improved clinical outcome in this series may be due to the small number of patients and/or worse outcome in patients treated beyond 8 hours from symptom onset. Randomized clinical trials, such as the ongoing MR RESCUE Trial, are needed to demonstrate definitively that endovascular mechanical embolectomy improves final patient outcome from stroke.
Footnotes
This work was supported in part by grants K24 NS 02092 (J.L.S.) and P50 NS044378 (J.L.S., C.S.K., S. Selco, R.J., D.K.), from NIH-NINDS. S. Starkman received research grants from Concentric Medical, Inc. G.R.D. is a medical advisor and holds stocks in Concentric Medical, Inc.
References
- 1.Tissue plasminogen activator for acute ischemic stroke: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–87 [DOI] [PubMed] [Google Scholar]
- 2.Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol 2004;61:1066–70. Erratum in: Arch Neurol 2004;61:1599 [DOI] [PubMed] [Google Scholar]
- 3.Nilasena DS, Kresowik TF, Wiblin RT, et al. Assessing patterns of tPA use in acute stroke (abstract). Stroke 2002;33:354 [Google Scholar]
- 4.Broderick JP, Lu M, Kothari R, et al. Finding the most powerful measures of the effectiveness of tissue plasminogen activator in the NINDS tPA stroke trial. Stroke 2000;31:2335–41 [DOI] [PubMed] [Google Scholar]
- 5.Furlan A. CVA: reducing the risk of a confused vascular analysis. The Feinberg lecture. Stroke 2000;31:1451–56 [DOI] [PubMed] [Google Scholar]
- 6.Gobin YP, Starkman S, Duckwiler G, et al. MERCI 1: a phase 1 study of mechanical embolus removal in cerebral ischemia. Stroke 2004;35:2848–54. Epub 2004 Oct 28 [DOI] [PubMed] [Google Scholar]
- 7.Smith WS, Sung G, Starkman S, et al, and the MERCI Trial Investigators. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 8.Felten RP, Ogden NRP, Pena C, et al. The Food and Drug Administration medical device review process: clearance of a clot retriever for use in ischemic stroke. Stroke 2005;36:404–06 [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke 2003;34:2729–35 [DOI] [PubMed] [Google Scholar]
- 10.Kidwell C, Starkman S, Jahan R, et al. Pretreatment MRI penumbral pattern predicts good clinical outcome following mechanical embolectomy. Stroke 2004;35:A294 [Google Scholar]
- 11.Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: The PROACT II trial. Neurology 2001;57:1603–10 [DOI] [PubMed] [Google Scholar]
- 12.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke 2001;32:1330–35 [DOI] [PubMed] [Google Scholar]
- 13.Marchal G, Beaudouin V, Rioux P, et al. Prolonged persistence of substantial volumes of potentially viable brain tissue after stroke: a correlative PET-CT study with voxel-based data analysis. Stroke 1996;27:599–606 [DOI] [PubMed] [Google Scholar]
- 14.Kidwell C, Alger J, Saver J. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke 2004;35:2662–65 [DOI] [PubMed] [Google Scholar]
- 15.Neumann-Haefelin T, Wittsack HJ, Wenserski F, et al. Diffusion- and perfusion-weighted MRI: the DWI/PWI mismatch region in acute stroke. Stroke 1999;30:1591–97 [DOI] [PubMed] [Google Scholar]
- 16.Schaller B, Graf R. Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab 2004;25:351–71 [DOI] [PubMed] [Google Scholar]