Abstract
Background
Recent studies have identified susceptibility genes of HBV clearance, chronic hepatitis B, liver cirrhosis, hepatocellular carcinoma, and showed the host genetic factors play an important role in these HBV-related outcomes.
Methods
Collected samples from different outcomes of HBV infection and performed genotyping by Affymetrix 500 k SNP Array. GCTA tool, PLINK, and Bonferroni method were applied for analysis of genotyping and disease progression. ANOVA was used to evaluate the significance of the association between biomarkers and genotypes in healthy controls. PoMo, FST, Vcftools and Rehh package were used for building the racial tree and population analysis. FST statistics accesses 0.15 was used as a threshold to detect the signature of selection.
Results
There are 1031 participants passed quality control from 1104 participants, including 275 HBV clearance, 92 asymptomatic persistence infection (ASPI), 93 chronic hepatitis B (CHB), 188 HBV-related decompensated cirrhosis (DC), 214 HBV-related hepatocellular carcinoma (HCC) and 169 healthy controls (HC). In the case–control study, one novel locus significantly associated with CHB (SNP: rs1264473, Gene: GRHL2, P = 1.57 × 10−6) and HCC (SNP: rs2833856, Gene: EVA1C, P = 1.62 × 10−6; SNP: rs4661093, Gene: ETV3, P = 2.26 × 10−6). In the trend study across progressive stages post HBV infection, one novel locus (SNP: rs1537862, Gene: LACE1, P = 1.85 × 10−6), and three MHC loci (HLA-DRB1, HLA-DPB1, HLA-DPA2) showed significant increased progressive risk from ASPI to CHB. Underlying the evolutionary study of HBV-related genes in public database, the derived allele of two HBV clearance related loci, rs3077 and rs9277542, are under strong selection in European population.
Conclusions
In this study, we identified several novel candidate genes associated with individual HBV infectious outcomes, progressive stages, and liver enzymes. Two SNPs that show selective significance (HLA-DPA1, HLA-DPB1) in non-East Asian (European, American, South Asian) versus East Asian, indicating that host genetic factors contribute to the ethnic disparities of susceptibility of HBV infection. Taken together, these findings provided a new insight into the role of host genetic factors in HBV related outcomes and progression.
Supplementary information
The online version contains supplementary material available at 10.1186/s12920-021-00907-0.
Keywords: HBV infection, Disease progression, GWAS, Host genetic factors, SNPs
Background
Hepatitis B virus (HBV) infection is one of the most common infectious diseases, with about 248 million HBsAg positive individuals worldwide and the largest HBsAg positive population in China [1]. HBV infection can develop a wide spectrum of liver diseases, including chronic hepatitis B, liver cirrhosis, hepatocellular carcinoma [2–4]. Previous studies showed the host genetic factor played a critical role in HBV infection susceptibility and identified associated SNPs with significant contribution, including major histocompatibility complex (MHC) genes, i.e. HLA-DPA1 (rs3077), HLA-DPB1 (rs9277535), HLA-C (rs3130542), HLA-DQ (rs2856718, rs7453920) [5–7], and non-MHC genes, i.e. UBE2L3 (rs4821116), INTS10 (rs7000921) [8, 9]. In advanced stages of HBV disease, host genetic factors influence the outcome of HBV infection [7, 10, 11], including HLA-DQ (rs9275319), HLA-DRB1 (rs2647073, rs3997872), STAT4 (rs7574865), C2 (rs9267673), PNPLA3 (rs738408, rs738409), SLC17A2 (rs80215559), HFE (rs1800562) [12, 13] for liver cirrhosis and KIF1B (rs17401966), HLA-DQA1/DRB1 (rs9272105), HLA-DQ (rs9275319), STAT4 (rs7574865) for hepatocellular carcinoma [14–16]. However, these reported HBV-related genes confer relatively small increments in risk and explain a small proportion of heritability. For example, although MHC genes are important for immune response to HBsAg, more than half the heritability is determined by non-MHC genes [17]. Moreover, previous studies showed that the MHC genes share a common influence on HBV infection, liver cirrhosis, hepatocellular carcinoma [6, 12, 15, 16] as well as associate with different risk in these outcomes [18]; i.e. HLA-DQ, STAT4, C2, HLA-DRB1 for liver cirrhosis and HCC [12], HLA-DQ for CHB [6]. These consistent [12] or different [18] risks indicated shared but also modified effects for progressive HBV-related outcomes. These results raised our interest to identify host genetic factor which increases the risk of progressive stages post HBV infection. To reveal new susceptibility genes for HBV infection and the HBV-related outcomes, we performed a genome-wide association study (GWAS) in 1031 participants, including 275 HBV clearance subjects, 92 asymptomatic persistence infection carriers (ASPI), 93 chronic hepatitis B patients (CHB), 188 HBV-related decompensated cirrhosis patients (DC), 214 HBV-related hepatocellular carcinoma patients (HCC) and 169 healthy controls (HC) (Table 1).
Table 1.
