Abstract
BACKGROUND AND PURPOSE: After an early progression of signal intensity changes in T2-weighted MR images, also known as “neurofibromatosis bright objects,” in patients with neurofibromatosis type 1 (NF-1), there is a tendency toward regression or even disappearance in early adulthood. The purpose of this study was to investigate whether adult patients with NF-1 exhibit generalized microstructural alterations even in normal-appearing brain regions.
MATERIALS AND METHODS: Conventional and diffusion tensor MR imaging of the brain was obtained in 10 adult patients with NF-1 and 10 age-matched healthy volunteers. Apparent diffusion coefficient (ADC) and fractional anisotropy (FA) were measured in brain stem, basal ganglia, thalamus, corpus callosum, and frontal and parietooccipital white matter regions.
RESULTS: Significantly increased ADC and decreased FA values were found in all regions of interest and in all patients with NF-1, irrespective of their scholastic achievement and subsequent professional performance, compared with control subjects (P < .001). There were no significant correlations with the age (P > .1) or with the lateralization between brain hemispheres (P > .05).
CONCLUSION: Diffusion tensor imaging reveals globally elevated FA and decreased ADC values in the mature brains of patients with NF-1, which is most likely a consequence of diffuse and basic alterations in cerebral microstructure that result from the underlying gene mutation.
Neurofibromatosis type 1 (NF-1) is the most prevalent phakomatosis and is characterized by multiple neurofibromas of various organs, changes in skin pigmentation, and deformation of the skeleton. NF-1 is an autosomal dominant disorder, occurring with a prevalence of 2–3 cases per 10,000 population1; half of the cases represent new mutations.2
Brain lesions in NF-1 include optic pathway gliomas, cerebral astrocytomas, aqueductal stenosis, and, predominantly in children, focal hyperintense areas in T2-weighted MR imaging also known as “neurofibromatosis bright objects” (NBO) or “focal areas of signal intensity”.3 The latter is considered a characteristic feature in children with NF-1,4 but it cannot be used as a diagnostic criterion.5 “Typical” NBOs do not exert mass effect, do not enhance with contrast agents, and are not visible on CT. In particular, they are not surrounded by edema.4,6–10 In most cases, NBOs are found in basal ganglia, thalamus, cerebellum, brain stem, and subcortical white matter (WM),4,5,11 with an incidence of 43%–77%.4,6,8,11,12 The frequency of occurrence seems to be related to both the presence of optic pathway gliomas and the age13; NBOs begin to appear at the age of 3 years and increase in number and size until the age of 10 years, whereupon they decrease and most often disappear completely until adulthood.8,14,15 The exact nature and the relevance of NBOs are still unclear. It has been suggested that abnormal myelin might be their source.11,14 The commonly accepted theory is that spongiotic and vacuolating alterations of myelin structure are responsible, which is based on the few histopathologic examinations reported by DiPaolo et al,16 who also found microcalcifications in the globus pallidus.
Patients with NF-1 often present with learning disabilities and megalencephaly,1,17,18 pointing to the possibility of a more generalized alteration of the brain microstructure. To better understand the underlying anatomic disturbances, the purpose of this study was to investigate whether adult patients with NF-1 exhibit generalized microstructural alterations even in normal-appearing brain regions. Therefore, we determined the apparent diffusion coefficient (ADC) and the fractional anisotropy (FA) of different brain regions using diffusion tensor imaging (DTI).
Materials and Methods
Subjects.
In this context, we refer to subjects affected by NF-1 as “patients.” The patients participating in this study were known previously to our NF clinic and were recruited through personal contacts to the Swiss Neurofibromatosis Organization. They fulfilled the diagnostic criteria for NF-1 based on a National Institutes of Health 1988 Consensus5 and were not known to suffer from obvious pathologic brain conditions (eg, brain tumor, epilepsy). There were 10 unrelated patients (4 women and 6 men), ranging in age from 18 to 36 years (mean age ± SD: 25.8 ± 5.4 years). We focused on this age group because no myelin remodeling (NBO) is to be expected at this age.8,14,15
Two patients (P2, P6) attended special schools because of their marked and severe learning difficulties; the schooling of the other patients was age-appropriate. The professional training is as follows: P1 and P4 obtained a university degree. Six patients are in full-time employment and self-sufficient, 3 of them (P5, P9, P10) were in 3-year vocational training, and 3 (P3, P7, P8) in 2-year vocational training. P2 works part-time (manual work), whereas P6 lives in an institution for handicapped people. Ten age-matched healthy volunteers (4 women, 6 men; age range, 20–36 years; mean age ± SD, 26.3 ± 4.6 years) served as control subjects.
