Abstract
BACKGROUND AND PURPOSE: The aim of this study was to clarify the cause of hyperintense putaminal rim (HPR) on the basis of 3T MR imaging–pathologic correlations.
MATERIALS AND METHODS: We evaluated brain MR images from 75 subjects 13 to 85 years of age on T2-weighted fast spin-echo (FSE) images at 3T. We also assessed HPR on postmortem T2-weighted FSE images from 4 postmortem cases 1, 12, 63, and 83 years of age. To clarify the cause of HPR, we used 3 staining methods: the Klüver-Barrera method to observe the myelin sheath, the Berlin blue method to observe hemosiderin, and ferritin immunohistochemistry to observe ferritin. The postmortem MR images were compared with the histologic findings in each case.
RESULTS: HPR was absent or vague in subjects under 30 years of age but present in subjects in their 30s–60s and again became vague in those subjects older than 70 years of age. The postmortem MR imaging–pathologic correlations revealed that ferritin deposits were slight in the lateral marginal area of the putamen in the 63-year-old subject showing present HPR, but in the 83-year-old subject with no HPR, ferritin deposits were prominent in the lateral marginal area of the putamen as well as in other areas.
CONCLUSION: Age-related disproportion in ferritin deposits between the lateral marginal area and the remainder of the putamen causes hypointensity of the latter and the relative hyperintensity of the former, which is depicted as HPR with 3T MR imaging.
High-field MR imaging is a recent advance in medical imaging. High-spatial-resolution imaging, which is based on high signal-to-noise ratio (SNR), is a major advantage of high-field MR imaging. Although its efficacy in clinical practice is not fully understood, there are an increasing number of reports that document the priority of high spatial resolution.1,2 On T2-weighted fast spin-echo (FSE) images of normal brain on 3T MR images, Lee et al3 recently reported linear hyperintensity in the lateral marginal area of the putamen, which they call hyperintense putaminal rim (HPR), and they stressed its clinical importance with respect to differentiation from multiple system atrophy. They suggested that HPR is a nonspecific normal finding and may reflect an enlargement of the space between the putamen and the external capsule and that HPR may be detectable with a 3T system because of its higher SNR and higher sensitivity to paramagnetic effects. However, their report was based on only 10 middle-aged subjects and had no histopathologic proof. In this study, we assessed the frequency of HPR in 75 subjects of various ages and performed postmortem 3T-pathologic correlations to clarify the cause of HPR.
Materials and Methods
We selected 75 subjects who had undergone brain MR imaging by using the 3T MR imaging system (Signa Horizon, GE Healthcare, Milwaukee, Wis). The subjects ranged from 13 to 85 years of age. They were divided into 8 age groups from teens to 70s with 10 subjects in each group and 5 subjects in their 80s. All subjects were neurologically healthy. MR images of the brain were taken for screening of headache or brain metastasis of cancer and showed no organic brain lesions except for a few hyperintense spots in the white matter on T2-weighted FSE images.
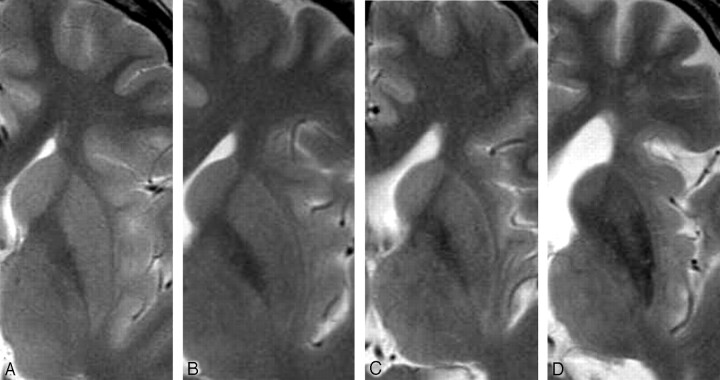
We obtained axial T2-weighted FSE images (TR/TE, 4000/82.7–84.2 ms; 320 × 512 matrix; 210-mm FOV; section thickness/intersection gap, 5/1.5 mm). Two neuroradiologists (S.F. and E.M.) independently assessed HPR in the bilateral putamina on axial T2-weighted FSE images and classified them into 3 grades: absent, vague, and present (Fig 1). In the few cases of initial disagreement between the 2 neuroradiologists, a consensus was reached.
Fig 1.
