Abstract
BACKGROUND AND PURPOSE: Our purpose was to report our experience with intracranial dural arteriovenous fistulas (DAVFs) with cortical venous drainage during a 12-year period.
PATIENTS AND METHODS: Between January 1994 and January 2006, 91 patients with intracranial DAVFs presented at our institution, and 29 (32%) had cortical venous drainage. There were 5 women and 24 men (mean age, 53.9 years; range, 24–77). Clinical presentation was intraparenchymal or subarachnoid hemorrhage in 18 patients (62%), seizures in 4 patients (14%), visual symptoms in 2 patients (7%), pulsatile bruit in 1 patient (3%), and the DAVF (14%) was incidentally discovered in 4 patients.
RESULTS: In 2 patients, the DAVF had been obliterated spontaneously at the time of scheduled embolization 10 and 2 months after hemorrhage, respectively. Five patients with an anterior fossa DAVF underwent successful surgery. In 14 patients, the DAVF was completely occluded with embolization alone, and in 7 patients, embolization was followed by surgery. Altogether, complete occlusion was angiographically confirmed in 28 of 29 DAVFs; the result of radiosurgery of 1 DAVF is pending. There were no complications of surgery; embolization was complicated by postembolization hemorrhage in 1 patient (3%).
CONCLUSION: Most DAVFs with cortical venous drainage have an aggressive clinical course. Treatment by a neurovascular team by using surgery, embolization, or a combination resulted in cure in all cases, with a very low complication rate.
Intracranial dural arteriovenous fistulas (DAVFs) with retrograde cortical venous drainage are aggressive lesions that can present with intracranial hemorrhage, seizures, progressive neurologic deficit, intracranial hypertension, or dementia.1–4 Prompt treatment is indicated because the natural history of untreated lesions is associated with a poor prognosis.5–6
Intracranial DAVFs can be classified according to the type of venous drainage.1, 7 Borden et al7 proposed a classification into 3 types of DAVF: type I with drainage to a dural sinus, type II with drainage to a dural sinus and reflux into cortical veins, and type III with drainage to the cortical veins only. Aggressive clinical presentation strongly correlates with Borden types: 2% of type I, 40% of type II, and 80% of type III DAVFs presented with intracranial hemorrhage or neurologic deficit.8
Treatment of DAVFs with cortical venous drainage is aimed at occlusion of the venous drainage or occlusion of all arterial supply and can be surgical, endovascular, or a combination.2, 9, 10 In this study, we report the incidence, clinical presentation, and treatment of intracranial DAVFs with cortical venous drainage.
Patients and Methods
Patients
From January 1994 onwards, all data from patients with dural fistulas who presented in our institution were prospectively recorded in a data base. Between January 1994 and January 2006, 130 patients with a DAVF were discussed in a joint meeting of neurosurgeons, neuroradiologists, and neurologists. There were 42 patients with DAVF type I with drainage to the transverse/sigmoid sinus, 39 patients with a spinal DAVF, 9 patients with a cavernous sinus DAVF, 4 patients with a DAVF draining to the vein of Galen only, 4 patients with a DAVF type II with drainage to the transverse/sigmoid sinus and reflux into cortical veins, 3 patients with a DAVF draining exclusively to the perimedullary veins, and 29 patients with a DAVF type III draining to cortical veins.
The 29 patients with DAVF type III are the subject of this study. There were 5 women and 24 men with a mean of 53.9 years of age (median, 53; range, 24–77 years).
