Abstract
BACKGROUND AND PURPOSE:There is mounting evidence of extratemporal volume changes associated with medically refractory temporal lobe epilepsy (TLE). This MR imaging study aimed to characterize volume changes in subcortical structures and cerebellar hemispheres with respect to lateralization of the seizure focus, onset and duration of epilepsy, and frequency of generalized tonic-clonic seizures (GTCS).
METHODS:Amygdalar, hippocampal, thalamic, caudate head, and cerebellar volume measurements were obtained in the preoperative MR images of 40 patients with TLE (20 right, 20 left), who underwent temporal lobe resection with good outcome, and in 20 right-handed control participants. All 3D MR images were spatially aligned and normalized before measurements were obtained. Standardized volumes and right-to-left volume ratios (VRs) were compared between control participants and right and left TLE groups. Multiple regression analyses were performed to study the effects of epilepsy onset and duration and GTCS frequency on ipsilateral-to-contralateral VRs with respect to the resected seizure focus.
RESULTS:Thalamic volumes were smaller bilaterally in patients with TLE. Hippocampal volumes were smaller ipsilateral to the seizure focus, but there was no significant volume loss involving the amygdala, caudate, or cerebellum. Hippocampal and amygdalar right-to-left VRs differed significantly between right and left TLE groups and controls, whereas thalamic right-to-left VRs differed only between the TLE groups. Thalamic ipsilateral-to-contralateral VRs were correlated positively with epilepsy onset and negatively with epilepsy duration. Caudate ipsilateral-to-contralateral VRs were positively, whereas amygdalar and cerebellar VRs were negatively, correlated with GTCS frequency.
CONCLUSIONS:Unilateral amygdalar and bilateral thalamic volume loss, in the absence of caudate head atrophy, is likely to reflect seizure-induced injury due to TLE. Correlations of VRs affecting the amygdala, caudate, and cerebellum with GTCS frequency may also reflect injury or a prediposition for secondary generalization. Potential effects of complex partial seizures, febrile seizures, or antiepileptic medications on subcortical structures need to be evaluated in future studies.
Medically refractory temporal lobe epilepsy (TLE) is associated with volume changes in the hippocampus ipsilateral to the seizure onset zone. It is still unclear to what extent the volume loss is related to a pre-existent injury or the result of recurrent seizures. Several cross-sectional volumetric studies showed that hippocampal volume loss is related to the duration of epilepsy.1–4 Asymmetries of structures functionally connected to the hippocampus were also demonstrated by MR imaging volumetry, including the amygdala,4–7 entorhinal cortex,8 fornix,7,9 mamillary bodies,7,10 and thalamus.11–13 Amygdalar atrophy ipsilateral to the temporal lobe seizure focus was associated with a prolonged duration of epilepsy.4 Thalamic volume reductions were found unilaterally11,13 or bilaterally,12 but there was no clear correlation of these changes to epilepsy onset, epilepsy duration, or seizure severity. TLE is also associated with volume changes in structures without direct functional connections to the hippocampus, such as the striatum11,12 and cerebellum.14–15 Cerebellar volume reductions were reported in a small percentage of children and adults with TLE, and these changes were correlated with age of onset or duration only in adults.
The studies evaluating extrahippocampal volume changes in TLE varied with respect to landmarks of the measured structures, resolution of scans, and intra- or inter-rater reliability. Furthermore, few studies reported volumetric data restricted to patients with prior temporal lobe resections.9,10,12,15 This is important because epilepsy surgery with a successful, ideally seizure-free, outcome is the gold standard for the localization of the epileptic zone. Finally, studies varied on whether the researchers adjusted volumetric measurements for total cerebral brain volume. This is not trivial because whole-brain size depends on age, sex, congenital insults with developmental delay, and even chronic epilepsy. To exclude the effect of whole-brain size, some study authors scaled their measurements to total brain volume,4–7,11,12,14,15 whereas others performed measurements directly in stereotaxic space.8,13 The latter method allows adjustment for differences in brain orientation, in addition to total brain volume, and facilitates identification of boundaries of the structures of interest by reducing variability in section orientation. Although this adjustment is performed in 3D, it also entails a nonlinear distortion of cerebral structures. In our study, volumetric measurements were performed for preoperative MR images that were stereotactically normalized to Talairach space. Only patients with TLE and good surgical outcome were included.