Classification of HPR on axial T2-weighted FSE images. A, Absent (15 years old). B, Vague (29 years old). C, Present (54 years old). D, Vague with marked and diffuse putaminal hypointenisty (77 years old).
MR images of the formalin-fixed brains (postmortem MR images) were obtained from 4 patients of 1, 12, 63, and 83 years of age who had died of non-neurologic disorders. The fixed brains were positioned in a standard way in the head coil. Axial and coronal T2-weighted FSE images (TR/TE, 4000/81; 320 × 512 matrix; section thickness/intersection gap, 4 and 1 mm, respectively) were obtained by using the 3T system. After MR imging, the brains were subjected to routine neuropathologic examination. Coronal sections of the brains were cut 5 mm thick. The putamen was macro- and microscopically normal in each case.
We analyzed the cause of HPR by using 3 staining methods: the Klüver-Barrera and Berlin blue methods and ferritin immunohistochemistry. The Klüver-Barrera method was used to evaluate myelinated fibers. The Berlin blue method was used for demonstration of free Fe3+, particularly hemosiderin. Ferritin immunohistochemistry was used to reveal ferritin deposits. These findings were compared with the postmortem MR images in each brain.
Results
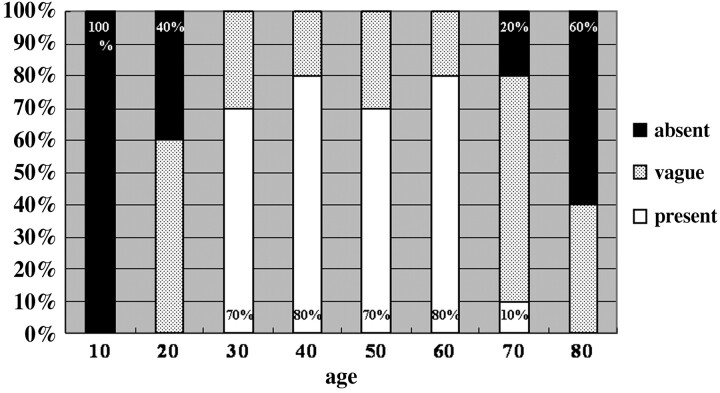
In 10 subjects younger than 20 years of age, the putamen showed no HPR on T2-weighted FSE images (Figs 1A and 2). Vague HPR appeared in 6 of 10 subjects in their 20s, which was noted as a relatively hyperintense narrow band in the lateral marginal area of the putamen, contrasting with the remaining areas showing mildly decreased intensity (Figs 1B and 2). In the groups from 30 to 69 years of age, HPR was present, contrasting with the remainder of the putamen with moderately decreased intensity (Figs 1C and 2). In the subjects older than 70 years of age, HPR tended to become vague again, and the signal intensity of the putamen decreased markedly and diffusely (Figs 1D and 2). Of the 10 subjects in their 70s, HPR was absent in 2 and vague in 7, whereas HPR was present in only 1. Of the 5 subjects older than 80 years of age, HPR was absent in 3 and vague in 2, and none had HPR present (Fig 2).
Fig 2.
Frequency of HPR in 8 age groups. HPR becomes gradually prominent after 20 years of age and tends to become vague or absent after 70 years of age.
On postmortem MR images, HPR was not demonstrated in the 1- and 12-year-old subjects (Fig 3A, -B). In contrast to these younger subjects, HPR was clearly visible as a hyperintense rim of the lateral marginal area of the putamen in the 63-year-old subject (Fig 4A, -B). In the 83-year-old subject, HPR was not demonstrated because of diffuse hypointensity of the putamen including the lateral marginal area (Fig 5A, -B).
Fig 3.
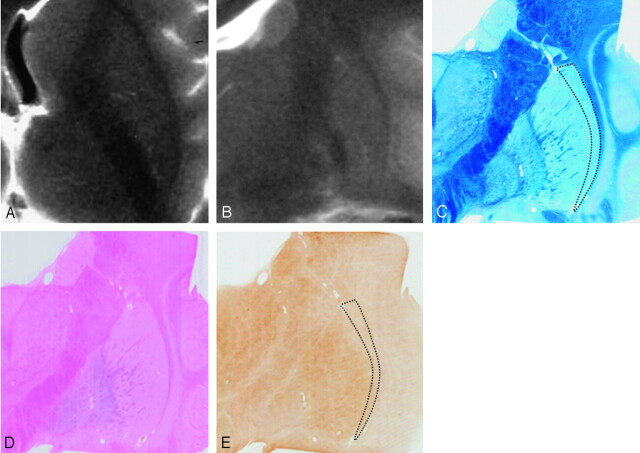
Comparison of postmortem MR images and histologic findings in a 12-year-old subject. Postmortem axial (A) and coronal (B) T2-weighted images. A section corresponding to B stained with Klüver-Barrera (C), Berlin blue (D), and ferritin immunohistochemistry (E). Postmortem MR images (A and B) show absent HPR. With Klüver-Barrera staining (C), myelinated fibers are small in number in the lateral marginal area of the putamen (dotted lines) in comparison with the remainder. With Berlin blue staining, hemosiderin is scattered in the inner portion of the putamen but is scarce in the outer portion (D). With ferritin immunohistochemistry, ferritin deposits are mild in the inner portion but are scarce in the lateral marginal area of the putamen (dotted lines, E).