Clinical Presentation
Clinical presentation was hemorrhage in 18 of 29 patients (62%), and 16 of these 18 patients had an intraparenchymal hematoma on CT scanning with the following locations: cerebellum in 4, occipital lobe in 4 (in 1 patient with a concomitant subdural hematoma), temporal lobe in 3, frontal lobe in 2, parietal lobe in 2, and basal ganglia in 1. In all patients, hematomas were located in the direct vicinity of the DAVF. Two patients presented with subarachnoid hemorrhage. In all patients who presented with hemorrhage, the DAVF was discovered on subsequent angiography. Four patients (14%) presented with seizures; 1 patient, with visual disturbances and conjunctival injection; 1 patient, with visual scotoma; and 1 patient, with pulsatile bruit. MR imaging in the 4 patients who presented with seizures showed dilated peripheral cortical veins suggestive of DAVF, confirmed on angiography. We discovered the following 4 DAVFs (14%) incidentally: 1 on angiography for radiosurgery of a cerebellar arteriovenous malformation (AVM), 1 during preoperative embolization of a meningeoma, 1 on angiography during carotid artery occlusion for a cavernous sinus aneurysm, and 1 on MR imaging performed for headaches. Venous drainage was to cortical cerebral veins in 20 patients, to cortical cerebellar veins in 5 patients, and to perimesencephalic or peripontine veins in 4 patients. Occlusion of the transverse/sigmoid sinus was apparent on MR imaging or angiography in 4 of 29 patients.
Treatment Strategy
Surgery was the treatment of choice for anterior fossa DAVFs. For all other DAVFs, embolization was the first treatment option, with the aim of complete cure. If complete cure could not be established, embolization was followed by surgery, consisting of disconnection of the venous drainage. Embolization was performed on a biplane angiographic unit (Integris BN 3000; Philips Medical Systems, Best, the Netherlands) in patients who were awake and under local anesthesia. After a complete angiographic work-up, arterial feeders were selectively catheterized with a microcatheter (Tracker 18, Tracker 10, Excel 14, or Excelsior SL-10, Boston Scientific, Fremont, Calif) to a point as close as possible to the fistula site. A mixture of 30% n-BCA (Histo-acryl; Braun, Melsungen, Germany) and 70% iodinated oil (Lipiodol; Guerbet, Rossy, France) was injected through the microcatheter, aimed at occlusion of the proximal part of the draining vein with the glue. If the venous side of the fistula could not be reached with glue (which was the case in most glue injections), other arterial feeders were catheterized and embolized, either until the venous drainage was occluded or until all or almost all arterial feeders were occluded. In some DAVFs supplied principally or only by the tentorial artery, this vessel was occluded with glue under protection of an occluding balloon (15 mm; Sentry, Boston Scientific) in the internal carotid artery at the level of the origin of the tentorial artery. In some patients with multiple arteries supplying the DAVF, some of the arterial feeders were occluded with coils in the process of embolization. Although occlusion of the draining vein with coils via a venous approach was considered in some cases, venous access was judged impossible or too difficult and this technique was not used. When angiographic cure was obtained, either with occlusion of all arterial feeders or with occlusion of the proximal draining vein, follow-up angiography after 6–12 weeks was scheduled to confirm stable obliteration of the DAVF. When angiographic cure could not be obtained with embolization, surgery was performed followed by angiography several days later to confirm complete obliteration of the DAVF.
Results
Incidence
DAVFs with cortical venous drainage accounted for 22% (29/130) of all central nervous system DAVFs and for 32% (29/91) of intracranial DAVFs.
Treatment Results
In 2 patients, the DAVF had been obliterated spontaneously at the time of scheduled embolization 10 and 3 months after hemorrhage. The first patient, a 31-year-old woman, had 2 right occipital intraparenchymal hemorrhages 10 and 2 months before referral to our institution. Although initial MR imaging findings had been misinterpreted as normal, in retrospect, dilated superficial veins were present on the right occipital pole consistent with a DAVF with cortical venous drainage. Eight months later, she had a 2nd hemorrhage and was referred to our hospital 2 months afterwards. Angiographic findings at the time of scheduled embolization were normal, except for some dilated occipital cortical veins in the late venous phase. On repeat MR imaging, the dilated cortical veins were no longer visible. Clinical and imaging findings were consistent with spontaneous regression of a DAVF with cortical venous drainage. The second patient was a 67-year-old man who presented at another institution with a right temporal hematoma. MR imaging and angiography demonstrated thrombosis of the right transverse and sigmoid sinus with a dural fistula to the cortical veins. Three months later, he was referred to our institution for treatment of the DAVF, but angiography showed recanalization of the sinus with disappearance of the fistula.