The primary objective of this study was to compare volume changes in subcortical structures and the cerebellar hemispheres in patients with medically refractory left or right TLE and controls. To better understand the effects of seizures on subcortical networks, we obtained volumetric measurements for limbic structures functionally connected to the hippocampus, such as the amygdala or thalamus, and for extralimbic structures indirectly connected to the hippocampus, including the caudate head and cerebellum. Volume changes were expected in the limbic structures ipsilateral to the seizure onset zone and not in extralimbic structures. The second objective was to correlate volume changes and asymmetries of these structures with specific historic factors, such as the age at onset of epilepsy, epilepsy duration, and frequency of generalized tonic-clonic seizures (GTCS).
Methods
Patients.
Forty patients who subsequently underwent epilepsy surgery, 20 with right TLE and 20 with left TLE, were included in this study. These patients were drawn from a group of 70 consecutive patients who underwent temporal lobectomy at the University of Texas Health Science Center at San Antonio. Patients were included who had undergone 3D high-resolution MR imaging and who had a greater than 90% seizure reduction at 2-year follow-up. Patients who had arteriovenous malformations, dual pathology by video electroencephalography (EEG) or structural neuroimaging, previous epilepsy surgery, or epileptogenic structural lesions extending beyond the temporal lobe were excluded. Infectious etiologies were only included if the patients had unilateral hippocampal atrophy on MR imaging. The demographics of the 40 patients are listed in Table 1.
Table 1:
Demographics of control participants and patients with epilepsy
| Control Participants (n = 20) | RTLE (n = 20) | LTLE (n = 20) | |
|---|---|---|---|
| Handedness (right-handed) | 20 (100) | 20 (100) | 18 (90) |
| Sex (male) | 9 (45) | 10 (50) | 9 (45) |
| Age at onset (years) | NA | 14 ± 13 | 13 ± 15 |
| Duration of epilepsy (years) | NA | 20 ± 11 | 23 ± 10 |
| GTCS | NA | 11 (55) | 12 (60) |
| GTCS frequency (per year) | NA | 11 ± 15 | 14 ± 20 |
| MRI Findings | |||
| HA | None | 17 (85) | 13 (65) |
| Nonlesional, no HA | None | 2 (10) | 4 (20) |
| Extrahippocampal lesions | None | 1 (5) | 3 (15) |
| Etiology | |||
| Unknown | NA | 9 | 7 |
| Meningitis, abscess | NA | 5 | 4 |
| Neoplasm | NA | 1 | 2 |
| Cortical dysplasia | NA | 4 | 3 |
| Trauma, infarct | NA | 1 | 3 |
| Cavernous hemangioma | NA | None | 1 |
Note:—TLE indicates temporal lobe epilepsy/resection; GTCS, generalized tonic-clonic seizures; HA, hippocampal atrophy; NA, not applicable. Values (in parentheses are percentages.
Data regarding clinical history and results of the presurgical evaluation were obtained from a chart review. All of the patients failed between 2 and 7 (mean, 5) antiepileptic medications, and all except 1 patient were exposed to phenytoin. GTCS frequency was estimated for a period of 12 months before surgery. Complex partial seizure (CPS) frequency was not quantified because CPS is difficult to differentiate from simple partial seizure (SPS) in some patients and tends to be underreported by patients because of the lack of injury or physical sequelae. All 40 patients underwent surface video-EEG monitoring and neuropsychologic testing. Patients and witnesses can more reliably keep count of GTCS. All patients underwent the intracarotid amobarbital procedure, whereas 39 underwent functional neuroimaging, including single-photon emission tomography or positron- emission tomography.
Control Participants.
MR images of 20 right-handed (11 women, 9 men) individuals were randomly selected from a data base of 170 healthy subjects.16 All participants had completed a 10-item handedness questionnaire, with questions drawn from the Edinburgh Laterality Inventory and Reitan Handedness Scale.17
MR Imaging.
In this study, volumetric measurements were performed of the amygdala, hippocampus, caudate head, thalamus, and cerebellum on 3D high-resolution MR images within 1 year of epilepsy surgery. Spatial normalization entails the coregistration of individual 3D brain scans with the Talairach brain.18 The individual brains were first spatially aligned with the stereotaxic brain and then scaled anisotropically in the 3 principal axes. The measurements were performed on spatially normalized datasets to remove brain size effects mediated by age and sex. Manual volumetric assessment was chosen because automated voxel-based morphometry did not reliably render the amygdala and thalamus, structures with less-defined borders.
Image Acquisition and Processing.