Fig 4.
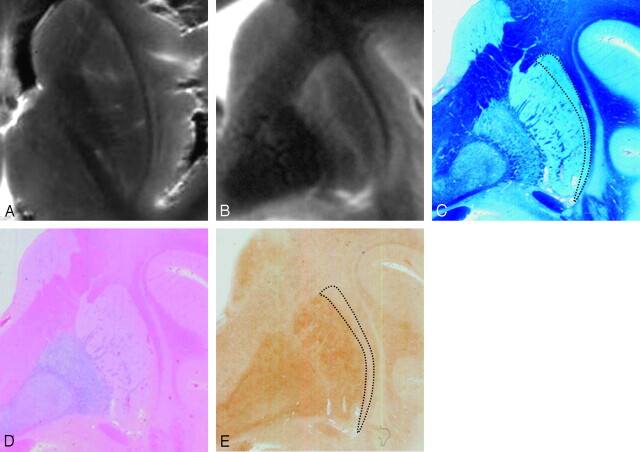
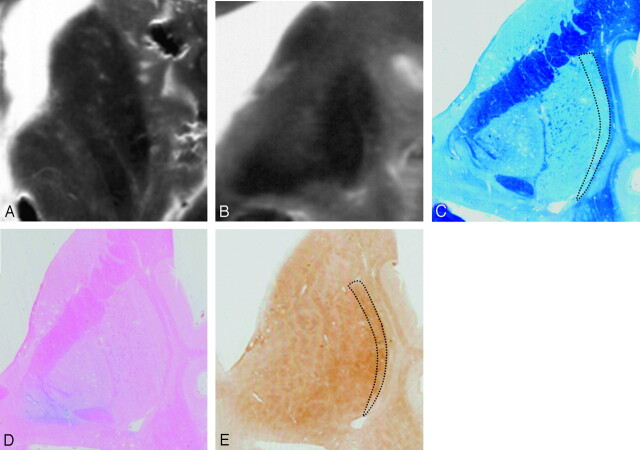
Comparison of postmortem MR images and histologic findings in a 63-year-old subject. Postmortem axial (A) and coronal (B) T2-weighted images. Sections corresponding to B are stained with Klüver-Barrera (C), Berlin blue (D), and ferritin immunohistochemistry (E). Postmortem MR images (A and B) show present HPR. With Klüver-Barrera staining (C), HPR on a T2-weighed FSE image corresponds to the lateral marginal area of the putamen (dotted lines), where myelinated fibers are small in number in comparison with the remainder of the putamen. With Berlin blue staining (D), hemosiderin-stained blue is scattered in the inner portion of the putamen but is scarce in the lateral marginal area. With ferritin immunohistochemistry (E), ferritin deposits are mild in the lateral marginal area of the putamen (dotted lines), corresponding to HPR on the postmortem MR images (B). By contrast, the deposits are increased in the remainder of the putamen corresponding to the area of decreased signal intensity on the postmortem MR images (B).
Fig 5.
Comparison of postmortem MR images and histologic findings in an 83-year-old subject. Postmortem axial (A) and coronal (B) T2-weighted images. A section corresponding to B stained with Klüver-Barrera (C), Berlin blue (D), and ferritin immunohistochemistry (E). A and B, Postmortem MR images show diffuse putaminal hypointensity, and HPR is not detectable. With Klüver-Barrera staining (C), myelinated fibers are few in the lateral marginal area of the putamen (dotted lines). With Berlin blue staining (D), hemosiderin is scattered in the inner portion of the putamen but is scarce in the outer portion. Ferritin immunohistochemistry reveals diffuse ferritin deposits in the putamen, including the lateral marginal area (E).