In 1 patient with a temporal DAVF incidentally discovered on angiography for radiosurgery for a cerebellar AVM, the DAVF was treated with radiosurgery during the same session, and results are pending. Five patients with an anterior fossa DAVF underwent successful surgery. In 14 patients, the DAVF was completely occluded with embolization alone, and in 7 patients, embolization was followed by surgery. Altogether, complete occlusion of the DAVF was angiographically confirmed in 28 of 29 DAVFs; the result of radiosurgery of 1 DAVF is not yet known.
Complications
There were no complications of surgery. Embolization was complicated by postembolization hemorrhage in a 61-year-old man with a left temporal DAVF admitted with a left temporal hematoma causing dysphasia and right-arm paresis. Angiography showed a left temporal DAVF supplied by the middle meningeal artery only and draining to the temporal cortical veins. Glue embolization was straightforward, with complete angiographic occlusion of the fistula. Several hours after embolization, the patient experienced headache with increased dysphasia and hemiparesis. A CT scan demonstrated enlargement of the temporal hematoma. Because the patient was stable, therapy was conservative. He gradually recovered with remaining mild dysphasia and right-arm dysfunction.
Representative Cases
Case 1.
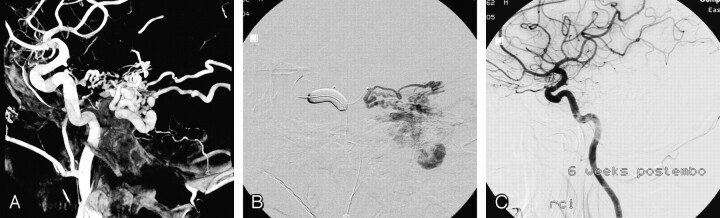
A 43-year-old man was referred to our institution for angiography (Fig 1). On MR imaging performed for headaches, dilated abnormal vessels in the right cerebellopontine angle were noted. Angiography showed a dural fistula with supply from the tentorial artery, lateral clival dural branches, middle meningeal artery, and occipital artery and draining into dilated lateral brain stem veins. The supply from the occipital artery and middle meningeal artery was embolized with glue, but there was no penetration into the veins. A microcatheter (Excelsior SL-10) was positioned into the tentorial artery with control of flow and reflux by an occlusion balloon. Subsequently, glue was injected that penetrated the draining veins. This resulted in complete occlusion of the fistula, confirmed with angiography 6 weeks later.
Fig 1.
A 43-year-old man with an incidentally discovered tentorial dural fistula.
A, Lateral view of 3D right internal carotid artery angiogram demonstrates a tentorial dural fistula mainly supplied by the tentorial artery. Additional arterial supply was by lateral clival branches, the occipital artery, and the middle meningeal artery.
B, Angiogram via microcatheter in the tentorial artery during balloon occlusion of the internal carotid artery at the level of the tentorial artery. The balloon prevents reflux, stabilizes the microcatheter, and induces flow control. From this point, glue was injected with deep penetration into the veins.
C, Follow-up angiogram 6 weeks later demonstrates complete closure of the fistula.
Case 2.