Characteristics of participants in the genome-wide association cohorts
| Disease categories | HBV clearance | ASPI | CHB | DC | HCC | HC |
|---|---|---|---|---|---|---|
| Sample size | 275 | 92 | 93 | 188 | 214 | 169 |
| Mean age ± SD | 49.56 ± 8.8 | 46.89 ± 6.9 * | 46.46 ± 5.7 ** | 50.65 ± 8.4 | 51.34 ± 10.5 * | 48.82 ± 7.1 |
| Male/female | 105/170 | 33/59 | 62/31 *** | 148/40 *** | 183/31 *** | 73/96 |
| ALT, U/L, mean ± SD | 26.95 ± 30.42 | 24.5 ± 8.79 | 169.53 ± 243.76 *** | 89.57 ± 115.79 *** | 214 ± 381.67 ** | 23.11 ± 7.97 |
| AST, U/L, mean ± SD | 25.89 ± 21.89 * | 23.16 ± 6.45 | 100.04 ± 127.54 *** | 102.46 ± 118.36 *** | 120.51 ± 286.05 *** | 22.23 ± 7.00 |
| TBiL, μmol/L, mean ± SD | 13.68 ± 9.93 | 12.28 ± 2.91 | 23.12 ± 35.60 *** | 65.87 ± 83.22 *** | 50.11 ± 90.93 *** | 12.95 ± 4.09 |
| DBiL, μmol/L, mean ± SD | 5.86 ± 18.39 | 3.59 ± 1.68 | 9.36 ± 14.29 ** | 35.15 ± 51.33 *** | 25.87 ± 55.04 *** | 4.54 ± 9.46 |
| ALP, U/L, mean ± SD | 74.30 ± 35.86 | 73.57 ± 44.58 | 108.89 ± 42.20 *** | 132.22 ± 61.42 *** | 141.63 ± 104.32 *** | 70.85 ± 25.33 |
| GGT, U/L, mean ± SD | 28.34 ± 30.77 | 29.88 ± 21.19 | 88.76 ± 106.63 *** | 89.94 ± 132.10 *** | 158.08 ± 183.92 *** | 27.58 ± 24.88 |
| ALB, g/L, mean ± SD | 43.33 ± 6.11 | 44.16 ± 6.71 | 42.40 ± 42.25 * | 32.28 ± 7.13 ** | 37.19 ± 7.27 *** | 43.93 ± 4.96 |
| AFP, μg/L, mean ± SD | 21.39 ± 47.39 * | 5.16 ± 4.79 | 34.00 ± 89.37 * | 87.74 ± 169.83 ** | 7315.22 ± 37,329.94 | 3.76 ± 4.34 |
| PTA, %, mean ± SD | 95.22 ± 52.69 | 91.67 ± 14.30 | 90.06 ± 21.76 | 63.97 ± 28.07 *** | 87.31 ± 33.96 | 90.84 ± 18.71 |
| PLT, 109/L, mean ± SD | 147.69 ± 55.40 | 146.84 ± 44.51 | 150.27 ± 49.61 | 73.96 ± 45.30 *** | 145.72 ± 79.24 | 148.94 ± 49.10 |
Abbreviations: ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; HC, healthy controls; SD, standard deviation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; ALP, alkaline phosphatase; GGT, glutamyl transpeptidase
Notes: *, statistical significance of the difference between each case group and HC group (***: p = [0, 0.001], **: p = (0.001, 0.01], *: p = (0.01, 0.05]). The significances of gender and other characteristics were calculated by Fisher's exact test and ANOVA test, respectively
Methods
Study participants
A total of 1104 unrelated, age- and gender- matched, Chinese participants were recruited in the study, enrollment criteria were consistent with a previous report [19]. The population of HBV-related phenotypes was composed of five subgroups: HBV clearance subjects, asymptomatic persistence infection (ASPI) carriers, chronic hepatitis B (CHB) patients, HBV-related decompensated cirrhosis (DC) patients, HBV-related hepatocellular carcinoma (HCC) patients. Healthy controls (HC) who were HBV serum marker-negative (HBsAg, anti-HBc) and had no serological evidence of co-infection with HCV, HDV, and HIV were also included. HBV chronic infection patients were diagnosed based on seropositivity of HBsAg at least 6 months. Then ASPI was defined as HBsAg and anti-HBc positive at least 6 months and serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) in normal values without abnormal before. CHB is defined as HBsAg and anti-HBc positive at least 6 months and ALT, AST abnormal before or at enrollment. DC was defined as HBsAg and anti-HBc positive at least 6 months with decompensated portal hypertension (gastroesophageal bleeding, ascites, edema or encephalopathy) or decompensated liver function (albumin < 35 g/L and total bilirubin > 35umol/L). HCC was defined at least one of following: (a) liver biopsy; or (b) abnormal alpha fetoprotein (AFP) and sonographic, CT or MRI space occupying evidence.
Clinical parameters
Clinical parameters including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), alkaline phosphatase (ALP), glutamyl transpeptidase (GGT), albumin (ALB), globulin (Glo), alpha fetoprotein (AFP), prothrombin time activity (PTA), platelets (PLT), HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc were collected from hospital information system. Other baseline characteristics were recorded during each patient’s clinical examination. In brief, liver biochemistry and virological tests were carried out by Bechman Coulter AU chemistry analyzers, chemiluminescence immunoassays (AxSYM or ARCHITECT I2000, Abbott, USA) or Ortho/Chemi-luminescent assay (Johnson and Johnson Co., USA) with commercially available kits; Anti-HAV IgM antibody, HDV antigen (HDAg) and anti-HDV antibody, and anti-HEV antibody were determined by commercially ELISA kits in China. For HBV DNA level, it was quantified using commercial real-time polymerase chain reaction kit with a lower limit of detection (LLOD) of 100 IU/ml (Daan company, China) or Roche Cobas Ampliprep/Cobas Taqman™ PCR assay with LLOD of 20 IU/ml (Roche, USA).
Genome-wide SNP genotyping and quality control
Genotyping was performed on Affymetrix 500k Genome-Wide Human SNP Array 6.0 (http://www.affymetrix.com/Auth/analysis/downloads/na35/genotyping/GenomeWideSNP_6.na35.annot.csv.zip). SNPs met the following quality control procedures were excluded: (1) call rate < 95%; (2) minor allele frequency (MAF) < 1%; (3) genotype in controls deviated from the Hardy Weinberg equilibrium (HWE test P-value < 10–5).
Statistics analysis
GCTA tool [20] was used to perform principal component analyses for estimating population substructure. The first two eigenvectors, pc1 and pc2, were used to display the population structure. PLINK 1.9 [21] software was used to perform logistic regression for identifying susceptibility SNPs of HBV infection and HBV-related outcomes. Gender and age were used as covariates in logistic regression. Chi-square test for trend in proportions was used to identify SNPs with increased effectiveness on disease progression. We used the Bonferroni method to adjust the false positive rate caused by multiple test. The number of independent LD block was used to represent the number of independent multiple test. We calculated a total of 21,077 independent LD blocks via GEC [22] and then set 0.05/21077 as the threshold of genome-wide significance. The genomic control method was used to measure population stratification by calculating the genomic inflation factor (λ) from median P-value. ANOVA was used to evaluate the significance of the association between biomarkers and genotypes in healthy controls. Using the SNPs in HBV infection-related loci in 1000 Genomes Project [23], we performed evolutional analyses, including building phylogenetic tree, detecting the signatures of selection, displaying the core haplotypes, estimating effective population size. Derived allele and ancestral allele of SNPs were accessed from Ensemble human ancestral genome (http://ftp.1000genomes.ebi.ac.uk/vol1/ftp/phase1/analysis_results/supporting/ancestral_alignments). PoMo [24], an allele frequency-based approach, was used to build the racial tree based on the allele frequency of SNPs in each population. FST [25], a classical metrics of population differentiation, was widely employed in detecting signatures of selection [26] in human genome [27, 28] and animal genome [29–31]. In our study, FST was implemented to detect the selective signature between East Asian population and each other population. Vcftools [32] was used to calculate the FST statistics of SNPs in paired populations. FST statistics accesses 0.15 [33] was used as a threshold to detect the signature of selection. Rehh package [34, 35] was used to display the haplotype bifurcation diagrams of the associated SNPs in different populations. Relate [36], a method for genome-wide genealogy estimation for thousands of samples, was used to estimate the historical population size at default setting.
Results
There are 1031 participants passed quality control from 1104 participants. The demographic and clinical characteristics of 1031 study participants included in our association study are presented in Table 1. All participants were genotyped by Affymetrix 500k SNP Array. A total of 607,153 SNPs passed through quality control (Additional file 1: Figure S1). These SNPs filtered minor allele frequency of < 1% and a call rate of < 95%.