Comparison of the postmortem MR images and histologic findings revealed that HPR corresponded to the lateral marginal area of the putamen (Fig 4C). With Klüver-Barrera staining of the putamen, which stains myelinated fibers blue, myelinated fibers were scarce in the lateral marginal area of the putamen in comparison with the other areas (Figs 3C, 4C, and 5C). There was no enlargement of the space between the putamen and the external capsule (Figs 3C, 4C, and 5C). With Berlin blue staining, which shows hemosiderin as blue, hemosiderin was not found in the putamen in the 1-year-old subject. In the other 3 subjects, hemosiderin was scattered in the inner portion of the putamen (Figs 3D, 4D, and 5D) but was not proportional to age. Thus, in all subjects, hemosiderin deposits were scarce in the lateral marginal area of the putamen (Figs 3D, 4D, and 5D). With ferritin immunohistochemistry of the putamen, which shows ferritin as brown, ferritin deposits were minimal in the 1-year-old subject and mild in the 12-year-old subject (Fig 3E). In the 63-year-old subject, who showed present HPR, ferritin deposits were prominent in the remainder of the putamen corresponding to the area of decreased signal intensity but mild in the lateral marginal area of the putamen that corresponds to HPR (Fig 4E). In the 83-year-old subject, who showed diffuse putaminal hypointensity without HPR, ferritin deposits were prominent and diffuse throughout the whole putamen including the lateral marginal area (Fig 5E).
Discussion
On T2-weighted FSE images at 3T, linear hyperintensity is frequently found in the lateral marginal area of the putamen. Lee et al3 called this hyperintensity HPR. They examined HPR in 10 healthy subjects ranging from 44 to 61 years of age and concluded that HPR is a nonspecific normal finding. In the present study, HPR was absent in the subjects younger than 20 years of age, vague or absent in the subjects in their 20s, and was present in the subjects in their 30s–60s. It again became vague or absent in the subjects in their 70s and 80s. Thus, the appearance of HPR seems to be related to aging.
T2-weighted MR images show iron deposits as decreased signal intensity.4 This T2 shortening mechanism is thought to be caused by intravoxel incoherent motion due to the local magnetic field inhomogeneities created by the presence of these iron particles.5 The decreased signal intensity is in proportion to the iron concentration and becomes more prominent with age.6,7 Abundant iron exists in the globus pallidus and in the putamen.8 A large portion (exceeding 80%) of the iron remains in the form of ferritin.8 In the globus pallidus, the iron deposits increase rapidly during the first 2 decades of life, but no further increase appears to occur after 30 years of age. In the putamen, the iron deposits increase somewhat more slowly with aging and do not reach maximum values at 50–60 years of age.8 These findings suggest that the appearance of HPR is connected to age-related iron deposits in the putamen.
In this study, we used the Berlin blue method and ferritin immunohistochemistry to observe iron deposits in the putamen because most of the nonheme iron in the brain exists in a mineralized form such as hemosiderin or ferritin, and concentrations of free iron aqua ions are negligible at physiologic pH values.9 Although there are many iron-bearing proteins within the cytosol and labile iron pool, wherein iron ions are presumably chelated with adenosine 5′-triphosphate, amino acids, citrate, and such, these total iron concentrations have been considered to be too low to affect MR imaging.10 Therefore, MR imaging contrast is considered to be mainly affected by ferritin and hemosiderin.9
In the present postmortem MR imaging–pathologic correlations, hemosiderin was scarce in the lateral marginal area of the putamen in all subjects; this scarcity suggests that hemosiderin deposits might have no effect on the appearance of HPR. The ferritin immunohistochemistry of the putamen in the 63-year-old subject showing present HPR revealed that ferritin deposits were mild in the lateral marginal area, contrasting with heavy deposits in the remainder of the putamen. By contrast, in the 83-year-old subject showing diffuse putaminal hypointensity without HPR, ferritin deposits were marked and diffuse in the putamen including the lateral marginal area. These findings suggest that the disproportion in ferritin deposits between the lateral marginal area and the remainder of the putamen in the subjects in their 30s–60s causes the hypointensity of the latter and the relative hyperintensity of the former, which is depicted as HPR on 3T MR images. Thus, HPR is a “pseudosign,” which is the perceived hyperintensity due to the remainder of the putamen becoming darker.
