Abstract
BACKGROUND AND PURPOSE: We investigated the association of multisection CT angiography (MSCTA) and perfusion CT (PCT) for the characterization of vasospasm secondary to aneurysmal subarachnoid hemorrhage.
Materials and METHODS: Among 27 patients with symptomatic cerebrovascular vasospasm investigated by digital subtraction angiography (DSA), 18 underwent both cerebral PCT and MSCTA. For the remaining 9, only PCT or MSCTA could be performed. MSCTA was compared with DSA for the detection and characterization of vasospasm on 286 intracranial arterial segments. PCT maps were visually reviewed for mean transit time, relative cerebral blood flow, and relative cerebral blood volume abnormalities and were qualitatively compared with the corresponding regional vasospasm detected by DSA.
RESULTS: Vasospasm was grouped into 2 categories: mild-moderate and severe. The depiction of vasospasm by MSCTA showed the best sensitivity, specificity, and accuracy at the level of the A2 and M2 arterial segments (100% for each), in contrast to the carotid siphon (45%, 100%, and 85% respectively). The characterization of vasospasm severity by MSCTA showed a sensitivity, specificity, and accuracy of 86.8%, 96.8%, and 95.2%, respectively, for mild-moderate vasospasm, and 76.5%, 99.5%, and 97.5%, respectively, for severe vasospasm. The PCT abnormalities were related to severe vasospasm in 9 patients and to mild-to-moderate vasospasm in 2. The sensitivity, specificity, and accuracy of PCT in detecting vasospasm were 90%, 100%, and 92.3%, respectively, for severe vasospasm, and 20%, 100%, and 38.5%, respectively, for mild-moderate vasospasm.
CONCLUSION: MSCTA/PCT can assess the location and severity of cerebrovascular vasospasm and its related perfusion abnormalities. It can identify severe vasospasm with risk of delayed ischemia and can thus guide the invasive treatment.
Cerebral vasospasm is a major cause of delayed neurologic morbidity after a subarachnoid hemorrhage (SAH). Cerebral ischemia secondary to vasospasm occurs in 20%–30% of these patients1 and has been correlated with a 1.5–3-fold increase in mortality in the first 2 weeks after SAH.2 The incidence of long-term ischemic deficits is estimated to be between 1% and 14%.3 Because aggressive treatment of vasospasm increases the risk of hemorrhage and brain edema,4–6 a noninvasive yet reliable, fast, and accurate diagnostic work-up is necessary to discern those patients who are in need of such an active therapy. Transcranial Doppler sonography is the most widely used imaging technique for detection of vasospasm,7 but it has clear limitations.8–13
The standard of reference for anatomic demonstration of cerebral vasospasm is digital subtraction angiography (DSA). DSA provides an accurate depiction of the intracranial vessels; however, it is an invasive procedure carrying a total complication rate of 0.2% when performed in patients without ischemic disease.14,15 Multisection CT angiography (MSCTA) has recently emerged as a reliable and accurate method for the anatomic depiction of intracranial vessels16 and offers the potential for the rapid diagnosis and monitoring of cerebral vasospasm. Because of the possibility of multiplanar segmentations, it allows an accurate depiction of the intracranial vasculature. Considering that a substantial percentage of patients with cerebrovascular vasospasm are often comatose or agitated, DSA often requires specialized anesthetic care that ensures sedation or general anesthesia. The very fast imaging afforded by MSCTA can overcome these limitations. Although MSCTA can precisely show the morphologic details of the subarachnoid vessels, it cannot provide any functional information about tissue perfusion.
Perfusion CT (PCT) imaging, in contrast to MSCTA, relies on cerebral hemodynamics rather than on anatomic vascular depiction; it provides an accurate noninvasive survey of the hemodynamic effects of vasospasm on the brain parenchyma. PCT is increasingly being used in the evaluation of acute ischemic stroke in emergent situations to assess the amount of irreversible ischemia (core) compared with reversible ischemia (penumbra).17 Angiographic vasospasm has been demonstrated to decrease regional cerebral blood flow, particularly when a severe vasospasm (luminal reduction superior to 50%) is present.18–20
PCT can thus be used to assess cerebral ischemia and infarction as a result of vasospasm after SAH. The goal of this study was is to compare MSCTA to DSA with respect to their ability to accurately detect significant cerebral vasospasm in patients with subarachnoid hemorrhage and to evaluate the contribution of PCT in this clinical setting.