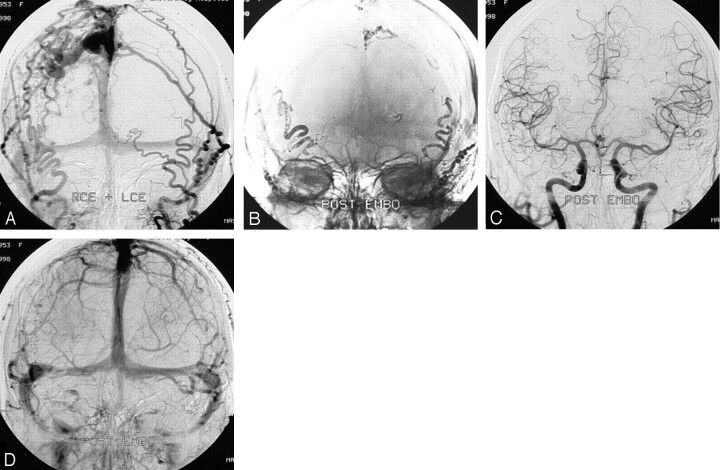
A 42-year-old woman (Fig 2) experienced sudden headache followed by left hemiparesis; a CT scan showed a right parietal hematoma. Angiography demonstrated a right parietal DAVF, supplied by right and left occipital and middle meningeal arteries and draining via cortical veins to the superior sagittal sinus. At embolization (in 1998), distal access in the tortuous feeding arteries could not be achieved. All 4 arterial feeders were proximally occluded with glue without occlusion of the draining veins. However, this resulted in angiographic cure, confirmed at follow-up angiography 3 months later. She made an uneventful recovery.
Fig 2.
A 42-year-old woman with a right parietal hematoma. In this early case, distal access was not possible, and glue injection resulted in rather proximal occlusion of the feeders instead of occlusion of the draining vein. Although unusual, this resulted in complete cure.
A, Frontal view of bilateral external carotid angiogram shows a right parietal DAVF supplied by right and left occipital and middle meningeal arteries and draining via cortical veins to the superior sagittal sinus.
B, Glue cast after embolization shows that all 4 arterial feeders are occluded without occlusion of the draining veins.
C and D, Arterial (C) and venous (D) phases of bilateral common carotid artery angiograms after embolization show complete occlusion of the DAVF, confirmed at follow-up angiography 3 months later.
Case 3.
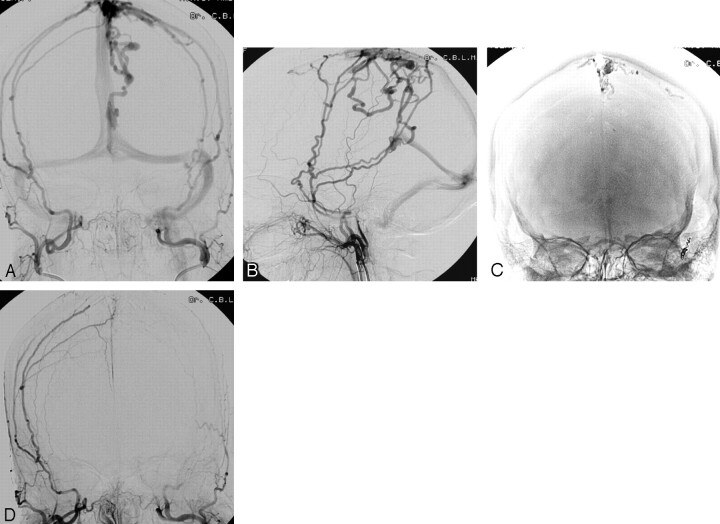
A 43-year-old woman (Fig 3) presented with a left parietal hematoma; angiography showed a DAVF supplied by right and left middle meningeal arteries with drainage to cortical veins and subsequently to the superior sagittal sinus and deep venous system. The anterior branch of the left middle meningeal artery was proximally occluded with coils. Subsequently, the posterior branch was catheterized, and the microcatheter could be advanced close to the fistula site. Glue was injected with good penetration into the draining veins, thereby occluding the fistula. Follow-up angiography 3 months later confirmed stable occlusion of the DAVF.
Fig 3.
A 43-year-old woman presenting with a left parietal hematoma.