The MR imaging sequence used for volumetric analysis was a 3D T1-weighted gradient-echo acquisition of the whole brain (TR/TE, 24/6; flip angle, 25°; field of view, 256 × 256 mm; matrix, 256 × 192). The right-sided marker, visible in all scans, was placed caudally from the subject’s right ear. Pixels were 1.0 × 1.0 mm and section width, 1 mm without interleave gap. All volumes were painted with a 1.0-mm3 resolution cursor on an SGI workstation by using Display (Montreal Neurological Institute, Canada), which allowed simultaneous visualization of the structures in 3 planes. Thresholds were selected for each structure to optimize gray-white contrast, and the same thresholds were used for all patients with epilepsy and controls. The boundaries of the amygdalar, hippocampal, thalamic, caudate, and cerebellar measurements were described previously.16,17 Caudate measurements included the caudate head (ie, the volume bounded posteriorly by the anterior commissure). Cerebellar measurements excluded the cerebellar peduncles. Examples of measurements are provided in Fig 1. Normal ranges and intra- and inter-rater reliability for volumetric measurements of these structures in control participants were published previously.16,17
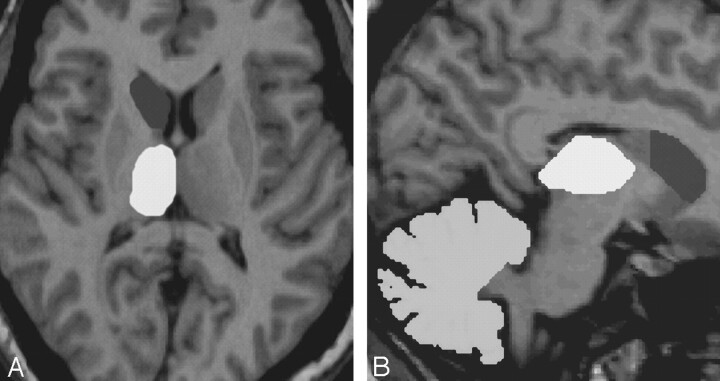
Fig 1.
MR imaging volumetry in a patient with right TLE. Axial view of the right caudate head and thalamus (A) and sagittal image of the right caudate head, thalamus, and cerebellar hemisphere (B).
Statistical Analysis.
The standardized volumes of structures were compared by 2-tailed t tests corrected for multiple comparisons (α < 0.05). Volume ratios reduced the effect of methodologic error introduced by spatial normalization and enhanced our ability to detect more subtle changes or structural asymmetries and their correlations with historic factors. The hippocampal, amygdalar, thalamic, caudate, and cerebellar right-to-left volume ratios (VRs) were compared for controls and right and left TLE groups by using an analysis of variance (ANOVA) test with a 3 × 5 factorial design. The TLE groups were then merged, and VRs were converted to ipsilateral-to-contralateral VRs, ipsilateral referring to the side of the temporal lobe seizure focus. Amygdalar, thalamic, caudate head, and cerebellar ipsilateral-to-contralateral VRs were compared between patients with hippocampal atrophy ipsilateral to the seizure focus (n = 30) and those without hippocampal atrophy (n = 10) by using an ANOVA test with a 2 × 4 factorial design. Atrophy of the hippocampus and other structures was defined as volumes over 2 SDs smaller than the control mean.
The distribution of ipsilateral-to-contralateral VRs for hippocampal, amygdalar, thalamic, caudate head, and cerebellar hemispheres, as well as epilepsy onset, epilepsy duration, and frequency of GTCS were examined for normality by using the Shapiro-Wilk test. Amygdalar and hippocampal ipsilateral-to-contralateral VRs, epilepsy onset, and GTCS frequency were non-normally distributed. First-order correlations were performed for the ipsilateral-to-contralateral VRs of each structure with epilepsy onset, epilepsy duration, and GTCS frequency. The Pearson coefficient correlation was used for normally distributed measurements, and the Spearman coefficient correlation was used for non-normally distributed measurements. Multiple regression analysis was performed to find the associations between VRs of the different structures and the onset of epilepsy, duration of epilepsy, and frequency of GTCS. A logarithmic transformation was performed for epilepsy onset, GTCS frequency, and amygdalar and hippocampal volumes to satisfy the assumption of normality for regression analysis. The coefficient of multiple determination indicated that 21% of variability was explained for epilepsy onset, 38% of variability was explained for epilepsy duration, and 67% of variability was explained for GTCS frequency by using this model. All correlation analyses were performed with SAS Version 8.2 (SAS Institute, Cary, NC).
Results
The means and SDs for the standardized volumes and right-to-left VRs are listed for the control participants and right and left TLE groups in Table 2.
