Abstract
BACKGROUND AND PURPOSE: To test a new contrast-specific sonography imaging method that offers visualization of the intracranial vasculature in a manner similar to that seen on angiography.
MATERIALS AND METHODS: Thirty patients (35 sonography studies total) were included in the study after they provided written informed consent. The patients were scanned through the temporal bone window from both sides after intravenous injection of an ultrasound contrast agent (UCA; perflexane lipid microspheres [Imagent]). The goal was to visualize the intracranial arteries, including the middle (M1–M3), anterior (A1 and A2), and posterior (P1–P3) cerebral arteries, using an axial scanning plane. The studies were performed using a contrast-specific imaging mode, based on a phase inversion technique (transcranial ultrasound angiography [tUSA]). For sensitivity, the results were compared with x-ray angiography as the “gold standard.” For interobserver reliability, 24 of 35 sonography studies were evaluated by 2 physicians with little training in transcranial sonography and by a seasoned sonographer.
RESULTS: The sensitivity of tUSA ranged between 0.778 (95% confidence interval [CI] of 0.577–0.914) and 0.963 (95% CI of 0.810–0.999). The sensitivities were similar among physicians with little training in transcranial sonography and the seasoned sonographer, indicating high inter-rater reliability. Overall, tUSA provided high anatomic resolution and vascular delineation even of small vessels in the millimeter range. At peak intensity, no UCA-related artifacts were observed.
CONCLUSION: tUSA provides images of the intracranial arteries similar to those obtained at angiography with high anatomic resolution, reasonable sensitivity, and interobserver reliability.
Real-time imaging of the intracranial arteries with sonography was first described by Bogdahn et al1 in the early nineties. The main difference compared with conventional transcranial Doppler (TCD) is the color-coded representation of arterial blood flow to allow the unequivocal identification of the circle of Willis within the anatomic grayscale (B-mode) image of the brain parenchyma. Further, with the use of Doppler mode, the blood flow can be analyzed semiquantitatively, as in conventional TCD, but with the added advantage of visual control by tracking the target vessel using the color flow map. However, the major limitation of all transcranial sonography techniques has been the massive acoustic signal intensity absorption while insonating through the intact skull. Furthermore, the phase aberration of the sonography beam, as a result of the convexity of the temporal bone, its surface roughness, and the multiple impedances (from outside to inside of the skull: pars compacta–pars spongiosa–pars compacta), limits sonography imaging of the adult brain by significantly reducing the signal-to-noise ratio (SNR).2, 3
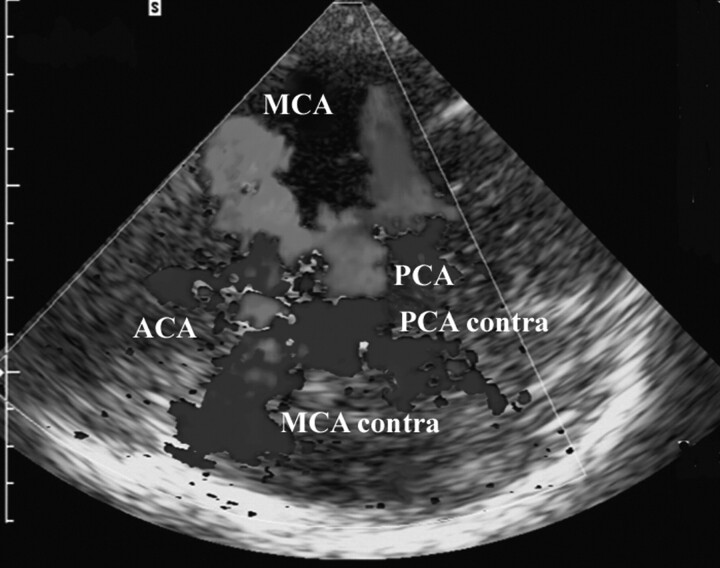
One approach to improving the SNR while imaging intracranial vasculature with color or power Doppler uses ultrasound contrast agents (UCAs). Intravenously injected, transpulmonary stable UCAs are well tolerated and allow the detection of the circle of Willis, peripheral branches of the anterior, middle, and posterior cerebral arteries, and the vertebrobasilar arteries.4, 5 The diagnostic benefits of contrast-enhanced transcranial sonography have been demonstrated in multiple studies.6–9 However, besides the potential diagnostic benefits contrast-enhanced sonography of the intracranial arteries is limited because of UCA specific artifacts that occur mainly in the early phase after IV bolus injection. Although color and power Doppler mode—the common sonography techniques for vascular imaging—are sensitive to detect flow, the spatial resolution of the received acoustic signal is relatively poor. Strong acoustic signals, encoded as color pixels on the screen, may appear “outside” the anatomic delineation of the vessel, especially in the early phase after UCA microbubble injection. The enhancement appears as an overamplification of the color or power Doppler signals on the screen of the ultrasound machine and is termed “blooming” (Fig 1).10 The experience and skills of the sonographer are needed to adjust the machine settings and to optimize the image to control the effect of blooming. To overcome UCA-specific artifacts such as blooming, IV infusion is an option. A UCA infusion provides a prolonged useful enhancement with fewer artifacts compared with a bolus injection.11 However, in clinical practice, such as in an acute stroke setting with time limitations, an infusion technique might not be appropriate.