A and B, Frontal (A) and lateral (B) views of bilateral external carotid artery angiograms reveal a DAVF supplied by the right and left middle meningeal arteries with drainage on the cortical veins and subsequently into the superior sagittal sinus and deep venous system.
C, Glue cast after embolization shows occlusion of the draining veins.
D, Bilateral external carotid angiogram confirms complete obliteration of the DAVF.
Case 4.
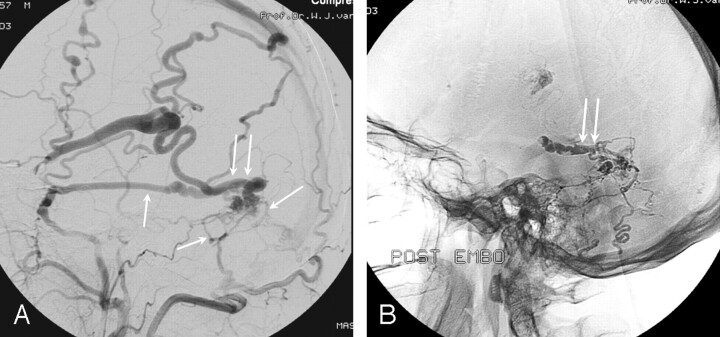
A 46-year-old man presented in another hospital with a seizure (Fig 4). MR imaging revealed dilated veins on the left cerebral convexity, and he was referred to our institution. Angiography showed a DAVF on the left sigmoid sinus supplied by the middle meningeal and occipital arteries with drainage to the vein of Labee. After occlusion of the occipital artery, glue injection in the middle meningeal artery resulted in occlusion of the draining vein of Labee.
Fig 4.
A 46-year-old man presenting with a seizure.
A, Lateral external carotid angiogram shows a DAVF on the left sigmoid sinus supplied by the middle meningeal and occipital arteries (single arrows), with drainage to the vein of Labee (double arrows).
B, Glue cast after embolization demonstrates occlusion of the draining vein of Labee (double arrows).
Discussion
DAVFs with cortical venous drainage are rare, and this type accounted for one third of all intracranial DAVFs treated in our institution. There was a striking preponderance of men versus women (24:5) harboring DAVFs with cortical venous drainage. Also in another large study,10 men were more often involved than women. On the other hand, benign DAVFs without cortical venous drainage more often are encountered in women.11, 12
In this study, angiographic cure was confirmed in 28 of 29 intracranial DAVFs with cortical venous drainage. The result of radiosurgery of 1 incidentally discovered DAVF is pending. Cure was obtained with surgery only in 5 anterior fossa DAVFs, with embolization only in 14 DAVFs and with a combination of embolization and surgery in 7 DAVFs. Two DAVFs were cured spontaneously after a long referral delay.
Our strategy of treatment included embolization as the first option for all DAVFs except for anterior fossa DAVFs. Arterial supply of anterior fossa DAVFs is usually derived from the ophthalmic artery, and embolization of this vessel has inherent risks. We used glue as the preferred embolic agent, and complications of occlusion of dural arteries supplying the fistula, such as cranial nerve dysfunction, did not occur. Although the goal of embolization was occlusion of the draining vein with glue, this could not be accomplished in all patients. During the 12 years of the study period, several technical advances in microcatheters and guidewires, such as braiding and hydrophilic coating, were introduced. With early material, distal access in long and tortuous feeders was often not possible, and glue injection often resulted in rather proximal occlusion of the feeders. However, in some patients, occlusion of all arterial supply resulted in complete cure (Fig 2). If complete obliteration could not be obtained with embolization, subsequent surgery consisting of disconnection of the venous outlet was successful in all cases without complications. Occlusion of the draining veins with glue is more likely to occur when the microcatheter is close to the fistula site, preferably in a wedge position with flow control.13 Because dural feeders are usually long and tortuous, navigation of the microcatheter should be done carefully to avoid dissection and spasm of small arteries. With modern microcatheters and guidewires, distal access is more often accomplished.