The study was carried out according to the ethic guidelines of the hospital as defined by the Helsinki Declaration. Written informed consent was obtained from all subjects or from their parents if appropriate.
MR measurements were carried out on a 1.5T whole-body MR system. Standard diagnostic T1-weighted (3D echo-spoiled gradient echo; TR, 27 ms; TE, 6 ms; number of acquisitions, 1; image resolution, 0.94 × 0.94 × 1.6 mm3) and T2-weighted (fast spin-echo; TR, 8060 ms; TE, 104 ms; number of acquisitions, 2; image resolution, 0.51 × 0.51 × 4.0 mm3) images were acquired. DTI was measured using a diffusion-weighted, single-shot, echo-planar imaging (EPI) sequence, with an image size of 128 × 128 and an in-plane resolution of 2 × 2 mm2. The whole brain was covered with 38 contiguous axial sections at a section thickness of 3 mm. The TR and TE were 6.5 seconds and 80 ms, respectively. A noise-optimized diffusion encoding schema with 55 diffusion encoding directions was used.19,20 The b factor was 1000 s/mm2, and 5 b0 images were measured.
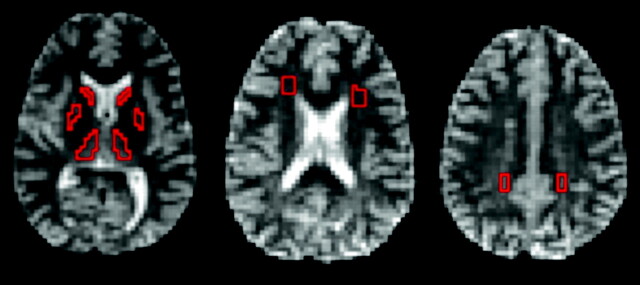
Calibration of DTI measurements was done on a water phantom. We defined the ADC as the trace of the diffusion tensor (D′), equivalent to the ADC of isotropic media. ADCs were compared with temperature-corrected literature data.21 Diffusion tensor maps were calculated pixel-by-pixel using custom software on the MATLAB platform (The Mathworks, Natick, Mass). ADC and FA were estimated in deep gray matter (ie, in caudate nucleus, lentiform nucleus [putamen and globus pallidus], and thalamus) and in WM (parietooccipital, frontal), pons, and corpus callosum (Fig 1). To avoid partial volume effects, regions of interest (ROIs) were selected pixel-by-pixel based on ADC, FA, and b0 images rather than using predefined ROIs. All ROIs for patients and control subjects were selected by the same investigator (S.L.Z.) individually based on anatomic landmarks in each subject. The ROIs from the corpus callosum were only chosen in a single plane to avoid partial volume effects. High-resolution T1- and T2-weighted MR images were used as references, but ROI selection was done on the ADC, FA, and b0-images to avoid misregistration between EPI and high-resolution images caused by the intrinsic susceptibility sensitivity of EPI.
Fig 1.
Images of a 20-year-old woman with NF-1. Axial B0 images showing the pixel-wise chosen ROIs framed in red: caudate nucleus, thalamus, lentiform nucleus (putamen and globus pallidus) (left), frontal WM (middle), and parietooccipital WM (right).
Statistical Procedure.
Statistical analyses were performed using SPSS version 11.5.1 (SPSS, Chicago, Ill). Mean ROI ADC and FA values of the different brain areas were analyzed separately in a multivariate analysis of variance with the between subject factor “group” (“patients with NF-1” and “healthy volunteers”). The within-group age dependence of ADC and FA values was tested separately for each region of interest by using a nonparametric bivariate correlation analysis (Kendall τ-b and Spearman). A paired t test was performed on all bilaterally measured ROI ADC and FA values to test for hemispheric differences within the groups.