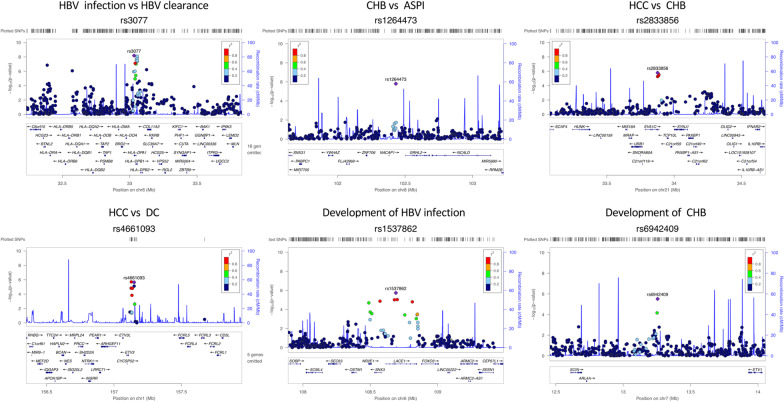
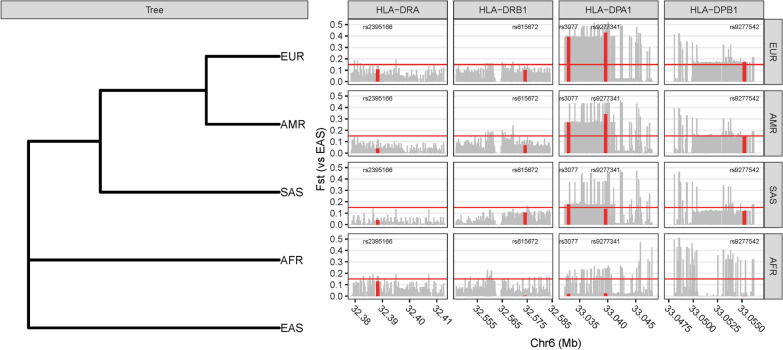
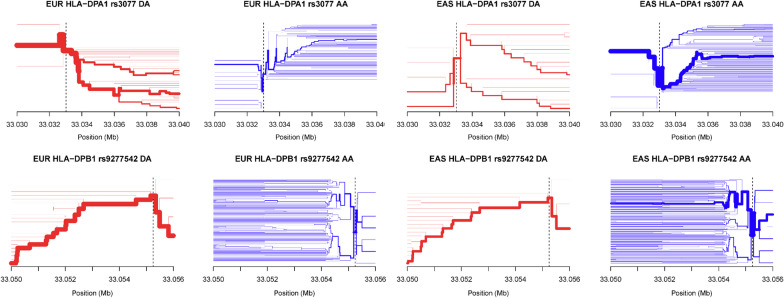
To demonstrate that there is no genetic stratification in the population, we performed a principal component analysis on the SNPs of all participants. The first two principal components show absence of population structure (Additional file 1: Figure S2). To identify susceptibility SNPs for HBV infection, we performed a GWAS in HBV infection similar with previous design [8, 9]. HBV clearance was used as a control group versus ASPI, CHB, DC, HCC as HBV chronic infection (case group). We observed associations of two novel MHC loci with progression to certain HBV stages (SNP: rs2395166, Gene: HLA-DRA, P = 1.42 × 10–7; SNP: rs615672, Gene: HLA-DRB1, P = 8.54 × 10–7) and two reported MHC loci (SNP: rs3077, Gene: HLA-DPA1, P = 6.60 × 10–9; SNP: rs9277542, Gene: HLA-DPB1, P = 1.53 × 10–8) (Table 2; Fig. 1). These MHC loci variants replicated association results of previous studies affirming that MHC gene alleles confer risks of susceptibility of HBV infection in East Asian. Interestingly, we found that these reported MHC loci (rs2395166:C, rs615672:G, rs3077:A, rs9277542:T, rs9277341:T) present significant differences in allele frequency between East Asian and non-East Asian population in gnomAD database (Table 3), as well as the differences between HBV infection group and HBV clearance group. Since different groups may not present an identical minor allele, here, we used the derived allele against the ancestral allele for studying the allele frequency across different populations. The derived allele frequencies in East Asian are much closer to the HBV chronic infection group, while other populations, such as European, are much closer to the HBV clearance group. These genetic differences may suggest a selective signal in non-East Asian population versus East Asian population. To confirm this, we firstly build a phylogenetic tree based on these loci and then showed the genetic diversity in world-wide populations, in which the East Asian population is at the root. We set the East Asian as the ancestral group in these loci according to the derived allele frequencies and the phylogenetic tree. Subsequently, we identified two strong phylogenetic signals (HLA-DPA1, HLA-DPB1) in the European population (Fig. 2) via FST method. Haplotype bifurcation diagrams of the two core SNPs (rs3077, rs9277542) presented that the resisted allele led to a long-range, and a high frequency homozygosity in European population (Fig. 3), confirming the natural genetic selection. These evidences revealed that the resisted alleles were under positive selection in European population strongly. We estimated the historic population size and then showed these two loci (HLA-DPA1, HLA-DPB1) were under selection during the past 26,000 years (Additional file 1: Figure S3). These results may provide a context for the racking influence of HBV infectious diseases in history.
Table 2.
The significance of HBV-related outcomes study
| Case–control studies | SNP | Gene | P value | OR | Minor Allele | Minor Allele Frequency | Report | λ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case (n) | Control (n) | Case | Control | ||||||||
| Infection (587) | Clearance (275) | rs2395166 | HLA-DRA | 1.42 × 10–7 | 0.4534 | C | 0.1269 | 0.2182 | MHC region | 1.003 | |
| rs615672 | HLA-DRB1 | 8.54 × 10–7 | 0.5697 | G | 0.405 | 0.5276 | MHC region | ||||
| rs3077 | HLA-DPA1 | 6.60 × 10–9 | 0.5007 | A | 0.2675 | 0.4145 | (Kamatani et al., 2009) | ||||
| rs9277542 | HLA-DPB1 | 1.53 × 10–8 | 0.5353 | T | 0.3735 | 0.5347 | (Kamatani et al., 2009) | ||||
| CHB (93) | ASPI (92) | rs1264473 | GRHL2 | 1.57 × 10–6 | 3.931 | C | 0.4402 | 0.1957 | Novel | 1.052 | |
| HCC (214) | CHB (93) | rs2833856 | EVA1C | 1.62 × 10–6 | 0.3515 | C | 0.2243 | 0.4086 | Novel | 1.022 | |
| HCC (214) | DC (188) | rs4661093 | ETV3 | 2.26 × 10–6 | 2.841 | A | 0.2104 | 0.0882 | Novel | 1.022 | |
Abbreviations: OR, odds ratio; ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; MHC, major histocompatibility complex; λ: statistics of genomic control
Fig. 1.
Regional plots shown –log10 P-values of SNPs in association study. Marker SNPs are shown as purple diamonds, other SNPs are shown as dots. R-square of Marker SNPs and other SNPs are shown against dark blue, blue, green, yellow and red colors, indicating the linkage disequilibrium. The structure of genes within the region are shown as rectangles and arrows. Abbreviation: ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma
Table 3.