Table 2:
Volumetric data of control participants and patients with temporal lobe epilepsy
| Structures | Subjects |
||
|---|---|---|---|
| Control Participants | Right TLE | Left TLE | |
| Hippocampus | |||
| Mean R volume | 3011 (408) | 2101 (506)* | 3078 (501) |
| Mean L volume | 2857 (409) | 2943 (510) | 2238 (729)* |
| R/L volume ratio | 1.06 (0.05)‡ | 0.73 (0.22)‡ | 1.47 (0.38)‡ |
| Amygdala | |||
| Mean R volume | 1608 (167) | 1533 (248) | 1667 (237) |
| Mean L volume | 1497 (186) | 1610 (185) | 1440 (400) |
| R/L volume ratio | 1.08 (0.06)‡ | 0.95 (0.16)‡ | 1.24 (0.41)‡ |
| Thalamus | |||
| Mean R volume | 6360 (445) | 5217 (797)* | 5811 (1056)‡ |
| Mean L volume | 6484 (575) | 5716 (862)* | 5361 (884)† |
| R/L volume ratio | 0.98 (0.07) | 0.91 (0.09)‡ | 1.09 (0.11)‡ |
| Caudate head | |||
| Mean R volume | 3263 (445) | 3236 (362) | 3248 (440) |
| Mean L volume | 3297 (394) | 3382 (449) | 3271 (514) |
| R/L volume ratio | 0.99 (0.06) | 0.96 (0.05) | 0.99 (0.06) |
| Cerebellar hemispheres | |||
| Mean R volume | 71451 (8589) | 66890 (9465) | 66756 (8907) |
| Mean L volume | 72210 (8347) | 68093 (9164) | 68134 (9258) |
| R/L volume ratio | 0.99 (0.03) | 0.98 (0.03) | 0.99 (0.03) |
Note:—Volumes expressed as mm3, ( ) indicate standard deviations, R indicates right; L, left; R/L, right divided by left; TLE, temporal lobe epilepsy/resection.
Statistically significant difference between the patient groups and control subjects (p < .0001).
Statistically significant difference between one patient group and control subjects (p < .02).
Statistically significant difference between 2 or 3 groups (p < .05).
Standardized Volumes.
The mean volume of the right-sided hippocampus was smaller in right TLE patients than for control participants (P < .0001), whereas the same left-sided structures were smaller for left TLE patients than for control participants (P < .0001). Thalamic volumes were significantly smaller bilaterally for the right (both thalami, P < .0001) and left (right thalamus, P < .02; left thalamus, P < .0001) TLE groups than for controls. Cerebellar volumes were bilaterally smaller in patients with TLE, but this difference was not statistically significant. Caudate head volumes did not differ among the 3 groups. Of 30 patients with hippocampal atrophy, only 7 (23%) had ipsilateral amygdalar atrophy. Twenty-four of 40 (60%) patients had thalamic atrophy, 13 bilateral, and 11 only ipsilateral to the seizure onset zone. Only 3 (8%) patients had bilateral cerebellar atrophy, all 3 of whom had bilateral thalamic atrophy as well. Only 1 patient had caudate head atrophy ipsilateral to the seizure onset zone. Standardized amygdalar, hippocampal, thalamic, caudate, or cerebellar volumes were not correlated with epilepsy onset, epilepsy duration, or GTCS frequency.
Right-to-Left VRs.
An interaction of condition and structure was detected between control participants and the TLE groups only with respect to right-to-left hippocampal and amygdalar VRs. Thalamic VRs differed between the 2 TLE groups only (P < .05).
Ipsilateral-to-Contralateral Volume.
Comparison between patients with and without hippocampal atrophy ipsilateral to the seizure onset zone demonstrated a significant difference for amygdalar VRs (P < .0001). No significant difference was found for thalamic, caudate, or cerebellar VRs.
The results of the multiple regression analysis seeking associations between ipsilateral-to-contralateral VRs of each structure and the historic factors are shown in Table 3. Only thalamic ipsilateral-to-contralateral VRs were positively correlated with epilepsy onset and negatively correlated with epilepsy duration. GTCS frequency was positively correlated with caudate head ipsilateral-to-contralateral VRs and negatively correlated with amygdalar and cerebellar ipsilateral-to-contralateral VRs.