Fig 1.
Blooming artifact. Contrast-enhanced transcranial sonography of the circle of Willis (axial scanning plane via the temporal bone window). The vessel delineation is diminished because of the strong contrast signal intensity (blooming) after IV bolus injection of the UCA. Red color-coding represents flow toward the transducer, blue color-coding flow away from it. MCA, middle cerebral artery; MCA contra, middle cerebral artery contralateral; PCA, posterior cerebral artery, PCA contra; posterior cerebral artery contralateral; ACA, anterior cerebral artery.
The purpose of this study was to develop a contrast-specific imaging technique that is easy to use and that enables visualization of the intracranial vessels in an angiography-like display with high spatial resolution and fewer UCA specific artifacts. We sought to validate the accuracy of physicians with little training in transcranial sonography, in preparation for wider clinical utility of the technique.
Materials and Methods
Study Population
In a prospective study, patients scheduled for a cerebral contrast angiogram for any reason were asked to participate and were enrolled consecutively after providing written informed consent, according to local Institutional Review Board approval. Patients had to be 18 years of age or older and had to be scheduled for cerebral x-ray angiography for any clinical indication. Patients with a known history of severe emphysema, pulmonary vasculitis, history of pulmonary embolism, or chronic renal failure were excluded from the study
Sonography Devices and Study Design
Sonoline Elegra and a Sonoline Antares (Siemens, Erlangen, Germany) sonography systems were used for the transcranial studies. Both devices are equipped with 2.5-MHz phased-array transducers (2.5PL20, Elegra; PX4-1, Antares).
Each side of the head was imaged with the patient in a supine position and the transducer held at the temporal bone acoustic window. The goal was to assess the intracranial vessel segments of the middle cerebral artery (M1, M2, and M3), the anterior cerebral artery (A1 and A2), and the posterior cerebral artery (P1, P2, and P3) in an axial scanning plane.
Transcranial Ultrasound Angiography (tUSA)
Because the aim was to develop an imaging technique that enables visualization of the intracranial arteries with high spatial and temporal resolution with fewer artifacts in an angiographic view, the term “transcranial ultrasound angiography (tUSA)” was chosen. tUSA is based on a phase-inversion technique using high-resolution wideband harmonic imaging to detect UCA microbubbles. To minimize microbubble destruction as a result of high acoustic intensity imaging, the minimum output power was used depending on the quality of the bone window. The goal was to lower the acoustic power to a point at which known anatomic landmarks (ie, brain stem, third ventricle) could barely be seen. The transmit power ranged between 16% and 70% of maximum (mechanical index: 1.4 at 100% output power). In cases of sufficient to optimal bone windows, the transmit frequency was increased to a maximum of 2.0 MHz. This method of tUSA has been described in detail recently.12
UCA and UCA Administration
We used AF0150 perflexane lipid microspheres (Imagent; IMCOR Pharmaceutical, San Diego, Calif). Imagent was FDA approved for endocardial border delineation, but as of June 2005, the agent is no longer commercially available.
The agent was supplied as 200 mg of powder in a sealed vial. It was reconstituted by adding 10 mL of water and gently mixing. The resultant microbubble suspension encapsulated perfluorohexane vapor and nitrogen in a thin phospholipid membrane and contained 5 × 108 microbubbles/mL with a mean diameter of 2–3 μm.