Divided allele frequency of significant SNPs in MHC region
| SNP | Study | Population | Derived Allele | Derived Allele Frequency | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Case–control studies | gnomAD | East Asian vs non-East Asian | |||||||
| Infection | Clearance | Healthy | East Asian | non-East Asian | |||||
| rs2395166 | our study | Chinese | C | 0.127 | 0.218 | 0.183 | 0.128 | 0.367 | 4.367 × 10–195 |
| rs615672 | our study | Chinese | G | 0.405 | 0.528 | 0.512 | 0.388 | 0.590 | 6.003 × 10–111 |
| rs3077 | our study | Chinese | A | 0.268 | 0.415 | 0.379 | 0.283 | 0.723 | |
| Kamatani et al., 2009 [5] | Japanese | 0.245 | - | 0.392 | |||||
| Guo et al., 2011 [55] | Chinese | 0.314 | 0.447 | 0.443 | 0 | ||||
| Nishida et al., 2012 [56] | Japanese, Korean | 0.213 | 0.393 | - | |||||
| Wong et al., 2013 [57] | Southern Chinese | 0.206 | 0.276 | 0.288 | |||||
| rs9277341 | our study | Chinese | T | 0.142 | 0.242 | 0.196 | 0.159 | 0.582 | 0 |
| Guo et al., 2011 [55] | Chinese | 0.133 | 0.237 | 0.237 | |||||
| rs9277542 | our study | Chinese | T | 0.374 | 0.535 | 0.482 | 0.339 | 0.627 | 2.005 × 10–225 |
| Kamatani et al., 2009 [5] | Japanese | 0.246 | - | 0.437 | |||||
Abbreviations: East Asian, East Asian population in gnomAD database; non-East Asian, combined all other population excepted East Asian in gnomAD database; P value, compared allele frequency between East Asian and non-East Asian population via fisher exact test. Derived Allele was accessed from human ancestral genome (Ensembl-59)
Fig. 2.
The racial tree (Left) was based on the SNPs in HBV-infection related genes, including HLA-DRA, HLA-DRB1, HLA-DPA1 and HLA-DPB1. The genotype and minor allele frequency of each SNPs were
accessed from 1000 Genome Project. EAS, AFR, SAS, EUR, AMR refer to East Asian, African, South Asian, European and American of 1000 Genome Project, respectively. The racial tree indicated a genetic difference in HBV-infection related genes among EAS, AFR, SAS, EUR, AMR. The genetic difference (Right) of each SNPs was evaluated by FST value. X-axis refer to physical position in chromosome 6. Y-axis refer to FST value of paired SNP. FST values of all paired SNPs of AFR, SAS, AMR, EUR versus EAS were displayed in grey bar. FST values accessed 0.15 (Red horizontal line) indicated the signal of selective event. Red bars and rs IDs showed the reported HBV infection-related SNPs. The FST values of European versus East Asian showed the genetic difference in HLA-DPA1 and HLA-DPB1, indicating a genetic selection against the HBV infection. The racial tree showed that the East Asian population is at the root, indicating that why we used East Asian population as a comparative population but not the other population, and compared other four populations with East Asian population
Fig. 3.
Haplotype bifurcation diagrams of infection-related SNPs, including rs3077 on HLA-DPA1 (upper) and rs9277542 on HLA-DPB1 (lower), in European (left) and East Asian (right). EUR and EAS in plot title refer to European and East Asian. DA and AA in plot title refer to derived allele (red) and ancestral allele (blue). Black dash line refers to the position of core SNP. Each node refers to a haplotype. The edge width reflects the population-specific frequency. Haplotype bifurcation diagrams were displayed via Rehh package. Haplotype bifurcation diagrams showed that the derived allele led to a long-range high frequency haplotype in European population and the ancestral allele led to a high frequency haplotype in East Asian population; the ancestral allele led to more haplotypes than the derived allele. The long-range high frequency haplotype confirms the genetic selection in HLA-DPA1 and HLA-DPB1 in European population
To identify new susceptibility locus for HBV-related outcomes, we performed association studies for CHB, DC, and HCC. Significantly, we observed three associated gene SNP loci: (1) (SNP: rs1264473, Gene: GRHL2, P = 1.57 × 10–6) associated with CHB versus ASPI; (2) (SNP: rs2833856, Gene: EVA1C, P = 1.62 × 10–6) associated with HCC versus CHB; and (3) (SNP: rs4661093, Gene: ETV3, P = 2.26 × 10–6) associated with HCC versus DC (Table 2; Fig. 1). No SNP associated with DC versus CHB were apparent.
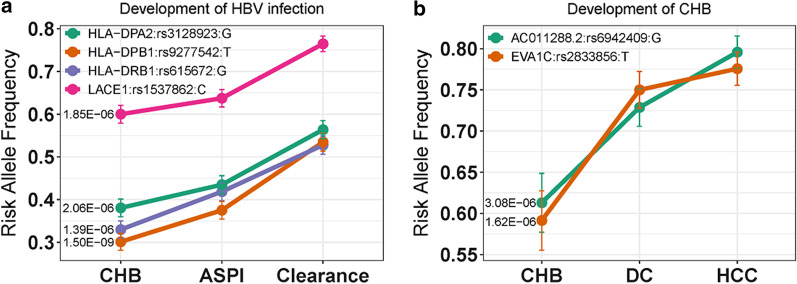
HBV clearance, ASPI, CHB, DC, and HCC are progressive stages post HBV infection [4]. We hypothesized that the host genetic factor contributes to the development of outcomes, as well as to the individual outcome. To investigate this hypothesis, we test two progressive stages upon HBV infection: 1.) HBV infection itself (CHB, ASPI, and HBV clearance) and 2.) development of CHB (CHB, DC, and HCC). We performed a chi-square test for trend in proportions of allele to identify SNPs increasing risk of HBV-related outcomes in the progressive stages. We observed association with one novel locus (SNP: rs1537862, Gene: LACE1, P = 1.85 × 10–6), one association with a reported locus (SNP: rs9277542, Gene: HLA-DPB1, P = 1.50 × 10–9), and two association variants at MHC genes (SNP: rs615672, Gene: HLA-DRB1, P = 1.39 × 10–6; SNP: rs3128923, Gene: HLA-DPA2, P = 2.06 × 10–6) with trend test of allele frequency across three outcomes (Table 4; Fig. 4A). The three reported MHC genes were demonstrated to play a critical role in the resistance of HBV infection, and two (HLA-DPB:rs9277542, HLA-DRB1:rs9277542) were identified to be associated with HBV clearance (Table 2). We did not observe any SNPs achieve genome-wide significant association with development of CHB; One additional locus (SNP: rs6942409, Gene: AC011288.2, P = 3.08 × 10–6) and the HCC associated locus (SNP: rs2833856, Gene: EVA1C, P = 1.62 × 10–5) were associated with increased risk of DC and HCC during the development of CHB (Table 5; Fig. 4b).
