Abstract
SUMMARY: In this study, we used a multiparametric MR imaging approach to assess a patient with Hirayama disease (HD). We found that cervical cord damage extends beyond cord T2-visible lesions. We also showed an altered pattern of cortical activations during movements of clinically unaffected limbs. Whereas this study suggests a more widespread cord involvement in HD than seen on routine MR imaging, its cause remains unclear (vascular damage versus a primary lower motor neuron disease).
Hirayama disease (HD) is a sporadic juvenile muscular atrophy of the distal upper limbs, which affects predominantly young men and is characterized by insidious unilateral or bilateral muscular atrophy and weakness of the hands and forearms, without sensory or pyramidal signs. HD clinical course is initially progressive, followed by spontaneous stabilization within several years after onset.1 The pathogenesis of the disease is unclear and pathologic studies are lacking. Routine MR imaging of patients with HD has shown segmental cord atrophy, rarely associated with T2 abnormalities.1–4 Previous studies of other cord diseases have shown that the extent of cord tissue damage is usually underestimated by using routine MR imaging and that brain plasticity might limit the clinical consequences of cord injury.5 In this study, we used a multiparametric MR imaging approach to investigate, in a patient with HD, the overall extent of cervical cord damage and the pattern of brain activations during simple movements of the dominant upper limb.
Case Report
This 50-year-old right-handed man was admitted to our hospital for the presence of severe atrophy and weakness of the distal upper extremities. Family history was negative for neuromuscular disorders. Symptoms began when he was 13 years old with a progressive bilateral muscle weakness and atrophy of the forearms and hands, which stabilized after 6 years. Cold paresis was also reported. Neurologic examination showed a marked bilateral atrophy of intrinsic hand and forearm muscles, more pronounced on the left side, with sparing of all other muscles, including the bulbar ones. No pyramidal and sensory signs or bladder dysfunction was observed. Results of routine blood analysis were normal. Neurophysiologic examinations showed the following findings: 1) bilateral severe chronic neurogenic changes in C7-C8-T1 myotomes, 2) unrecordable left ulnar nerve conduction velocity (NCV) (with normal motor and sensory NCV of the other limb nerves), and 3) no somatosensory- and motor-evoked central conduction abnormalities (4 limbs). No significant changes of motor-evoked potential and somatosensory evoked potential parameters were measured during neck flexion.
At this stage, the following MR imaging sequences were performed in the patient and a group of 15 age-matched controls: Cord— 1) axial, coronal, and sagittal dual-echo fast spin-echo (FSE); 2) sagittal T1-weighted SE; 3) axial 2-dimensional gradient-echo (GE) with and without a saturation pulse to calculate the magnetization transfer ratio; and 4) sagittal pulsed-gradient diffusion-weighted sensitivity encoded single-shot echo-planar to calculate mean diffusivity and fractional anisotropy; Brain—1) dual-echo FSE, 2) 3D T1-weighted magnetization-prepared rapid acquisition of gradient echo (MPRAGE), and 3) T2-weighted single-shot echo-planar imaging during the performance of a simple motor task consisting of flexion-extension of the last 4 fingers of the right hand. A detailed description of these sequences is given elsewhere.6 Motor functional assessment was performed at the time of MR imaging acquisition by using the 9-hole peg test (9HPT) and maximal finger-tapping frequency. The time to complete the 9HPT and the finger tapping rate of the patient did not differ from that of controls.
Magnetization transfer ratio, mean diffusivity (MD), and fractional anisotropy histograms of the whole cervical cord were derived, as described previously.6 For each histogram, the average magnetization transfer ratio, mean diffusivity, and fractional anisotropy were measured. Functional MR imaging (fMRI) analysis was performed by using statistical parametric mapping software (SPM99; available at: http://www.fil.ion.ac.uk/spm).7 MPRAGE images from each subject were coregistered with the corresponding fMRI datasets and normalized into SPM standard space. Then, fMRI results were superimposed on these high-resolution normalized images. No abnormalities were seen on brain routine MR images. Sagittal and coronal spinal T2-weighted images showed bilateral hyperintense lesions, more evident on the left side, extending from C4 to C7. Such signal intensity abnormalities were located in the anterior horns on axial dual-echo scans. At the same level, T1-weighted scans showed hypointense signal intensity and cord atrophy (Fig 1). Average cervical cord magnetization transfer ratio and fractional anisotropy were 2 SDs below, and average MD was 2 SDs above the corresponding mean values of controls (Table). During the performance of the motor task, the patient showed a brain pattern of activations that involved the classic sensorimotor network, including the primary sensorimotor cortex, secondary sensorimotor cortex, supplementary motor area, cerebellum, insula, and prefrontal areas. A second-level random effect analysis was performed to assess the differences between the patient and healthy subjects in the movement-associated brain pattern of cortical activations. The patient with HD had increased activations of the ipsilateral primary sensorimotor cortex, contralateral secondary sensorimotor cortex, and supplementary motor area (Fig 2).