Where possible, retrograde transvenous occlusion with coils of the most proximal venous outlet is a safe endovascular option for curative treatment of aggressive DAVFs.14, 15 In selected cases, access to the draining vein is possible via arterial feeders,16 and in these cases, coil occlusion of the venous drainage is a safe alternative to glue embolization. In our patients, neither transvenous nor transarterial access to the draining vein was judged possible. With advances in microcatheters and guidewires, such distal venous and arterial access routes may become more feasible.
In conclusion, DAVFs with cortical venous drainage have an aggressive clinical course. Treatment by a neurovascular team by using surgery, embolization, or a combination results in cure in all patients, with a very low complication rate.
References
- 1.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671–80 [DOI] [PubMed] [Google Scholar]
- 2.Mullan S. Reflections upon the nature and management of intracranial and intraspinal vascular malformations and fistulae. J Neurosurg 1994;80:606–16 [DOI] [PubMed] [Google Scholar]
- 3.Cognard C, Casasco A, Toevi M, et al. Dural arteriovenous fistulas as a cause of intracranial hypertension due to impairment of cranial venous outflow. J Neurol Neurosurg Psychiatry 1998;65:308–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst RW, Bagley LJ, Galetta S, et al. Dementia resulting from dural arteriovenous fistulas: the pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol 1998;19:1267–73 [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk JM, terBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002;33:1233–36 [DOI] [PubMed] [Google Scholar]
- 6.Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg 1999;90:78–84 [DOI] [PubMed] [Google Scholar]
- 7.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995;82:166–79 [DOI] [PubMed] [Google Scholar]
- 8.Davies MA, TerBrugge K, Willinsky R, et al. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg 1996;85:830–37 [DOI] [PubMed] [Google Scholar]
- 9.Hoh BL, Choudhri TF, Connolly ES Jr, et al. Surgical management of high-grade intracranial dural arteriovenous fistulas: leptomeningeal venous disruption without nidus excision. Neurosurgery 1998;42:796–804 [DOI] [PubMed] [Google Scholar]
- 10.van Dijk JM, TerBrugge KG, Willinsky RA, et al. Selective disconnection of cortical venous reflux as treatment for cranial dural arteriovenous fistulas. J Neurosurg 2004;101:31–35 [DOI] [PubMed] [Google Scholar]
- 11.Satomi J, van Dijk JM, Terbrugge KG, et al. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg 2002;97:767–70 [DOI] [PubMed] [Google Scholar]
- 12.Raupp S, van Rooij WJ, Sluzewski M, et al. Type I cerebral dural arteriovenous fistulas of the lateral sinus: clinical features in 24 patients. Eur J Neurol 2004;11:489–91 [DOI] [PubMed] [Google Scholar]
- 13.Nelson PK, Russell SM, Woo HH, et al. Use of a wedged microcatheter for curative transarterial embolization of complex intracranial dural arteriovenous fistulas: indications, endovascular technique, and outcome in 21 patients. J Neurosurg 2003;98:498–506 [DOI] [PubMed] [Google Scholar]
- 14.Defreyne L, Vanlangenhove P, Vandekerckhove T, et al. Transvenous embolization of a dural arteriovenous fistula of the anterior cranial fossa: preliminary results. AJNR Am J Neuroradiol 2000;21:761–65 [PMC free article] [PubMed] [Google Scholar]
- 15.Kallmes DF, Jensen ME, Cloft HJ, et al. Percutaneous transvenous coil embolization of a Djindjian type 4 tentorial dural arteriovenous malformation. AJNR Am J Neuroradiol 1997;18:673–76 [PMC free article] [PubMed] [Google Scholar]
- 16.Layton KF, Nelson MD, Kallmes DF. Transarterial coil embolization of the venous component of aggressive type 4 dural arteriovenous fistulas. AJNR Am J Neuroradiol 2006;27:750–52 [PMC free article] [PubMed] [Google Scholar]