Results
Lateralization
ROI data were analyzed with regard to a lateralization effect to exclude a local (ie, hemispheric specific) effect and proof that the alteration of ADCs and FAs is global. No differences in ADC or FA (P > .05) were found between left- and mirrored right-sided anatomic locations in the control group (Table 1). The patients with NF-1, on the contrary, showed differences in the ADC values between the 2 hemispheres (P < .05), without revealing a consistent asymmetry. On the other hand, the FA values in these patients did not show hemispheric differences (P > .05), except for the parietooccipital WM regions (P < .05). Based on these heterogeneous laterality findings, we decided to proceed with the analysis with averaged region of interest data of both hemispheres.
Table 1:
Lateralization of regional ADC and FA in patients with NF-1 and healthy volunteers (mean ± SD and significance of difference)
| ROI and Hemisphere | Patients with NF-1 |
Healthy Volunteers |
||||||
|---|---|---|---|---|---|---|---|---|
| ADC | P(R vs L) | FA | P(R vs L) | ADC | P(R vs L) | FA | P(R vs L) | |
| Caudate nucleus | ||||||||
| R | 0.94 ± 0.02 | .006 | 0.13 ± 0.02 | .056 | 0.78 ± 0.02 | .539 | 0.18 ± 0.00 | .596 |
| L | 0.96 ± 0.03 | 0.12 ± 0.02 | 0.79 ± 0.03 | 0.18 ± 0.00 | ||||
| Lentiform Nucleus | ||||||||
| R | 0.95 ± 0.02 | .086 | 0.10 ± 0.01 | .160 | 0.76 ± 0.03 | .302 | 0.14 ± 0.01 | .063 |
| L | 0.97 ± 0.02 | 0.10 ± 0.01 | 0.76 ± 0.02 | 0.15 ± 0.01 | ||||
| Thalamus | ||||||||
| R | 0.99 ± 0.03 | .033 | 0.19 ± 0.02 | .683 | 0.77 ± 0.02 | .703 | 0.28 ± 0.01 | .719 |
| L | 0.98 ± 0.02 | 0.19 ± 0.02 | 0.77 ± 0.02 | 0.28 ± 0.01 | ||||
| Frontal WM | ||||||||
| R | 0.95 ± 0.02 | .0002 | 0.26 ± 0.04 | .906 | 0.79 ± 0.04 | .355 | 0.34 ± 0.01 | .098 |
| L | 0.99 ± 0.02 | 0.26 ± 0.06 | 0.80 ± 0.03 | 0.37 ± 0.01 | ||||
| Parietooccipital WM | ||||||||
| R | 0.96 ± 0.04 | .009 | 0.36 ± 0.04 | .026 | 0.77 ± 0.03 | .617 | 0.48 ± 0.02 | .111 |
| L | 0.93 ± 0.03 | 0.33 ± 0.05 | 0.76 ± 0.03 | 0.45 ± 0.02 | ||||
Note:—ADC indicates apparent diffusion coefficient; FA, fractional anisotropy; NF-1, neurofibromatosis type 1; ROI, region of interest; WM: white matter; R, right; L, left.
Apparent Diffusion Coefficient and Fractional Anisotropy
Patients with NF-1 showed a significant increase in ADC values (P < .001) and a significant decrease in FA values (P < .001) compared with the healthy volunteers in all selected brain regions (Table 2). To exclude a systematic difference between the 2 populations, we also analyzed ADC values of the CSF, which did not show significant differences between groups (P > .5). FA of CSF was within noise level as expected for isotropic media.
Table 2:
Comparison of regional ADC and FA in patients with NF-1 and healthy volunteers (mean ± SD and significance of difference)
| ROI | Patients with NF-1 |
Healthy Volunteers |
||||
|---|---|---|---|---|---|---|
| ADC | FA | P < | ADC | FA | P < | |
| Caudate nucleus | 0.95 ± 0.03 | 0.13 ± 0.02 | .001 | 0.79 ± 0.03 | 0.18 ± 0.01 | .001 |
| Lentiform nucleus | 0.96 ± 0.02 | 0.10 ± 0.01 | .001 | 0.76 ± 0.02 | 0.14 ± 0.02 | .001 |
| Thalamus | 0.99 ± 0.03 | 0.19 ± 0.02 | .001 | 0.77 ± 0.02 | 0.28 ± 0.02 | .001 |
| Pons | 0.97 ± 0.03 | 0.30 ± 0.03 | .001 | 0.77 ± 0.03 | 0.42 ± 0.03 | .001 |
| Corpus callosum | 1.03 ± 0.05 | 0.56 ± 0.07 | .001 | 0.82 ± 0.04 | 0.76 ± 0.03 | .001 |
| Frontal WM | 0.97 ± 0.03 | 0.26 ± 0.05 | .001 | 0.79 ± 0.03 | 0.35 ± 0.05 | .001 |
| Parietooccipital WM | 0.95 ± 0.04 | 0.35 ± 0.05 | .001 | 0.76 ± 0.03 | 0.46 ± 0.06 | .001 |
| CSF | 3.06 ± 0.39 | .769 | 3.01 ± 0.43 | |||
Note:—ADC indicates apparent diffusion coefficient; FA, fractional anisotropy; NF-1, neurofibromatosis type 1; ROI, region of interest; WM: white matter.