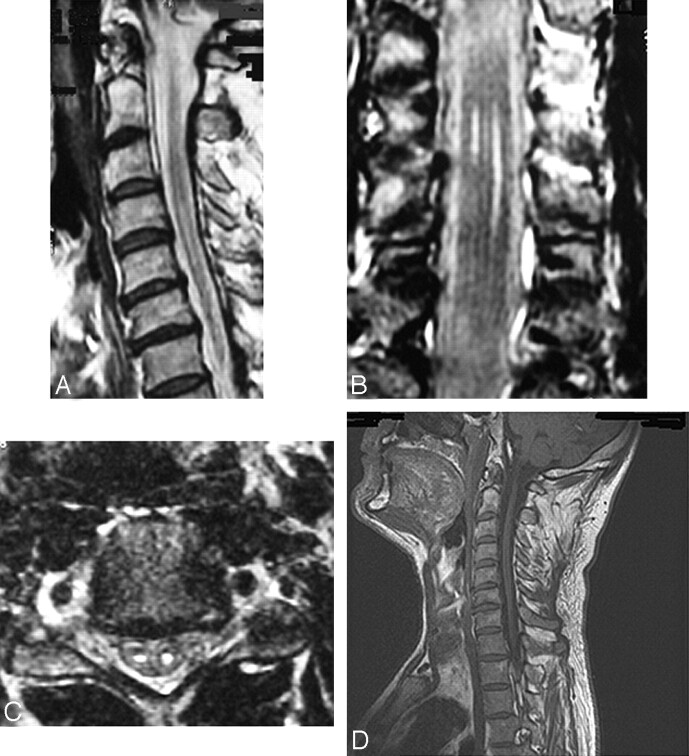
Fig 1.
Sagittal (A), coronal (B), and axial (C) T2-weighted and sagittal T1-weighted (D) cervical cord images from a patient with HD. T2-weighted images show the presence of bilateral abnormalities, more evident on the left side, extending from C4 to C7. On the T1-weighted scans, these lesions appear as hypointense; atrophy of the cord is also seen.
Cervical spinal cord MTR, MD, and FA histogram-derived metrics from a patient with Hirayama disease and age-matched healthy control subjects
| Control Subjects Mean (SD) | Normality Ranges (mean ± SD) | HD Patient | |
|---|---|---|---|
| Average MTR (%) | 35.3 (1.2) | 32.9–37.7 | 30.2 |
| Average MD (×10−3 mm2s−1) | 1.22 (0.08) | 1.06–1.38 | 1.44 |
| Average FA | 0.43 (0.03) | 0.37–0.49 | 0.32 |
Note:—MTR indicates magnetization transfer ratio; MD, mean diffusivity; FA, fractional anisotropy; HD, Hirayama disease.
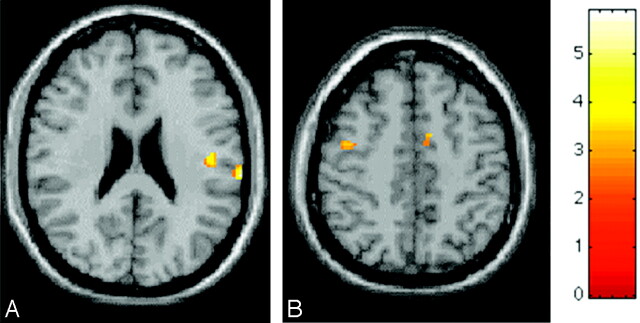
Fig 2.
Random effect between-group analysis (corrected P values <0.05) showing, on a high-resolution T1-weighted image in the standard SPM space, regions of relative increased cortical activations (color-coded for t values) in a patient with HD during the performance of a simple motor task with the right hand, in comparison with healthy volunteers. A, Contralateral secondary sensorimotor cortex. B, Ipsilateral primary sensorimotor cortex and supplementary motor area. The increased activation of these regions in a patient with HD might be due to a perceived complexity/novelty of the experimental simple task, as a consequence of cord injury. Such cortical reorganization might contribute to maintaining a normal level of upper limb function in this patient.