AF0150 was administered intravenously as a bolus injection into an antecubital or forearm vein by using a 20G catheter, followed by a 5-mL saline flush. The UCA bolus dose was 0.5 mL if the intracranial image quality was optimal, 1.0 mL if not optimal but clinically useful, and 2.0 mL if the image quality was insufficient (Table 1). The UCA dose injection was given twice, once for each hemisphere. Because the maximum allowable dose for this agent is 0.2 mL/kg, an average 70-kg adult can receive a total of 14 mL. If needed, up to 2 more UCA injections were allowed in this study.
Table 1:
Assessment of transcranial image quality
| Image quality | Contralateral Skull | 3rd Ventricle | Brain stem |
|---|---|---|---|
| Optimal | + | + | + |
| Clinically useful | + | +/− | +/− |
| Insufficient | +/− | − | − |
Note:—Image quality was defined by the number of anatomic structures visualized precontrast.
Statistical Analysis
The visualization (yes/no) of the cerebral artery segments (MCA: M1, M2, M3, ACA: A1, A2, PCA: P1, P2, P3) was assessed by an experienced sonographer who was blinded to the cerebral x-ray angiography data. The presence of all cerebral artery segments was confirmed on all x-ray angiograms. The sensitivity of visualizing the segments was calculated using the x-ray angiogram as the “gold standard.” The 95% confidence intervals (CIs) were determined for each of these values using an exact binomial distribution.13
In addition to sensitivity, the interobserver agreement was also assessed. For the 24 of the 35 sonography studies for which videos of the studies were available, 2 physicians with little sonography training and an experienced sonographer evaluated the scans for bilateral visualization. All 3 readers were blinded to the tUSA and x-ray data. The sensitivity of visualization was calculated for all readers independently. The McNemar χ2 test for paired proportions was used to determine the difference in sensitivities between pairs of readers. The Holm method was used to adjust for multiple comparisons.14
Results
Overall Description
Thirty patients (21 women, 9 men) were studied with tUSA. The age ranged between 18 and 81 years of age with a mean age of 50 years. Three of 30 patients underwent 2 scans, and 1 patient underwent 3 scans. These 4 patients also underwent repeated neuroradiologic interventions, including preinterventional cerebral x-ray angiography, because of arteriovenous malformation treatment, as well as postinterventional angiography. The individual study conditions, based on the quality of the temporal bone window, were as follows: 7 of 35, optimal; 21 of 35, clinically useful; and 7 of 35, insufficient study quality. Ten of the 35 scans were performed using a Sonoline Elegra sonography scanner and the remaining 25 with a Sonoline Antares system. The total UCA volume per single case ranged between 1.0 and 8.0 mL, with a mean volume of 2.8 mL of contrast agent per patient. No serious adverse events were described as a result of UCA administration; in 2 of 35 cases, a mild, transient (<60 seconds) metallic taste was reported immediately after UCA injection, and in 1 case of 35, the tUSA study failed because of IV catheter misplacement.
Sensitivity
On the right hemisphere, the sensitivity to visualize the aforementioned vessel segments ranged between 0.788 (A2 segment) and 0.970 (P1 and P2 segments). The corresponding 95% CIs were 0.611–0.910 (A2 segment) and 0.842–0.999 (P1 and P2 segments).
For the left hemisphere, the results were comparable. The sensitivity ranged between 0.829 (A2 segment) and 0.971 (M1, P1, and P2 segments) with 95% CIs of 0.664–0.993 (A2 segment) and 0.851–0.999 (M1, P1, and P2 segments), respectively (Table 2). In both hemispheres, the highest sensitivities were seen in the proximal vessel segments (M1, P1, and A1 segments), whereas the lowest values were reached in the peripheral vessel segments (M3, P3, and A2). Among the latter, the sensitivity for A2 was slightly lower compared with M3 and P3 segments.