Table 4.
The significance of progressive HBV infection study
| SNP | Gene | P value | Resistant Allele | Resistant Allele Frequency | Related Risk | |||
|---|---|---|---|---|---|---|---|---|
| CHB | ASPI | Clearance | CHB (vs ASPI) | Clearance (vs ASPI) | ||||
| rs615672 | HLA-DRB1 | 1.39 × 10–6 | G | 0.3297 | 0.4185 | 0.5276 | 0.82 | 1.12 |
| rs9277542 | HLA-DPB1 | 1.50 × 10–9 | T | 0.3011 | 0.375 | 0.5347 | 0.84 | 1.17 |
| rs3128923 | HLA-DPA2 | 2.06 × 10–6 | G | 0.3804 | 0.4348 | 0.5636 | 0.89 | 1.14 |
| rs1537862 | LACE1 | 1.85 × 10–6 | C | 0.6 | 0.6374 | 0.7647 | 0.92 | 1.19 |
Abbreviations: ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B. RR was calculated with the comparison of CHB and ASPI, Clearance and ASPI respectively
Fig. 4.
The raising allele frequency in HBV related outcomes during the progression. Four SNPs with increased resistance in CHB, ASPI, HBV clearance during HBV infection (a) and two SNPs with increased risk in the CHB, DC, HCC during the development of CHB (b). Abbreviation: ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma
Table 5.
The suggestive significance of progressive CHB study
| SNP | Gene | P value | Risk Allele | Risk Allele Frequency | Related Risk | |||
|---|---|---|---|---|---|---|---|---|
| CHB | DC | HCC | DC (vs CHB) | HCC (vs CHB) | ||||
| rs6942409 | AC011288.2 | 3.08 × 10–6 | G | 0.6129 | 0.7287 | 0.7958 | 1.20 | 1.37 |
| rs2833856 | EVA1C | 1.62 × 10–5 | T | 0.5914 | 0.75 | 0.7757 | 1.30 | 1.35 |
Abbreviations: CHB, chronic hepatitis B; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma. RR was calculated with the comparison of HCC and CHB, DC and CHB respectively
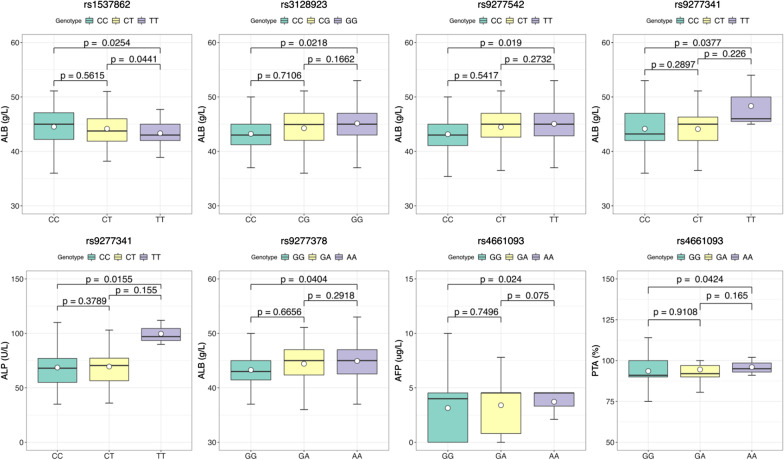
Host genetic factors were demonstrated to influence concentrations of liver enzymes in plasma, which are widely used to indicate liver disease [37, 38]. Here, to investigate the functional change in liver influenced by the HBV related loci described above, we performed a variance analysis in 10 clinical parameters of serum liver enzymes (ALT, AST, TBIL, DBIL, ALP, GGT, ALB, AFP, PTA, and PLT) between different genotypes in healthy controls (Additional file 1: Figure S4-9). Six loci (rs1537862, rs3128923, rs9277542, rs9277341, rs9277378, rs4661093) showed modest associations with concentrations of liver enzymes, including ALB, ALP, AFP, and PTA (Fig. 5). These associations suggest pathways linking the host genetic factors, metabolism, and liver function for understanding the mechanisms of infection and disease progression.
Fig. 5.
The association between HBV related loci and serum liver enzyme levels in health controls. P values were calculated by ANOVA test. White-circle refer to the mean liver enzymes level with different genotypes. The significant differences indicate that these SNPs contribute to liver enzyme activity
In sum, our study identified susceptibility SNPs associated with HBV related outcomes and SNPs increased the risk of progressive outcomes from HBV clearance to HBV chronic infection, DC, and HCC in a Chinese population (Additional file 1: Figure S10).
Discussion
HBV infection leads to a wide spectrum of clinical outcomes, including spontaneous clearance, asymptomatic carrier, chronic hepatitis B, liver cirrhosis, and hepatocellular carcinoma. Previous studies showed that MHC genes played an important role in outcomes of HBV infection [7]. Alleles associated with HBV infection versus HBV clearance affect infection risk, and a low-risk allele indicated an effect on virus clearance. By contrast loci associated with CHB versus ASPI indicated risk for the severe progression, while a low-risk allele affected tolerance of virus. The tolerance-related gene, GRHL2, was demonstrated to influence the inflammation in hepatocytes by regulating microRNA 122 (MIR122) and the target of MIR122, HIF1α [39]. Levels of GRHL2 were increased in liver tissues of patients with alcoholic liver disease and correlated with decreases in levels of MIR122. Increased levels of MIR122 in hepatocytes of mice with ethanol-induced liver disease and advanced fibrosis reduced levels of HIF1α and reduced serum levels of alanine aminotransferase (ALT). Taken together, we propose that the low-risk allele rs1264473:T at GRHL2 ablates severe persistent inflammation through increased the levels of MIR122.
Our previous studies [40, 41] showed that NTCP S267F mutation significantly affected the disease progression to cirrhosis (P = 0.017), and hepatocellular carcinoma (P = 0.023) versus CHB [40] and the rs3077:T allele was associated with decreased risk of chronic HBV infection (OR = 0.62, P = 0.001) [41]. In this study, we searched for host genetic factor with increased risk of the development-related outcomes in GWAS. One novel locus, LACE1, and three infection-related MHC loci were associated the progression of HBV infection. These results showed that the host genetic factors, both MHC and non-MHC genes, increased the risk of progressive outcomes post HBV infection, as well as HBV mutation. It is reported that HBV infection altered the mitochondrial metabolism and mitochondrial dynamics, which result in mitochondrial injury and liver disease [42]. LACE1 was reported to affect mitochondrial protein homeostasis [43]. Knockdown of LACE1 converted the expression of a crucial component of regulating mitochondrial dynamics, OPA1 [43–45]. In addition, we found that the risk allele, LACE1:rs1537862:T, decreased the level of ALB significantly (P = 0.025, Fig. 5). ALB is a critical marker decreasing with the deterioration of chronic liver diseases [46–48]. Biosynthesis of ALB was affected by proinflammatory cytokines [49, 50] and excess amounts of oxidative agents released by mitochondria from injured liver [46, 51]. Taken together, we proposed LACE1 may affect hepatic infection by changing the hepatic mitochondrial metabolism and leading to the progression of HBV infection.