It is evident that high spatial resolution based on high SNR contributes to the appearance of HPR,3 because the SNR increases 30%–60% in semisolid tissues.11 On the basis of our results, we consider that the higher susceptibility effects play a more important role in the appearance of HPR on high-field MR imaging. MR imaging at 3T increases the sensitivity to susceptibility effects and improves the depiction of iron-containing brain structures in comparison with 1.5T MR imaging; mean contrast/noise ratio is significantly decreased by 90%–119% in the deep nuclei on FSE images.12 MR imaging at 3T can depict decreased intensity because of minimal iron deposits, which is not apparent on 1.5T MR imaging. Consequently, the appearance of HPR is strongly associated with hypointensity, which can be depicted with 3T MR imaging because of higher susceptibility effects by the iron deposits in the putamen. Thus, HPR is clearly depicted on 3T MR images but not on 1.5T MR images because HPR is the perceived hyperintensity due to the remainder of the putamen becoming darker by susceptibility effects.
Lee et al3 supposed that HPR reflects the space between the putamen and the external capsule in a normal subject. They also reported that HPR becomes indistinct or absent on fluid-attenuated inversion recovery (FLAIR) images because of extracellular fluid accumulation in the CSF-equivalent space.3 However, our results reveal that HPR is located in the lateral marginal area of the putamen and that there is no enlargement of the space between the putamen and the external capsule. We consider that the indistinct appearance of HPR on FLAIR images is due to decreased signal intensity of the gray matter in comparison with T2-weighted images.
Conclusion
HPR is absent in subjects younger than 2 years of age, appears vague in subjects in their 20s, and becomes clearly present in subjects in their 30s–60s. In subjects older than 70 years of age, HPR becomes indistinct again. HPR reflects age-related changes of ferritin deposits in the putamen. It is important to recognize that HPR is observed clearly in clinically healthy subjects in their 30s–60s on 3T MR images.
Acknowledgments
We are grateful to Takefumi Yamane, Eiji Yamashita, and Naoki Iwata for technical support in obtaining high-quality MR images for this study.
References
- 1.Nakada T, Kwee IL, Fujii Y, et al. High-field, T2 reversed MRI of the hippocampus in transient global amnesia. Neurology 2005;64:1170–74 [DOI] [PubMed] [Google Scholar]
- 2.Nakada T. High-field, high-resolution MR imaging of the human indusium griseum. AJNR Am J Neuroradiol 1999;20:524–25 [PMC free article] [PubMed] [Google Scholar]
- 3.Lee WH, Lee CC, Shyu WC, et al. Hyperintense putaminal rim sign is not a hallmark of multiple system atrophy at 3T. AJNR Am J Neuroradiol 2005;26:2238–42 [PMC free article] [PubMed] [Google Scholar]
- 4.Schenker C, Meier D, Wichmann W, et al. Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology 1993;35:119–24 [DOI] [PubMed] [Google Scholar]
- 5.Le Bihan D, Turner R. Intravoxel incoherent motion imaging using spin echoes. Magn Reson Med 1991;19:221–27 [DOI] [PubMed] [Google Scholar]
- 6.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem 1958;3:41–51 [DOI] [PubMed] [Google Scholar]
- 7.Aoki S, Okada Y, Nishimura K, et al. Normal deposits of brain iron in childhood and adolescence: MR imaging at 1.5T. Radiology 1989;172:381–85 [DOI] [PubMed] [Google Scholar]
- 8.Laz Haque T, Miki Y, Kanagaki M, et al. MR contrast of ferritin and hemosiderin in the brain: comparison among gradient-echo, conventional spin-echo and fast spin-echo sequences. Eur J Radiol 2003;48:230–36 [DOI] [PubMed] [Google Scholar]
- 9.Schenck JF. Magnetic resonance imaging of brain iron. J Neurol Sci 2003;207:99–102 [DOI] [PubMed] [Google Scholar]
- 10.Crichton R. Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences. 2nd ed. New York: Wiley;2001. :167–68
- 11.Frayne R, Goodyear BG, Dickhoff P, et al. Magnetic resonance imaging at 3.0 tesla: challenges and advantages in clinical neurological imaging. Invest Radiol 2003;38:385–402 [DOI] [PubMed] [Google Scholar]
- 12.Allkemper T, Schwindt W, Maintz D, et al. Sensitivity of T2-weighted FSE sequences towards physiological iron deposits in normal brains at 1.5 and 3.0 T. Eur Radiol 2004;14:1000–04 [DOI] [PubMed] [Google Scholar]