Table 2:
Transcranial ultrasound angiography sensitivity analysis in comparison to the “gold standard” cerebral x-ray angiography
| Vessel Segments | Sensitivity | 95% Confidence Interval |
|---|---|---|
| Right | ||
| MCA | ||
| M1 | 0.96 | 0.798–0.993 |
| M2 | 0.96 | 0.798–0.993 |
| M3 | 0.92 | 0.757–0.981 |
| PCA | ||
| P1 | 0.96 | 0.842–0.999 |
| P2 | 0.96 | 0.842–0.999 |
| P3 | 0.88 | 0.718–0.966 |
| ACA | ||
| A1 | 0.96 | 0.757–0.981 |
| A2 | 0.84 | 0.611–0.910 |
| Left | ||
| MCA | ||
| M1 | 0.96 | 0.851–0.999 |
| M2 | 0.96 | 0.808–0.993 |
| M3 | 0.92 | 0.733–0.968 |
| PCA | ||
| P1 | 0.96 | 0.851–0.999 |
| P2 | 0.96 | 0.851–0.999 |
| P3 | 0.96 | 0.733–0.968 |
| ACA | ||
| A1 | 0.96 | 0.808–0.993 |
| A2 | 0.92 | 0.664–0.934 |
Note:—MCA indicates middle cerebral artery; PCA, posterior cerebral artery; ACA, anterior cerebral artery.
Interobserver Reliability
Twenty-four of 35 tUSA studies were videotaped. These tapes were used for interobserver reliability and to assess the difference between inexperienced and experienced observers. The sensitivities for the visualization by the 2 physicians with little sonography training and an experienced sonographer are shown in Table 3. No statistically significant differences in sensitivities were detected between any pairs of the 3 readers, thus indicating a high interobserver agreement of visualization.
Table 3:
Interobserver reliability analysis: comparison of an experienced sonographer and 2 ultrasound-inexperienced stroke fellows.
| Vessel Segments | Stroke Fellow 1 (n = 24) |
Stroke Fellow 2 (n = 24) |
Sonographer (n = 24) |
|||
|---|---|---|---|---|---|---|
| No. Visualized | Sensitivity | No. Visualized | Sensitivity | No. Visualized | Sensitivity | |
| Right | ||||||
| MCA | ||||||
| M1 | 22 | 0.92 | 22 | 0.92 | 23 | 0.96 |
| M2 | 22 | 0.92 | 22 | 0.92 | 23 | 0.96 |
| M3 | 21 | 0.88 | 22 | 0.92 | 22 | 0.92 |
| PCA | ||||||
| P1 | 22 | 0.92 | 22 | 0.92 | 23 | 0.96 |
| P2 | 22 | 0.92 | 22 | 0.92 | 23 | 0.96 |
| P3 | 18 | 0.75 | 19 | 0.79 | 21 | 0.88 |
| ACA | ||||||
| A1 | 21 | 0.88 | 21 | 0.84 | 23 | 0.96 |
| A2 | 21 | 0.88 | 22 | 0.92 | 20 | 0.84 |
| Left | ||||||
| MCA | ||||||
| M1 | 23 | 0.96 | 23 | 0.96 | 23 | 0.96 |
| M2 | 23 | 0.96 | 23 | 0.96 | 23 | 0.96 |
| M3 | 17 | 0.71 | 20 | 0.83 | 22 | 0.92 |
| PCA | ||||||
| P1 | 23 | 0.96 | 23 | 0.96 | 23 | 0.96 |
| P2 | 23 | 0.96 | 23 | 0.96 | 23 | 0.96 |
| P3 | 19 | 0.79 | 20 | 0.83 | 23 | 0.96 |
| ACA | ||||||
| A1 | 20 | 0.83 | 19 | 0.79 | 23 | 0.96 |
| A2 | 23 | 0.96 | 23 | 0.96 | 22 | 0.92 |
Note:—MCA indicates middle cerebral artery; PCA, posterior cerebral artery; ACA, anterior cerebral artery.