Age Effect
There was no correlation with age for either ADC or FA in all selected brain regions of patients and control subjects (P > .1).
T2-Weighted Imaging
Two of the 10 patients with NF-1 showed hyperintense lesions on the T2-weighted imaging. P1 had an NBO in the thalamus bilaterally. P4 had faint T2 hyperintensities in the region of the caudate nucleus, the left substantia nigra, and the right thalamus. Yet the mean ADC and FA values in P1 and P4 lay within the corresponding confidence intervals for these regions.
MR imaging in P6 revealed ventricular dilation. The FA and ADC values of the right putamen, the right thalamus, the corpus callosum, and the left frontal WM were outside the 95% confidence intervals of the patient group. However, excluding P6 from the statistical analysis did not change the significances of the differences between groups. None of the control subject had hyperintense lesions in the brain.
Discussion
Elevated ADC and decreased FA values were found in every investigated anatomic brain location of patients with NF-1 compared with the age-matched control subjects (P < .001). We could corroborate the findings of former studies concerning the elevated ADC. Alkan et al22 found elevated ADC values in NBO and in some normal-appearing regions (hippocampus and thalamus), whereas Eastwood et al23 revealed elevated ADC values in hyperintense lesions as well as in normal-appearing basal ganglia compared with the control group. In a longitudinal study Sheikh et al also found increased ADC values in NBOs.24
Moreover, diffusion-weighted imaging (DWI) and DTI have successfully been applied to detect microstructural changes in patients with different brain disorders. Ashtari et al25 reported decreased FA values in several brain regions (normal-appearing WM: frontal, parietal, temporal, occipital, splenium of the corpus callosum, posterior limb of the internal capsule, and WM lesions frontal and parietal) of patients with attention-deficit/hyperactivity disorder. Fukuda et al26 investigated patients with myotonic dystrophy and described lower FA values in the cerebral WM of the patients compared with healthy control subjects.
Although Alkan et al,22 Eastwood et al,23 and Sheikh et al24 did not determine FA in their NF-1 patient groups, our study revealed reduced FA values in all selected ROIs and in all patients. Our results indicate microstructural changes of brain tissue. A reduction in FA and an increase in ADC might indicate a disintegration of the myelin sheaths and/or an axonal disruption.27 The observed increase in diffusion in the brains of patients with NF-1 may originate from a myelin disorder, such as an intramyelinic edema.16 Sheikh et al24 explained their findings as a result of increased number and/or size of myelin vacuoles. On the other hand Alkan et al22 saw their results in the context of a possible demyelination. Eastwood et al23 also explained it as demyelination or “as myelin disturbance, such as diminished myelin amount or increased myelin turnover.”
Using DWI, Tognini et al28 found increased ADCs in normal-appearing brain as well as in regressed NBO. DiPaolo et al16 did not observe a “frank demyelination” in their histopathologic findings of NBOs in 3 patients with NF-1. Instead, they reported a spongiform myelinopathy or a vacuolar change of myelin. To our knowledge, this is the only realized histopathologic investigation into hyperintense lesions in patients with NF-1. These findings, however, may not be truly representative for the entire NF-1 population, because 2 of their patients had severe clinical symptoms: 1 was a premature baby with an intraventricular hemorrhage and seizures, and the other received chemotherapy for treatment of fibrosarcoma. Only the 3rd patient, who died as a result of complications during a scoliosis surgery, seemed to represent the “usual” NF-1 patient without comorbidity. Nevertheless, DiPaolo et al reported identical histopathologic findings in all 3 patients.