Table 3:
Multiple regression analysis for I/C volume ratios with historical factors
| Model | Onset R2 = 0.21 |
Duration R2 = 0.38 |
GTCS R2 = 0.67 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | T | P | β | SE | T | P | β | SE | T | P | |
| Intercept | 0.71 | 7.12 | 0.10 | 0.92 | 139.7 | 66.10 | 2.11 | 0.04 | 24.69 | 8.41 | 2.93 | 0.01 |
| Hippocampus | 0.30 | 2.08 | 0.15 | 0.88 | −13.07 | 19.38 | −0.67 | 0.50 | 2.17 | 2.45 | 0.88 | 0.39 |
| Amygdala | −4.01 | 2.60 | −1.54 | 0.13 | −9.55 | 24.18 | −0.39 | 0.69 | −12.02 | 4.10 | −2.93 | 0.01* |
| Thalamus | 5.98 | 2.33 | 2.56 | 0.01* | −62.93 | 21.69 | −2.90 | 0.007* | −1.50 | 3.17 | −0.47 | 0.64 |
| Caudate | −1.90 | 3.06 | −0.62 | 0.53 | 1.44 | 28.48 | 0.05 | 0.96 | 7.72 | 3.54 | 2.18 | 0.04* |
| Cerebellum | 0.16 | 5.84 | 0.03 | 0.97 | −46.93 | 54.24 | −0.87 | 0.39 | −23.00 | 7.30 | −3.15 | 0.008* |
Note:—GTCS indicates generalized tonic-clonic seizures. All results are expressed as coefficients (β), standard error (SE), T-statistic (T), and P value (P).
Statistical significance.
Discussion
Several studies used MR imaging volumetric measurements to demonstrate volume loss in cortical and subcortical structures functionally associated with an atrophied hippocampus in patients with medically refractory TLE. The affected structures included the amygdala,4–7 entorhinal cortex,8 fornix,7,9 mamillary bodies,7,10 and thalamus.11–13 Volume loss in extralimbic basal ganglia and cerebellum was reported less consistently. This study evaluated volume change in subcortical structures, including the hippocampus, amygdala, thalamus, caudate head, and cerebellum in spatially standardized MR images of patients with TLE who subsequently had a good outcome from epilepsy surgery. Volumes of individual structures and right-to-left VRs were compared between right and left TLE patients and controls. Ipsilateral-to-contralateral VRs were compared between patients with and without hippocampal atrophy. Finally, correlations of ipsilateral-to-contralateral VRs and standardized volumes were examined with historic factors, including epilepsy onset, epilepsy duration, and GTCS frequency.
Amygdala.
Amygdalar volumes were not significantly atrophied in either right or left TLE groups compared with controls. Only 7 patients had significant amygdalar atrophy, all of whom had hippocampal atrophy. Nonetheless, the right-to-left ratios differed significantly for right and left temporal lobectomy groups, even compared with controls. Surprisingly, in contrast to previous studies,1–4,6 neither hippocampal nor amygdalar volumes were correlated with the onset or duration of epilepsy. The absence of these correlations may represent an artifact of the nonlinear distortion associated with spatial normalization, though not even the VRs, which are not affected by standardization, were correlated with these factors. It remains unclear from these data, whether amygdalar injury is due to a pre-existent injury involving the medial temporal lobe, an early insult due to prolonged febrile or afebrile seizures, or chronic injury due to ictal propagation from the ipsilateral hippocampus. On the other hand, amygdalar asymmetry, with volume loss ipsilateral to the seizure onset zone, was correlated with GTCS frequency. Whether amygdalar atrophy predisposed patients to secondary generalization late in the course of their disease or was a result of injury due to GTCS is unclear. In either case, because of amygdalar connections to bilateral frontal lobes and basal ganglia, the amygdala may serve as a staging ground for secondary generalization in TLE.
Thalamus.
Evidence of bilateral thalamic atrophy was surprising in the setting of focal epilepsy, even though volume loss was greater ipsilateral to the seizure onset zone. Thalamic asymmetry was substantial enough to differentiate patients with left and right temporal lobe epilepsies, and in a larger sample, the ipsilateral thalamus may have been significantly smaller than the contralateral thalamus. Volume loss was reported only ipsilateral to the seizure onset zone by 2 studies,11,13 neither of which were performed in patients who had undergone surgery. The patients in this series, who all underwent epilepsy surgery, may have had more severe epilepsy and seizure-induced volume loss. The only other surgical series also demonstrated bilateral thalamic volume loss, but this study did not use stereotactic space.12 In addition to thalamic atrophy, the authors of that study also found bilateral caudate and pallidal atrophy, suggesting the possibility of diffuse cerebral volume reduction in some patients.