Discussion
Contrast-specific brain imaging with sonography is currently a challenging research field. Unlike conventional TCD, sonographic imaging techniques to visualize the intracranial vessels or to assess the parenchymal microcirculation are available in only a few specialized centers. Color-coded duplex sonography is the method of choice for blood flow imaging with sonography, such as in heart, liver, kidney, or fetal diagnostics. The first studies published in the early 1990s demonstrated the applicability of color-coded duplex sonography for the visualization of intracranial vessels9, 15, 16 as well. After the introduction of UCA, the diagnostic value of transcranial duplex sonography improved significantly. However, UCA-specific artifacts were described, limiting the diagnostic value of color-coded, contrast-enhanced sonography studies. The initial blooming artifact (see Materials and Methods) and its impact on the diagnostic usefulness of the signal intensity have been described.17, 18 Blooming displays on the screen as an overamplification of the Doppler signal intensity that is dramatically enhanced by UCA early after IV bolus injection. This leads to a loss of anatomic orientation that becomes hidden by the color overlay of main parts of the field of view. The effect, which depends on the UCA dosage and concentration as well as the individual quality of the bone window, diminishes over time. Although an optimal contrast signal intensity can be achieved in the course of the study, it was shown that the diagnostically useful window might be narrowed significantly as a result of blooming.11, 19
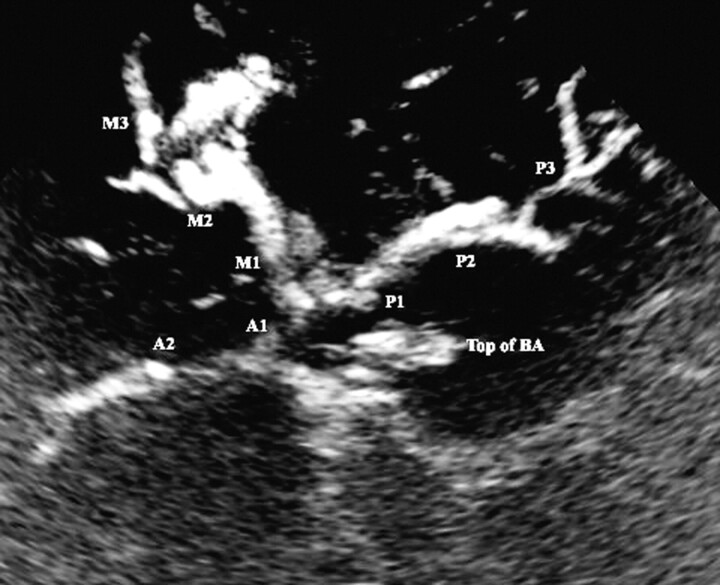
The rapidly increasing knowledge of the acoustic properties of UCA microbubbles led to the development of contrast-specific imaging techniques, such as wideband phase inversion and second or subharmonic imaging.20–23 We have reported the benefits of contrast-specific imaging of the extracranial carotid arteries and aimed to test a comparable technique for the visualization of the intracranial arteries.24 tUSA takes advantage of the UCA acoustic properties to produce a B-mode rather than a Doppler image that produces fewer UCA-specific artifacts and requires lower acoustic power settings. We have observed that blooming artifacts, which occur predominantly in the early phase after intravenous UCA injection with Doppler imaging, can be suppressed using tUSA, leading to an angiography-like view of the intracranial vessels during signal intensity enhancement as well as at peak signal intensity (Fig 2). Although no systematic comparisons with color and/or power mode were done in this study, the main benefits of tUSA seem to be a high spatial and temporal resolution and the anatomic delineation of the vessels. Peripheral vessel segments (ie, M3 and P3 segments), MCA bifurcations (M1/M2 cross-over), and smaller vessel segments within the millimeter range could be adequately visualized. In contrast to this, the acoustic sensitivity of tUSA appears to be lower compared with color and power mode. Once the visual contrast effect is no longer observed on tUSA, signal intensity enhancement can be detected when the imaging mode is switched to one of the Doppler imaging techniques.
Fig 2.
Transcranial ultrasound angiography (tUSA). tUSA image of the circle of Willis and its branches after UCA injection. M1, M2, and M3, middle cerebral artery segments; A1 and A2, anterior cerebral artery segments; P1, P2, and P3, posterior cerebral artery segments; BS, brain stem; top of BA, hyperechogenic distal part of the basilar artery.
The significance of TCD in general is well displayed by its broad acceptance for various indications such as stroke,25–27 embolus detection,28–30 or vasospasm monitoring.31–33 The main benefits of TCD are well known: it is inexpensive, it can be used at the bedside, and it enables assessment of the intracranial flow dynamics in real-time. The main disadvantage of TCD is the dependency on the experience/skill of the operator, accompanied with the requirement for the sonographer to interpret flow spectra instead of direct visualization of intracranial vessels.