One could speculate that the alterations of ADC and FA in patients with NF-1 reported here might have originated from globally increased brain water. However, the only existing histologic study in patients with NF-116 reported no conspicuousness concerning the brain water content in these patients but did report a spongiform myelinopathy or a vacuolar change of myelin. Notwithstanding these findings, we cannot exclude globally increased brain water as the reason for the altered ADC and FA values in NF-1 patients. However, the observed alterations in brain diffusion are more likely to indicate a dismyelination in patients with NF-1.
In many NF clinics neuroimaging is not considered an essential investigation at initial presentation of a patient with NF-1 who has normal neurologic states, including vision. Therefore, no previous MR imaging is available in our patient group. Thus, whether they had NBOs in some of the investigated regions during childhood is not known. Alkan et al22 reported significantly increased ADCs in normal-appearing hippocampus and thalamus on T2-weighted images, which they considered residual lesions from former NBOs. Based on these reports, it seems to be very unlikely that our ADC changes in each of the examined brain areas originate from such residual lesions.
The great variability of FA and ADC values of P6 with ventricular dilation, which were outside the normative confidence intervals, might be explained by partial volume effects between brain tissue and CSF.
Sheikh et al24 examined 12 patients with NF-1 between 2–20 years. They speculated that ADCs may approach more near-normal values with increasing age. Nevertheless, the data presented in their Fig 2 depict ADC values in adult patients with NF-1 that are higher than in the age-matched control subjects, which corresponds well to our findings.
Although Alkan et al,22 Sheikh et al,24 and Tognini et al28 reported similar alterations of ADC, a quantitative comparison with the results reported in this study is not appropriate because of methodologic dissimilarities and differences in the examined age range of patients with NF-1.
We did not observe any age dependency in our adult patients, which accords closely with findings of Snook et al29 and may be explained by the completed myelination process in adult brains. Longitudinal measurements from childhood to adulthood would be required to answer the question of whether there is a recovery of altered myelination in patients with NF-1.
Few data have been published on hemispheric differences in patients with NF-1. Eastwood et al23 did not observe significant ADC variations between hemispheres, except for the thalamus. Our data revealed hemispheric ADC differences in caudate, thalamus, and WM (parietooccipital and frontal), but no global left-right tendency was found. We did not detect hemispheric differences in FA, except in the parietooccipital WM. Although we have not performed neuropsychologic testing in our patients, it is remarkable that identical findings were obtained in university graduates and in patients who attended special school.
Conclusion
We found elevated ADC and decreased FA values in all selected brain regions of all patients with NF-1 compared with age-matched healthy volunteers. We interpret our findings to be a consequence of diffuse and basic alterations in cerebral microstructure in the mature NF brain, resulting from the underlying gene mutation.
Footnotes
S.L.Z. and T.L. contributed equally to this work.
References
- 1.Friedman JM, Gutmann DH, Riccardi VM. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. Baltimore: The Johns Hopkins University Press;1999
- 2.Viskochil D. Genetics of neurofibromatosis 1 and the NF-1 gene. J Child Neurol 2002;17:562–70; discussion 571–562, 646–51 [DOI] [PubMed] [Google Scholar]
- 3.Gonen O, Wang ZJ, Viswanathan AK, et al. Three-dimensional multivoxel proton MR spectroscopy of the brain in children with neurofibromatosis type 1. AJNR Am J Neuroradiol 1999;20:1333–41 [PMC free article] [PubMed] [Google Scholar]
- 4.North K. Neurofibromatosis type 1. Am J Med Genet 2000;97:119–27 [DOI] [PubMed] [Google Scholar]
- 5.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol 1988;45:575–78 [PubMed] [Google Scholar]