The only study using stereotaxic space for the measurements was able to correlate ipsilateral thalamic volume loss with epilepsy duration and showed that there was a significant association of smaller thalamic volumes with prolonged febrile seizures.13 The association of bilateral thalamic atrophy with febrile seizures was confirmed by a second study as well.12 Although ipsilateral thalamic volumes were not correlated with onset or duration in this study, asymmetry of the thalami did show a significant association with onset and duration. The association of an earlier onset may reflect a pre-existent injury in the ipsilateral hemisphere or a remote acute injury inflicted by febrile or afebrile status epilepticus. Bilateral thalamic atrophy was reproduced in a rodent model of generalized status epilepticus, the injury specifically localized to the mediodorsal nuclei.19 Whereas a pre-existent global or hemispheric injury may have affected some patients in this study, the absence of caudate head volume loss would suggest that any such damage would have been limited to the limbic system. The pattern of bilateral thalamic injury, more severe ipsilaterally, was also reported in association with unilateral hippocampal kindling in mice.20
The mechanism underlying bilateral thalamic volume loss in the setting of focal epilepsy needs to be better understood. Bilateral thalamic atrophy cannot be explained by the deafferentation of the thalamic nuclei due to unilateral hippocampal sclerosis. In this study, there was no significant difference between thalamic volumes or VRs between patients with or without hippocampal atrophy. Hence, seizure propagation from the medial temporal lobe through other pathways seems likely. Anatomic studies in the macaque demonstrated that the main thalamic projections from the medial temporal lobe arose from the subiculum and entorhinal cortex, and not from the hippocampus or amygdala.21 The subicular cortex projects through the fornix to the anterior thalamic nuclei and, to a lesser extent, to the rostral midline sites. Few projections from the entorhinal cortex travel through the fornix but use the inferior thalamic peduncle to project to the bilateral mediodorsal thalamic nuclei. Some projections from the subiculum or entorhinal cortex to the lateral dorsal nuclei and pulvinar remain ipsilateral. Higher resolution MR imaging may be required to demonstrate volume changes of specific thalamic nuclei. The pattern of atrophy in the thalamus may vary depending on the hippocampal or extrahippocampal location of the medial temporal lobe seizure-onset zone.
Caudate Head/Cerebellar Hemispheres.
No volume reduction was noted in the caudate head in medically refractory TLE. The projections from the medial temporal lobe to the caudate head are limited, and these are mainly indirect. Caudate atrophy is described by some studies,11,12 but these studies included the caudate body and tail in their measurements, parts of the caudate that are more vulnerable to seizure propagation due to their anatomic proximity and functional connections to the medial temporal lobe. The absence of volume change in the caudate head is an important finding because it helps to control for bilateral cerebral or hemispheric injuries that may have predated the onset of epilepsy, causing volume loss in all subcortical structures.
Cerebellar atrophy has been described in the setting of localization-related epilepsy in several studies.14,15,22 In TLE, cerebellar volume reduction affects both cerebellar hemispheres. Sandok et al15 reported that cerebellar volumes are significantly reduced in a subset of patients with TLE. In this study, bilateral volume reduction was identified, but this difference was not significant. Larger numbers of patients may have been needed to achieve statistical significance. Furthermore, in the process of standardization, the cerebellar volumes were passively adjusted to total brain volume; this adjustment may have excessively magnified cerebellar volumes in patients with diffuse cerebral atrophy.
It is unclear whether the cerebellar atrophy predates the onset of the epilepsy, is due to febrile or afebrile status epilepticus at an early age, is related to frequent GTCS, or is caused by phenytoin toxicity in some patients. De Marcos et al22 showed that cerebellar volume reduction has been associated with the duration of epilepsy and the years of phenytoin treatment. In this study, cerebellar volume reduction was not correlated with onset or duration of epilepsy or GTCS frequency. However, a surprising correlation of caudate and cerebellar asymmetries with GTCS frequency emerged, suggesting relative volume reduction in the contralateral caudate head and ipsilateral cerebellum. If this correlation had affected solely 1 of 2 structures, this finding may be brushed aside as an artifact. However, volume reduction in both structures probably reflects involvement of the contralateral cortical-subcortical-cerebellar network due to propagation of the ictal discharge during secondary generalization. Serial MR imaging in stereotaxic space may help to elucidate the progression of these changes.
Other Factors That May Contribute to Volume Loss.
This study did not address several factors that may affect volumes of subcortical structures, such as antiepileptic medications, CPS frequency, and febrile seizures. Phenytoin is known to cause volume loss in the cerebellum after chronic administration.22 However, in this study, the number of years of phenytoin exposure and phenytoin levels were not available to evaluate potential effects on cerebellar volume. Although other antiepileptic medications may cause hypoperfusion or hypometabolism in the cortex or cerebellum,23 their effect on the basal ganglia volumes is unknown.