We believe that tUSA might reduce the dependency on the skill of the sonographer and therefore improve reliability. The results of the blinded reading of 2 physicians with little training in transcranial sonography and an experienced sonographer and the comparison of the 3 readings among each other show high interobserver agreement (Table 3). Although duplex sonography systems equipped with contrast-specific imaging techniques are decreasing in initial cost, they remain more expensive than TCD machines. However, the angiogram-like display of the circle of Willis and arterial branches and the ability to track vessels and acquire flow velocity data if desired should speed the training of inexperienced personnel, perhaps justifying the extra equipment costs.
Conclusion
tUSA using contrast-specific imaging techniques enables visualization of the major portion of the intracranial vasculature at the patient's bedside. The clinical usefulness is the high sensitivity in visualizing the circle of Willis and the major branches of the anterior, middle, and posterior cerebral arteries without image artifacts and with little or no observer experience.
Footnotes
This work has been supported by United States National Institutes of Health grant P50-NS044148 (Specialized Program on Translational Research in Acute Stroke).
Preliminary results presented at the International Stroke Conference sponsored by the American Stroke Association, a division of American Heart Association; February 2–4, 2005; New Orleans, La. (Hölscher T, Wilkening W, Olson S, et al. Transcranial ultrasound angiography (tUSA): a new contrast-specific imaging mode. Stroke 2005;36:P258).
References
- 1.Bogdahn U, Becker G, Winkler J, et al. Transcranial color-coded real-time sonography in adults. Stroke 1990;21:1680–88 [DOI] [PubMed] [Google Scholar]
- 2.Clement GT, Sun J, Hynynen K. The role of internal reflection in transskull phase distortion. Ultrasonics 2001;39:109–13 [DOI] [PubMed] [Google Scholar]
- 3.Deverson S, Evans DH, Bouch DC. The effects of temporal bone on transcranial Doppler ultrasound beam shape. Ultrasound Med Biol 2000;26:239–44 [DOI] [PubMed] [Google Scholar]
- 4.Bogdahn U, Becker G, Schlief R, et al. Contrast-enhanced transcranial color-coded real-time sonography. Results of a phase-two study. Stroke 1993;24:676–84 [DOI] [PubMed] [Google Scholar]
- 5.Becker G, Lindner A, Bogdahn U. Imaging of the vertebrobasilar system by transcranial color-coded real-time sonography. J Ultrasound Med 1993;12:395–401 [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner RW, Arnold M, Gonner F, et al. Contrast-enhanced transcranial color-coded duplex sonography in ischemic cerebrovascular disease. Stroke 1997;28:2473–78 [DOI] [PubMed] [Google Scholar]
- 7.Bogdahn U, Frohlich T, Becker G, et al. Vascularization of primary central nervous system tumors: detection with contrast-enhanced transcranial color-coded real-time sonography. Radiology 1994;192:141–48 [DOI] [PubMed] [Google Scholar]
- 8.Gerriets T, Goertler M, Stolz E, et al. Feasibility and validity of transcranial duplex sonography in patients with acute stroke. J Neurol Neurosurg Psychiatry 2002;73:17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumgartner RW, Baumgartner I, Schroth G. Diagnostic criteria for transcranial colour-coded duplex sonography evaluation of cross-flow through the circle of Willis in unilateral obstructive carotid artery disease. J Neurol 1996;243:516–21 [DOI] [PubMed] [Google Scholar]
- 10.Forsberg F, Liu JB, Burns PN, et al. Artifacts in ultrasonic contrast agent studies. J Ultrasound Med 1994;13:357–65 [DOI] [PubMed] [Google Scholar]
- 11.Hölscher T, Schlachetzki F, Bauer A, et al. Echo-enhanced transcranial color-coded US: clinical usefulness of intravenous infusion versus bolus injection of SH U 508A. Radiology 2001;219:823–27 [DOI] [PubMed] [Google Scholar]