There is a limitation in our study, that is we do not have an additional cohort for replicate study. In spite of that, we showed the reported loci in MHC region are significantly related to HBV infection. These replicate results of previous studies confirm our findings are reliable and provide confidence for our study in this cohort. Here, we provide novel candidate genes related to individual outcomes, progressive stages, and liver enzymes. Moreover, we identified two SNPs that show selective significance (HLA-DPA1, HLA-DPB1) in non-East Asian (European, American, South Asian) versus East Asian. East Asian population seem more susceptible to HBV infection than non-East Asian, and the differences of susceptibility were affected by HBV genotype [52], immunity [53], and environmental exposure [53, 54]. Even in an identical environment (United States), Asian are more prevalent in chronic HBV infection than non-Asian [53]. It seems likely that host genetic factors contribute to the ethnic disparities of susceptibility of HBV infection. Taken together with the genetic associations and evolutionary signals, our findings provide a new insight for HBV study.
Conclusion
In case–control study, we identified one novel locus (SNP: rs1264473, Gene: GRHL2, P = 1.57 × 10–6) significantly associated with CHB, two novel loci (SNP: rs2833856, Gene: EVA1C, P = 1.62 × 10–6; SNP: rs4661093, Gene: ETV3, P = 2.26 × 10–6) significantly associated with HCC. In trend study across multiple outcomes, we identified one novel locus (SNP: rs1537862, Gene: LACE1, P = 1.85 × 10–6) and three MHC loci (HLA-DRB1, HLA-DPB1, HLA-DPA2) significantly increased progressive risk from CHB through ASPI to HBV clearance. In evolutionary study, we showed the derived allele of two HBV clearance related loci, rs3077 and rs9277542, are under strong selection in European population. We suggested these selected alleles may play a role in resisting the susceptibility of HBV in Europeans. Our findings provided a new insight into the role of host genetic factors in HBV related outcomes and progression.
Supplementary Information
Additional file 1: Figure S1. The summary of final SNPs characteristic, including MAF, call rate, and p-value of Hardy-Weinberg equilibrium test.. Figure S2. Principal component analyses indicated there are no population stratification among 6 subgroups. Abbreviation: ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B; DC, decompensated cirrhosis; HC: healthy controls; HCC, hepatocellular carcinoma. Figure S3. Effective population sizes inferred using Related-package across all individuals of each population in two loci (HLA-DPA1, HLA-DPB1). Recentsize histories (26000 years ago) in European (purple) population showed modest difference compared with East Asian (red) population. Abbreviation: EUR, European; AMR, American; SAS, South Asian; AFR, African. Figure S4. Boxplots of rs2395166 genotype and serum liver enzyme levels in HC. Figure S5. Boxplots of rs615672 genotype and serum liver enzyme levels in HC. Figure S6. Boxplots of rs3077 genotype and serum liver enzyme levels in HC. Figure S7. Boxplots of rs1264473 genotype and serum liver enzyme levels in HC. Figure S8. Boxplots of rs2833856 genotype and serum liver enzyme levels in HC. Figure S9. Boxplots of rs6942409 genotype and serum liver enzyme levels in HC. Figure S10. The summary of associated SNPs contributed to HBV-related outcomes and the progression. Abbreviation: PI, persistence infection; ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma.
Acknowledgements
We thank the participants for their involvement in this study.
HBV study consortium: Department of Infectious Diseases, Peking University First Hospital, Beijing, China (Zheng Zeng, Yanyan Yu, Xiaoyuan Xu, Haiying Lu); Institute of Liver Diseases Research, Beijing Military General Hospital, Beijing, China (Darong Hu); Beijing Ditan Hospital (Rongbing Wang, Yifan Chen); Department of Surgery, Beijing Institute of Tumor Prevention and Therapy, Beijing, China (Cunyi Hao); Department of Infectious Diseases, Shanxi Medical University, Taiyuan, China (Heping Zhou); Department of Infectious Diseases, Qinhuangdao No. 3 Hospital, Qinhuangdao, China (Zhonghou Han); Department of Surgery, Inner Mongolia Medical College, Hohhot, China (Lidao Bao, Xiping Zhang); Department of Infectious Diseases, Xuzhou No. 3 Hospital, Xuzhou, China (Dasi Guo); Department of Infectious Diseases, Xinjian Medical University, Urumoqi, China (Yaoxin Zhang); Department of Infectious Diseases, the Second Affiliate Hospital of China Medical University, Shenyang, China (Xiaoguang Dou); Institute of Liver Diseases Research, Peking University People’s Hospital, Beijing, China (Lai Wei); Department of Surgery, Peking Union Medical College, Beijing, China (Qiang Qu).
Abbreviations
- AFP
Alpha Fetoprotein
- AFR
African
- ALB
Albumin
- ALP
Alkaline Phosphatase
- ALT
Alanine Aminotransferase
- AMR
American
- anti-HBc
Antibody to Hepatitis B core Antigen
- anti-HBe
Antibody to Hepatitis B e Antigen
- anti-HBs
Antibody to Hepatitis B surface Antigen
- anti-HDV
Antibody to Hepatitis D Virus
- anti-HEV
Antibody to Hepatitis E Virus
- ASPI
Asymptomatic Persistence Infection
- AST
Aspartate Aminotransferase
- CHB
Chronic Hepatitis B
- DBIL
Direct Bilirubin
- DC
Decompensated Cirrhosis
- EAS
East Asian
- EUR
European
- Glo
Globulin
- GGT
Glutamyl Transpeptidase
- GWAS
Genome Wide Association Study
- HBeAg
Hepatitis B e Antigen
- HBsAg
Hepatitis B surface Antigen
- HBV
Hepatitis B Virus
- HCC
Hepatocellular Carcinoma
- HC
Healthy Controls
- HCV
Hepatitis C Virus
- HDV
Hepatitis D Virus
- HIV
Human Immunodeficiency Virus
- HWE
Hardy–Weinberg Equilibrium
- MAF
Minor Allele Frequency
- MHC
Major Histocompatibility Complex
- PI
Persistence Infection
- PLT
Platelets
- PTA
Prothrombin Time Activity
- SAS
South Asian
- SD
Standard Deviation
- SNP
Single Nucleotide Polymorphism
- TBIL
Total Bilirubin
Authors' contributions
ZZ, HKL, HYL, and HFX contributed equally. SJO and ZZ designed the study. ZZ, HYL, YYY, XYX, MY, XLT, HLX, and HBV study consortium collected samples and analyzed clinical data. ZZ and XLT maintained database. HKL, ZZ, HFX, TZ, LPG, and JGZ performed bioinformatic analyses. ZZ, SJO, and JGZ reviewed and revised the manuscript. SJO and ZZ supervised the research and made final decision. All authors have read and approves the final.