The effects of CPS were also not taken into consideration. Although the number of CPSs experienced by each patient appears to be associated with decreased hippocampal volume, such a study is difficult to replicate. Because patients are amnesic for their seizures, especially in the absence of any injury, or may not be able to differentiate SPS from CPS, it is difficult to quantify them over the long-term. The only prospective study looking at the effects of seizures on hippocampal volumes by performing serial MR imaging in patients with TLE was able to correlate GTCS, and not CPS, frequency with progression of hippocampal atrophy.24
Finally, this study was not able to identify patients with febrile seizures with certainty. Accurate histories of febrile seizures could not be verified by medical records in each patient. In several cases, in which medical records were available, it was not clear whether the patients had a prolonged febrile seizure or viral meningoencephalitis. From previous studies, it is apparent that patients with febrile seizures may have particular patterns of subcortical volume loss, but these findings need to be confirmed in larger cohorts.12,13
Future Directions.
MR imaging volumetry is a labor-intensive method of volumetric measurement and is not easily replicated or compared across various centers. In the past few years, voxel-based morphometry evolved as a promising and reliable replacement for MR volumetry. Voxel-based morphometry is an automated image-analysis technique allowing detection of regional differences in gray matter structures between groups of subjects. Recent studies have analyzed either gray matter concentration25,26 or gray matter volumetry,26,27 enabling quantification of gray matter attenuation and volume in cortex as well as in subcortical structures. These studies used stereotaxic space for group comparisons, and recent developments in image-processing software led to a reduction of nonlinear distortion due to spatial normalization. The pattern of decreased gray matter concentration or volume varies among studies. Most consistently, there is evidence of bilateral thalamic abnormalities. Furthermore, the cerebellar hemispheres are also bilaterally affected. However, these new imaging techniques have hardly been used to evaluate the effect of historical factors. These techniques have neither been tested serially to detect whether the gray matter attenuation or volumes actually decrease with time, nor have they been applied to a surgical population, in which the seizure onset zone is adequately localized.
Conclusion
MR imaging volumetry detected decreases in volumes of structures functionally connected to the hippocampus, such as the amygdala and thalamus and, to a far lesser degree, in more remotely connected structures, such as the cerebellar hemispheres. Thalamic volume loss was bilateral, a finding that was recently validated by several voxel-based morphometry studies.25–27 Despite bilateral thalamic volume loss, the asymmetry between the thalami was strongly correlated with the onset and duration of epilepsy. These findings reflect either a remote acute injury in the setting of status epilepticus or chronic injury due to seizure propagation. In either case, the sparing of the caudate head implicates seizure-induced injury to the limbic structures and cerebellum, rather than the result of a remote, global, or hemispheric structural or developmental abnormality that predated the onset of the epilepsy. The correlation of amygdalar VRs with GTCS frequency may reflect the seizure-induced injury or a predisposition for secondary generalization, whereas the correlation of caudate and cerebellar VRs with GTCS is likely to represent injury due to propagation of the ictal discharge to the contralateral hemisphere. Prospective serial MR imaging needs to be performed in spatially standardized datasets to detect volume changes. The use of voxel-based morphometry will help to pool larger numbers of well-selected patients to detect subtle changes in cortical and subcortical gray matter densities and volumes associated with the progression of epilepsy.
Footnotes
This work was supported by the EJLB Foundation and the National Institute of Mental Health (P20 DA52176) and an Internal Review Grant through UTHSCSA (00–0021). Presented at: 10th Annual Meeting of the Organization for Human Brain Mapping, June 13–17, 2004, Budapest, Hungary.