Discussion
In agreement with previous studies of patients with a stabilized HD,1–3 routine MR imaging showed bilateral T2-weighted signal intensity abnormalities located in the anterior cervical horns, associated with regional atrophy. These abnormalities appeared as hypointense on T1-weighted images. This finding was consistent with pathologic data8 showing loss of large and small neurons and gliosis in the cervical anterior horns. These data also suggest that HD might be secondary to microvascular changes following chronic trauma to the spinal cord during flexion and extension of the neck.9 The novelty of this study lies in the fact that magnetization transfer and diffusion-weighted MR imaging histograms of the cervical cord suggested that cord damage in HD extends beyond that seen on routine MR imaging scans (this is because the relatively limited portion of the cord that appears abnormal on routine MR imaging is unlikely to explain the dramatic changes that were observed for all the 3 histograms of the cervical cord). This again supports the ischemic theory of HD, because a microcirculatory disturbance in the anterior spinal artery territory might lead to damage to the corticospinal tracts because of their strategic location in the border zone. However, the extremely long duration of the disease of our patient (37 years) and the lack of involvement of other central nervous system areas do not allow ruling out an anterior horn cell degenerative disease as the underlying cause of the observed changes.
Because the patient performed well on behavioral tests of the upper dominant limb, this study also provides evidence for significant cortical reorganization. The notion that the presence and extent of spinal cord damage might modulate brain activity has been suggested by previous studies of patients with different spinal cord diseases.6,10 The correlation found in some of these studies between the severity of cord damage and the extent of cortical functional changes suggests that these functional changes might have an adaptive role in limiting the clinical consequences of structural cord damage.6,10 During the performance of the motor task, the patient had increased activations of the ipsilateral primary sensorimotor cortex, contralateral secondary sensorimotor cortex, and supplementary motor area, which might be due to a perceived complexity/novelty of the experimental simple task, as a result of cord injury. The most tempting speculation for this finding is to suggest a mild injury to corticospinal tracts (as suggested by magnetization transfer and diffusion tensor MR imaging data), which remains clinically silent thanks to the brain cortical reorganization.
References
- 1.Hirayama K. Non-progressive juvenile spinal muscular atrophy of the distal upper limb (Hirayama’s disease). In: De Jong JMBV, ed. Handbook of Clinical Neurology. Vol 15. Amsterdam: Elsevier;1991. :107–20
- 2.Pradhan S, Gupta RK. Magnetic resonance imaging in juvenile asymmetric segmental spinal muscular atrophy. J Neurol Sci 1997;146:133–38 [DOI] [PubMed] [Google Scholar]
- 3.Schröder R, Keller E, Flacke S, et al. MRI findings in Hirayama’s disease: flexion-induced cervical myelopathy or intrinsic motor neuron disease? J Neurol 1999;246:1069–74 [DOI] [PubMed] [Google Scholar]
- 4.Misra UK, Kalita J, Mishra VN, et al. A clinical, magnetic resonance imaging, and survival motor neuron gene deletion study of Hirayama Disease. Arch Neurol 2005;62:120–23 [DOI] [PubMed] [Google Scholar]
- 5.Rocca MA, Hickman SJ, Bo L, et al. Imaging spinal cord in multiple sclerosis. J Neuroimaging 2005;15:297–304 [DOI] [PubMed] [Google Scholar]
- 6.Rocca MA, Mezzapesa DM, Ghezzi A, et al. Cord damage elicits brain functional reorganization after a single episode of myelitis. Neurology 2003;61:1078–85 [DOI] [PubMed] [Google Scholar]
- 7.Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time-series revisited. NeuroImage 1995;2:45–53 [DOI] [PubMed] [Google Scholar]
- 8.Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology 2000;54:1922–26 [DOI] [PubMed] [Google Scholar]
- 9.Hirayama K, Tomonaga M, Kitano K, et al. Focal cervical poliopathy causing juvenile muscular atrophy of distal upper extremity: a pathological study. J Neurol Neurosurg Psychiatry 1987;50:285–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filippi M, Rocca MA. Cortical reorganisation in patients with MS. J Neurol Neurosurg Psychiatry 2004;75:1087–89 [DOI] [PMC free article] [PubMed] [Google Scholar]