Funding
This study was supported by the National Natural Science Foundation of China (No. 61972007, No. 30671855), the International Science & Technology Cooperation Program of China (No. 2014DFR31200), Federal funds from the National Cancer Institute, National Institutes of Health, USA (No. N01-CO-12400), Shenzhen Municipal of Government of China (JCYJ20170412153248372, JCYJ20180507183615145). These funding have no conflict of interest. The content of this publication does not necessarily reflect the view of policies of the Department of Health and Human Service, nor does mention of trade name, commercial products or organizations imply endorsement by the U.S. Government.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available as they are still being investigated but are available from the corresponding author (Dr. Zheng Zeng) on reasonable request and will be released in China National GeneBank (https://db.cngb.org/cmdb) (need to be approved by Human Genetic Resource Administration of China). The direct web links to the SNPs of 1000 Genomes Project (http://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20130502) and Ensemble human ancestral genome (http://ftp.1000genomes.ebi.ac.uk/vol1/ftp/phase1/analysis_results/supporting/ancestral_alignments).
Ethics approval and consent to participate
This study was carried out following the guidelines of the 1975 Declaration of Helsinki. Informed written consent was obtained from all participants and the study was performed with the approval of the Ethics Committee of Peking University First Hospital and local cooperation hospitals (Beijing Military General Hospital, Beijing Ditan Hospital, Beijing Institute of Tumor Prevention and Therapy, Shanxi Medical University, Qinhuangdao No. 3 Hospital, Inner Mongolia Medical College, Xuzhou No. 3 Hospital, Xinjian Medical University, the Second Affiliate Hospital of China Medical University, Peking University People’s Hospital, Peking Union Medical College).
Consent to publish
Not applicable.
Competing interests
The authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheng Zeng, Hankui Liu, Huifang Xu and Haiying Lu contributed equally to the manuscript
Contributor Information
Zheng Zeng, Email: zeng@bjmu.edu.cn.
Jianguo Zhang, Email: zhangjg@genomics.cn.
Stephen J. O’Brien, Email: lgdchief@gmail.com
References
- 1.Schweitzer A, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. The Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. Hepatology. 1995;21(1):77–82. doi: 10.1002/hep.1840210114. [DOI] [PubMed] [Google Scholar]
- 3.Liaw Y-F, Chu C-M. Hepatitis B virus infection. The lancet. 2009;373(9663):582–592. doi: 10.1016/S0140-6736(09)60207-5. [DOI] [PubMed] [Google Scholar]
- 4.Sundaram V, Kowdley K. Management of chronic hepatitis B infection. BMJ. 2015;351:h4263. doi: 10.1136/bmj.h4263. [DOI] [PubMed] [Google Scholar]
- 5.Kamatani Y, et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41(5):591. doi: 10.1038/ng.348. [DOI] [PubMed] [Google Scholar]
- 6.Mbarek H, et al. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Hum Mol Genet. 2011;20(19):3884–3892. doi: 10.1093/hmg/ddr301. [DOI] [PubMed] [Google Scholar]
- 7.Zeng Z. Human genes involved in hepatitis B virus infection. World journal of gastroenterology: WJG. 2014;20(24):7696. doi: 10.3748/wjg.v20.i24.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z, et al. New loci associated with chronic hepatitis B virus infection in Han Chinese. Nat Genet. 2013;45(12):1499. doi: 10.1038/ng.2809. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, et al. Genome-wide association study identifies 8p21 3 associated with persistent hepatitis B virus infection among Chinese. Nature Commun. 2016;7(1):1–11. doi: 10.1038/ncomms11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frodsham AJ. Host genetics and the outcome of hepatitis B viral infection. Transpl Immunol. 2005;14(3–4):183–186. doi: 10.1016/j.trim.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 11.He Y-L, et al. Host susceptibility to persistent hepatitis B virus infection. WJG. 2006;12(30):4788. doi: 10.3748/wjg.v12.i30.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang D-K, et al. Genetic variations in STAT4, C2, HLA-DRB1 and HLA-DQ associated with risk of hepatitis B virus-related liver cirrhosis. Sci Reports. 2015;5:16278. doi: 10.1038/srep16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen VL, et al. Genetic variants that associate with cirrhosis have pleiotropic effects on human traits. Liver Int. 2020;40(2):405–415. doi: 10.1111/liv.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H et al (2010) Genome-wide association study identifies as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nature Genetics 42(9): 755–758. [DOI] [PubMed]
- 15.Li S, et al. GWAS identifies novel susceptibility loci on 6p21. 32 and 21q21.3 for hepatocellular carcinoma in chronic hepatitis B virus carriers. PLoS Genetics. 2012:8:7. [DOI] [PMC free article] [PubMed]
- 16.Jiang D-K, et al. Genetic variants in STAT4 and HLA-DQ genes confer risk of hepatitis B virus–related hepatocellular carcinoma. Nat Genet. 2013;45(1):72. doi: 10.1038/ng.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höhler T, et al. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: a vaccination study in twins. The Lancet. 2002;360(9338):991–995. doi: 10.1016/S0140-6736(02)11083-X. [DOI] [PubMed] [Google Scholar]
- 18.Ji X, et al. Impacts of human leukocyte antigen DQ genetic polymorphisms and their interactions with hepatitis B virus mutations on the risks of viral persistence, liver cirrhosis, and hepatocellular carcinoma. Infection, Genetics and Evolution. 2014;28:201–209. doi: 10.1016/j.meegid.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Z, et al. A population-based study to investigate host genetic factors associated with hepatitis B infection and pathogenesis in the Chinese population. BMC Infect Dis. 2008;8(1):1. doi: 10.1186/1471-2334-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, et al. GCTA: a tool for genome-wide complex trait analysis. The American Journal of Human Genetics. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. The American journal of human genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M-X, et al. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet. 2012;131(5):747–756. doi: 10.1007/s00439-011-1118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Consortium, G.P. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Maio N, Schrempf D, Kosiol C. PoMo: an allele frequency-based approach for species tree estimation. Syst Biol. 2015;64(6):1018–1031. doi: 10.1093/sysbio/syv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984:1358–1370. [DOI] [PubMed]
- 26.Holsinger KE, Weir BS. Genetics in geographically structured populations: defining, estimating and interpreting F ST. Nat Rev Genet. 2009;10(9):639–650. doi: 10.1038/nrg2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabeti PC, et al.. Positive natural selection in the human lineage. Science, 2006;312(5780):1614–1620. [DOI] [PubMed]