- 12.Hölscher T, Wilkening W, Lyden P, et al. Transcranial ultrasound angiography (tUSA): a new approach for contrast specific imaging of intracranial arteries. Ultrasound Med Biol 2005;31:1001–06 [DOI] [PubMed] [Google Scholar]
- 13.Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–13 [Google Scholar]
- 14.Holm S. A simple sequentially rejection multiple test procedure. Scand J Statist 1979;6:65–70 [Google Scholar]
- 15.Baumgartner RW, Baumgartner I, Mattle HP, et al. Transcranial color-coded duplex sonography in unilateral flow-restrictive extracranial carotid artery disease. AJNR Am J Neuroradiol 1996;17:777–83 [PMC free article] [PubMed] [Google Scholar]
- 16.Seidel G, Kaps M, Gerriets T. Potential and limitations of transcranial color-coded sonography in stroke patients. Stroke 1995;26:2061–66 [DOI] [PubMed] [Google Scholar]
- 17.Nabavi DG, Droste DW, Kemeny V, et al. Potential and limitations of echocontrast-enhanced ultrasonography in acute stroke patients: a pilot study. Stroke 1998;29:949–54 [DOI] [PubMed] [Google Scholar]
- 18.Schminke U, Motsch L, Bleiss A, et al. Continuous administration of contrast medium for transcranial colour-coded sonography. Neuroradiology 2001;43:24–28 [DOI] [PubMed] [Google Scholar]
- 19.Albrecht T, Urbank A, Mahler M, et al. Prolongation and optimization of Doppler enhancement with a microbubble US contrast agent by using continuous infusion: preliminary experience. Radiology 1998;207:339–47 [DOI] [PubMed] [Google Scholar]
- 20.Burns PN. Harmonic imaging with ultrasound contrast agents. Clin Radiol 1996;51 Suppl 1:50–55 [PubMed] [Google Scholar]
- 21.Burns PN. Overview of echo-enhanced vascular ultrasound imaging for clinical diagnosis in neurosonology. J Neuroimaging 1997;7 Suppl 1:S2–14 [PubMed] [Google Scholar]
- 22.de Jong N, Ten Cate FJ, Lancee CT, et al. Principles and recent developments in ultrasound contrast agents. Ultrasonics 1991;29:324–30 [DOI] [PubMed] [Google Scholar]
- 23.Forsberg F, Shi WT, Goldberg BB. Subharmonic imaging of contrast agents. Ultrasonics 2000;38:93–98 [DOI] [PubMed] [Google Scholar]
- 24.Kono Y, Pinnell SP, Sirlin CB, et al. Carotid arteries: contrast-enhanced US angiography–preliminary clinical experience. Radiology 2004;230:561–68 [DOI] [PubMed] [Google Scholar]
- 25.Alexandrov AV, Demchuk AM, Felberg RA, et al. High rate of complete recanalization and dramatic clinical recovery during tPA infusion when continuously monitored with 2-MHz transcranial Doppler monitoring. Stroke 2000;31:610–14 [DOI] [PubMed] [Google Scholar]
- 26.Alexandrov AV, Demchuk AM, Felberg RA, et al. Intracranial clot dissolution is associated with embolic signals on transcranial Doppler. J Neuroimaging 2000;10:27–32 [DOI] [PubMed] [Google Scholar]
- 27.Demchuk AM, Burgin WS, Christou I, et al. Thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke 2001;32:89–93 [DOI] [PubMed] [Google Scholar]
- 28.Droste DW, Lakemeier H, Ritter M, et al. The identification of right-to-left shunts using contrast transcranial Doppler ultrasound: performance and interpretation modalities, and absence of a significant side difference of cardiac micro-emboli. Neurol Res 2004;26:325–30 [DOI] [PubMed] [Google Scholar]
- 29.Droste DW, Lakemeier S, Wichter T, et al. Optimizing the technique of contrast transcranial Doppler ultrasound in the detection of right-to-left shunts. Stroke 2002;33:2211–16 [DOI] [PubMed] [Google Scholar]
- 30.Meves SH, Schroder A, Muhs A, et al. Distribution of artificially-produced microembolic signals into the cerebral circulation. Ultrasound Med Biol 2001;27:285–87 [DOI] [PubMed] [Google Scholar]
- 31.Aaslid R. Transcranial Doppler assessment of cerebral vasospasm. Eur J Ultrasound 2002;16:3–10 [DOI] [PubMed] [Google Scholar]
- 32.Singh V, McCartney JP, Hemphill JC 3rd. Transcranial Doppler ultrasonography in the neurologic intensive care unit. Neurol India 2001;49 Suppl 1:S81–89 [PubMed] [Google Scholar]
- 33.Sloan MA, Alexandrov AV, Tegeler CH, et al. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2004;62:1468–81 [DOI] [PubMed] [Google Scholar]