References
- 1.Trenerry MRI, Jack CR, Sharbrough FW, et al. Quantitative MRI hippocampal volumes: association with onset and duration of epilepsy, and febrile convulsions in temporal lobectomy patients. Epilepsy Res 1993;15:247–52 [DOI] [PubMed] [Google Scholar]
- 2.O’Brien TJ, So EL, Meyer FB, et al. Progressive hippocampal atrophy in chronic intractable temporal lobe epilepsy. Ann Neurol 1999;45:526–29 [PubMed] [Google Scholar]
- 3.Theodore WH, Bhatia S, Hatta J, et al. Hippocampal atrophy, epilepsy duration and febrile seizures in patients with partial seizures. Neurology 1999;52:132–36 [DOI] [PubMed] [Google Scholar]
- 4.Salmenperä T, Kälviäinen R, Partanen K, et al. Hippocampal and amygdaloid damage in partial epilepsy: a cross-sectional MRI study of 241 patients. Epilepsy Res 2001;46:69–82 [DOI] [PubMed] [Google Scholar]
- 5.Cendes F, Andermann F, Gloor P, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology 1993;43:719–25 [DOI] [PubMed] [Google Scholar]
- 6.Kälviäinen R, Salmenperä T, Partanen K, et al. MRI volumetry and T2 relaxometry of the amygdala in newly diagnosed and chronic temporal lobe epilepsy. Epilepsy Res 1997;28:39–50 [DOI] [PubMed] [Google Scholar]
- 7.Bilir E, Craven W, Hugg J, et al. Volumetric MRI of the limbic system: anatomical determinants. Neuroradiology 1998;40:138–44 [DOI] [PubMed] [Google Scholar]
- 8.Bernasconi N, Bernasconi A, Andermann F, et al. Entorhinal cortex in temporal lobe epilepsy: a quantitative MRI study. Neurology 1999;52:1870–76 [DOI] [PubMed] [Google Scholar]
- 9.Marmourian AC, Cho CH, Saykin AJ, et al. Association between the size of the lateral ventricle and asymmetry of the fornix in patients with temporal lobe epilepsy. AJNR Am J Neuroradiol 1998;19:9–13 [PMC free article] [PubMed] [Google Scholar]
- 10.Marmourian AC, Rodichok L, Towfighi J. The asymmetric mamillary body: association with medical temporal lobe disease demonstrated with MRI. AJNR Am J Neuroradiol 1995;16:517–22 [PMC free article] [PubMed] [Google Scholar]
- 11.DeCarli C, Hatta J, Fazilat S, et al. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol 1998;43:41–45 [DOI] [PubMed] [Google Scholar]
- 12.Dreifuss S, Vingerhoets FJG, Lazeyras F, et al. Volumetric measurements of sub-cortical nuclei in patients with temporal lobe epilepsy. Neurology 2001;57:1636–41 [DOI] [PubMed] [Google Scholar]
- 13.Natsume J, Bernasconi N, Andermann F, et al. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology 2003;60:1296–300 [DOI] [PubMed] [Google Scholar]
- 14.Lawson JA, Vogrin S, Bleasel AF, et al. Cerebral and cerebellar volume reduction in children with intractable epilepsy. Epilepsia 2000;41:1456–62 [DOI] [PubMed] [Google Scholar]
- 15.Sandok EK, O’Brien TJ, Jack CR, et al.. Significance of cerebellar atrophy in intractable temporal lobe epilepsy: a quantitative MRI study. Epilepsia 2000;41:1315–20 [DOI] [PubMed] [Google Scholar]
- 16.Szabo CA, Xiong J, Lancaster JL, et al. Amygdalar and hippocampal volumetry in control participants: differences regarding handedness. AJNR Am J Neuroradiol 2001;22:1342–45 [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo CA, Xiong J, Lancaster JL, et al. MRI volumetry of subcortical structures and cerebellum in normal individuals. AJNR Am J Neuroradiol 2003;24:644–47 [PMC free article] [PubMed] [Google Scholar]
- 18.Lancaster JL, Glass TG, Lankipalli BR, et al. A modality independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapp 1995;3:209–23 [Google Scholar]
- 19.Kubova H, Druga R, Lukasiuk K, et al. Status epilepticus causes necrotic damage in the mediodorsal nucleus of the thalamus in immature rats. J Neurosci 2001;21:3593–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertram EH, Mangan PS, Zhang D, et al. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia 2001;42:967–78 [DOI] [PubMed] [Google Scholar]
- 21.Aggleton JP, Desimone R, Mishkin M. The origin, course, and termination of the hippocampothalamic projections in the macaque. J Comp Neurol 1986;243:409–21 [DOI] [PubMed] [Google Scholar]
- 22.De Marcos FA, Ghizoni E, Kobayashi E, et al. Cerebellar volume and long-term use of phenytoin. Seizure 2003;12:312–15 [DOI] [PubMed] [Google Scholar]
- 23.Sechi G, Casu AR, Rosati G, et al. Cerebral and cerebellar diaschisis following carbamazepine therapy. Prog Neuropsychopharmacol Biol Psychiatry 1995;19:889–901 [DOI] [PubMed] [Google Scholar]
- 24.Briellman RS, Berkovic SF, Syngeniotis A, et al. Seizure-associated hippocampal volume loss: a longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol 2002;51:641–44 [DOI] [PubMed] [Google Scholar]
- 25.Bonilha L, Rorden C, Castellano G, et al. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol 2004;61:1379–84 [DOI] [PubMed] [Google Scholar]
- 26.Keller SS, Wilke M, Wieshmann UC, et al. Comparison of standard and optimized voxel-based morphometry for analysis of brain changes associated with temporal lobe epilepsy. NeuroImage 2004;23:860–68 [DOI] [PubMed] [Google Scholar]
- 27.Bernasconi N, Duchesne S, Janke A, et al. Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. NeuroImage 2004;23:717–23 [DOI] [PubMed] [Google Scholar]