- 28.Voight BF, et al. A map of recent positive selection in the human genome. PLoS Biol. 2006;4(3):e72. doi: 10.1371/journal.pbio.0040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kijas JW, et al. Genome-wide analysis of the world's sheep breeds reveals high levels of historic mixture and strong recent selection. PLoS Biol. 2012;10(2):e1001258. doi: 10.1371/journal.pbio.1001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, et al. Detection of selection signatures in Chinese Landrace and Yorkshire pigs based on genotyping-by-sequencing data. Frontiers in genetics. 2018;9:119. doi: 10.3389/fgene.2018.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z, et al. Whole-genome resequencing reveals signatures of selection and timing of duck domestication. GigaScience, 2018; 7(4):giy027. [DOI] [PMC free article] [PubMed]
- 32.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankham R, Briscoe DA, Ballou JD. Introduction to conservation genetics. Cambridge University Press, Cambridge; 2002
- 34.Sabeti PC, et al. Detecting recent positive selection in the human genome from haplotype structure. Nature. 2002;419(6909):832–837. doi: 10.1038/nature01140. [DOI] [PubMed] [Google Scholar]
- 35.Gautier M, Klassmann A, Vitalis R. rehh 2.0: a reimplementation of the R package rehh to detect positive selection from haplotype structure. Mol Ecol Resourc. 2017;17(1):78–90. [DOI] [PubMed]
- 36.Speidel L, et al. A method for genome-wide genealogy estimation for thousands of samples. Nat Genet. 2019;51(9):1321–1329. doi: 10.1038/s41588-019-0484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chambers JC, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet. 2011;43(11):1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masuda M, et al. Interaction of genetic markers associated with serum alkaline phosphatase levels in the Japanese population. Human genome variation. 2015;2(1):1–6. doi: 10.1038/hgv.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satishchandran A, et al. MicroRNA 122, regulated by GRLH2, protects livers of mice and patients from ethanol-induced liver disease. Gastroenterology. 2018;154(1):238–252.e7. [DOI] [PMC free article] [PubMed]
- 40.An P, Zeng Z, Winkler CA. The Loss-of-Function S267F variant in HBV receptor NTCP reduces human risk for HBV infection and disease progression. J Infect Dis. 2018;218(9):1404–1410. doi: 10.1093/infdis/jiy355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.An P, et al. A common HLA–DPA1 variant is a major determinant of hepatitis B virus clearance in Han Chinese. J Infect Dis. 2011;203(7):943–947. doi: 10.1093/infdis/jiq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SJ, et al. Hepatitis B virus disrupts mitochondrial dynamics: induces fission and mitophagy to attenuate apoptosis. PLoS Pathogens. 2013;9:12. [DOI] [PMC free article] [PubMed]
- 43.Cesnekova J, et al. The mammalian homologue of yeast Afg1 ATPase (lactation elevated 1) mediates degradation of nuclear-encoded complex IV subunits. Biochemical Journal. 2016;473(6):797–804. doi: 10.1042/BJ20151029. [DOI] [PubMed] [Google Scholar]
- 44.Frezza C, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126(1):177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Anand R, et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204(6):919–929. doi: 10.1083/jcb.201308006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar PA, SuBramanian K. The role of ischemia modified albumin as a biomarker in patients with chronic liver disease. JCDR. 2016. 10(3): BC09. [DOI] [PMC free article] [PubMed]
- 47.Yuen M-F, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54(11):1610–1614. doi: 10.1136/gut.2005.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walayat S, et al. Role of albumin in cirrhosis: from a hospitalist’s perspective. Journal of community hospital internal medicine perspectives. 2017;7(1):8–14. doi: 10.1080/20009666.2017.1302704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castell JV, et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 50.Carvalho JR, Machado MV. New insights about albumin and liver disease. Annals of hepatology. 2018;17(4):547–560. doi: 10.5604/01.3001.0012.0916. [DOI] [PubMed] [Google Scholar]
- 51.Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World journal of gastroenterology: WJG. 2014;20(25):8082. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teshale EH, et al. Genotypic distribution of hepatitis B virus (HBV) among acute cases of HBV infection, selected United States counties, 1999–2005. Clin Infect Dis. 2011;53(8):751–756. doi: 10.1093/cid/cir495. [DOI] [PubMed] [Google Scholar]
- 53.Kim H, et al. Racial/ethnic disparities in the prevalence and awareness of hepatitis B virus infection and immunity in the United States. J Viral Hepatitis. 2017;24(11):1052–1066. doi: 10.1111/jvh.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan AT, et al. Host ethnicity and virus genotype shape the hepatitis B virus-specific T-cell repertoire. J Virol. 2008;82(22):10986–10997. doi: 10.1128/JVI.01124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo X, et al. Strong influence of HLA-DP gene variants on development of persistent chronic HBV carriers in the Han Chinese population. Hepatology (Baltimore, Md.), 2011. 53(2):422. [DOI] [PMC free article] [PubMed]
- 56.Nishida N, et al. Genome-wide association study confirming association of HLA-DP with protection against chronic hepatitis B and viral clearance in Japanese and Korean. PLoS ONE. 2012;7(6):e39175. doi: 10.1371/journal.pone.0039175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong DKH, et al. Role of HLA-DP polymorphisms on chronicity and disease activity of hepatitis B infection in Southern Chinese. PLoS ONE. 2013;8:6. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The summary of final SNPs characteristic, including MAF, call rate, and p-value of Hardy-Weinberg equilibrium test.. Figure S2. Principal component analyses indicated there are no population stratification among 6 subgroups. Abbreviation: ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B; DC, decompensated cirrhosis; HC: healthy controls; HCC, hepatocellular carcinoma. Figure S3. Effective population sizes inferred using Related-package across all individuals of each population in two loci (HLA-DPA1, HLA-DPB1). Recentsize histories (26000 years ago) in European (purple) population showed modest difference compared with East Asian (red) population. Abbreviation: EUR, European; AMR, American; SAS, South Asian; AFR, African. Figure S4. Boxplots of rs2395166 genotype and serum liver enzyme levels in HC. Figure S5. Boxplots of rs615672 genotype and serum liver enzyme levels in HC. Figure S6. Boxplots of rs3077 genotype and serum liver enzyme levels in HC. Figure S7. Boxplots of rs1264473 genotype and serum liver enzyme levels in HC. Figure S8. Boxplots of rs2833856 genotype and serum liver enzyme levels in HC. Figure S9. Boxplots of rs6942409 genotype and serum liver enzyme levels in HC. Figure S10. The summary of associated SNPs contributed to HBV-related outcomes and the progression. Abbreviation: PI, persistence infection; ASPI, asymptomatic persistence infection; CHB, chronic hepatitis B; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available as they are still being investigated but are available from the corresponding author (Dr. Zheng Zeng) on reasonable request and will be released in China National GeneBank (https://db.cngb.org/cmdb) (need to be approved by Human Genetic Resource Administration of China). The direct web links to the SNPs of 1000 Genomes Project (http://ftp.1000genomes.ebi.ac.uk/vol1/ftp/release/20130502) and Ensemble human ancestral genome (http://ftp.1000genomes.ebi.ac.uk/vol1/ftp/phase1/analysis_results/supporting/ancestral_alignments).