- 6.Barkovich AJ. Pediatric Neuroimaging, 4th ed. Philadelphia: Lippincott Williams & Wilkins;2005
- 7.Hyman SL, Gill DS, Shores EA, et al. Natural history of cognitive deficits and their relationship to MRI T2-hyperintensities in NF-1. Neurology 2003;60:1139–45 [DOI] [PubMed] [Google Scholar]
- 8.Menor F, Marti-Bonmati L, Arana E, et al. Neurofibromatosis type 1 in children: MR imaging and follow-up studies of central nervous system findings. Eur J Radiol 1998;26:121–31 [DOI] [PubMed] [Google Scholar]
- 9.Mirowitz SA, Sartor K, Gado M. High-intensity basal ganglia lesions on T1-weighted MR images in neurofibromatosis. AJR Am J Roentgenol 1990;154:369–73 [DOI] [PubMed] [Google Scholar]
- 10.Terada H, Barkovich AJ, Edwards MS, et al. Evolution of high-intensity basal ganglia lesions on T1-weighted MR in neurofibromatosis type 1. AJNR Am J Neuroradiol 1996;17:755–60 [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh T, Magnaldi S, White RM, et al. Neurofibromatosis type 1: the evolution of deep gray and white matter MR abnormalities. AJNR Am J Neuroradiol 1994;15:1513–19 [PMC free article] [PubMed] [Google Scholar]
- 12.Moore BD, Slopis JM, Schomer D, et al. Neuropsychological significance of areas of high signal intensity on brain MRIs of children with neurofibromatosis. Neurology 1996;46:1660–68 [DOI] [PubMed] [Google Scholar]
- 13.Aoki S, Barkovich AJ, Nishimura K, et al. Neurofibromatosis types 1 and 2: cranial MR findings. Radiology 1989;172:527–34 [DOI] [PubMed] [Google Scholar]
- 14.Sevick RJ, Barkovich AJ, Edwards MS, et al. Evolution of white matter lesions in neurofibromatosis type 1: MR findings. AJR Am J Roentgenol 1992;159:171–75 [DOI] [PubMed] [Google Scholar]
- 15.Griffiths PD, Blaser S, Mukonoweshuro W, et al. Neurofibromatosis bright objects in children with neurofibromatosis type 1: a proliferative potential? Pediatrics 1999;104:e49. [DOI] [PubMed] [Google Scholar]
- 16.DiPaolo DP, Zimmerman RA, Rorke LB, et al. Neurofibromatosis type 1: pathologic substrate of high-signal-intensity foci in the brain. Radiology 1995;195:721–24 [DOI] [PubMed] [Google Scholar]
- 17.North K. Neurofibromatosis Type I in Childhood. London: MacKeith Press;1997
- 18.Moore BD 3rd, Slopis JM, Jackson EF, et al. Brain volume in children with neurofibromatosis type 1: relation to neuropsychological status. Neurology 2000;54:914–20 [DOI] [PubMed] [Google Scholar]
- 19.Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 1999;42:515–25 [PubMed] [Google Scholar]
- 20.Alexander DC, Barker GJ. Optimal imaging parameters for fiber-orientation estimation in diffusion MRI. Neuroimage 2005;27:357–67 [DOI] [PubMed] [Google Scholar]
- 21.Holz M, Heil SR, Sacco A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate H-1 NMR PFG measurements. Phys Chem Chem Phys 2000;2:4740–42 [Google Scholar]
- 22.Alkan A, Sigirci A, Kutlu R, et al. Neurofibromatosis type 1: diffusion weighted imaging findings of brain. Eur J Radiol 2005;56:229–34 [DOI] [PubMed] [Google Scholar]
- 23.Eastwood JD, Fiorella DJ, MacFall JF, et al. Increased brain apparent diffusion coefficient in children with neurofibromatosis type 1. Radiology 2001;219:354–58 [DOI] [PubMed] [Google Scholar]
- 24.Sheikh SF, Kubal WS, Anderson AW, et al. Longitudinal evaluation of apparent diffusion coefficient in children with neurofibromatosis type 1. J Comput Assist Tomogr 2003;27:681–86 [DOI] [PubMed] [Google Scholar]
- 25.Ashtari M, Kumra S, Bhaskar SL, et al. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry 2005;57:448–55 [DOI] [PubMed] [Google Scholar]
- 26.Fukuda H, Horiguchi J, Ono C, et al. Diffusion tensor imaging of cerebral white matter in patients with myotonic dystrophy. Acta Radiol 2005;46:104–09 [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 2002;15:435–55 [DOI] [PubMed] [Google Scholar]
- 28.Tognini G, Ferrozzi F, Garlaschi G, et al. Brain apparent diffusion coefficient evaluation in pediatric patients with neurofibromatosis type 1. J Comput Assist Tomogr 2005;29:298–304 [DOI] [PubMed] [Google Scholar]
- 29.Snook L, Paulson LA, Roy D, et al. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 2005;26:1164–73 [DOI] [PubMed] [Google Scholar]