Materials and Methods
Patients
During the period from September 2002 to September 2004, 27 consecutive patients (13 men and 14 women; median age, 50 years; range, 31–69 years) with clinical signs of intracranial arterial vasospasm after aneurysmal SAH were evaluated by DSA and CT within a 1–12-hour interval and included in the present study. Clinical vasospasm was characterized by a diminished level of consciousness in 11 patients, a frontal syndrome in 7, hemiplegia in 6, and aphasia in 3. Among the 27 patients, 18 underwent successive MSCTA and PCT during the same session; the remaining 9 could not undergo both MSCTA and PCT, so only one of the procedures was performed. This was due principally to an agitated state in 6 patients; in 3 patients the renal function was limited and only MSCTA was performed. All together, 22 patients underwent MSCTA and 23 underwent PCT. DSA was performed on all patients as part of the standard treatment and was never performed solely for the purpose of the present study. Before considering MSCTA and PCT in this study, all patients were admitted to our neurosurgical intensive care unit with a central intravenous catheter, intravenous hydration, and oral administration of nimodipine. Informed consent was obtained from the patients or their relatives in all cases. Our review board approved this study protocol, and institutional informed consent guidelines were followed.
Imaging Protocols
A 16-row CT unit was used. The CT protocol consisted of a baseline unenhanced cerebral CT with approximately 30 5-mm transverse sections acquired at 120 kVp and 200 mA, immediately followed by PCT and MSCTA. PCT consisted of a 40-second acquisition, with 40 gantry rotations performed at a rate of 1 rotation per second in cine mode during intravenous administration, with the use of a power injector, of 50 mL of iohexol (300 mg/mL of iodine) at a rate of 5 mL/s into an antecubital vein. The acquisition parameters for PCT series were 80 kVp and 100 mA.21 Two acquisitions of 2 adjacent 10-mm sections were performed, generating 2 sets of images distributed on 2 anatomic planes of the brain. The first cerebral plane was selected above the orbit to protect the lenses, at the level of the third ventricle and the basal ganglia. The second plane was selected at the level of the lateral ventricles and the centrum semiovale. The PCT sections were matched with 4 unenhanced cerebral CT sections.
MSCTA was performed 5 minutes after the last series of PCT. The optimal timing of the MSCTA acquisition was selected according to the enhancement timing recorded during the PCT series. The MSCTA data acquisition was performed according to the following parameters: spiral mode; 0.5-second rotations; 16-row collimation at 0.625 mm; pitch, 1.375; section thickness, 0.625 mm; reconstruction interval; 0.5 mm, and acquisition parameters, 120 kVp/280 mA. A caudocranial scanning direction was selected, covering the volume extending from a point situated 1 cm below the foramen magnum up to the roof of the lateral ventricles. The injected volume was 50 mL with an injection rate of 5 mL/s. The overall effective dose equivalent required for the global CT protocol was calculated in each patient. Four-vessel DSA was performed via a transfemoral intra-arterial approach with multiples projections.
Perfusion CT Data Processing and Analysis
PCT data consist of time-contrast enhancement curves registered in each pixel, linearly related to time-concentration curves for the iodinated contrast material. PCT data were analyzed using the PCT software developed by Philips Medical Systems (Cleveland, OH). This software relies on the central volume principle, which is the most accurate for low injection rates of iodinated contrast material.22 The central volume principle applies a mathematical operation called deconvolution to calculate the mean transit time (MTT).23–25 The deconvolution operation requires a reference arterial input function, which is selected by the PCT software in a region of interest drawn by the user around the A2 segments of the anterior cerebral artery. The relative cerebral blood volume (rCBV) is calculated from the areas under the time-enhancement curves.26,27 A simple equation combining rCBV and MTT values allows the calculation of relative cerebral blood flow (rCBF): rCBF = rCBV/MTT.25 The PCT maps consisted of MTT, cerebral blood volume (CBV), and cerebral blood flow (CBF) maps. PCT images were reviewed individually by 3 neuroradiologists, and a consensus was planned thereafter. rCBV, rCBF, and MTT maps were evaluated for abnormal rCBV, rCBF, and MTT values on the anterior, middle, and posterior cerebral artery territories of each hemisphere to compare the presumed pathologic hemisphere with the presumed healthy one by visual observation of the generated maps. The abnormalities on the PCT maps were correlated with the vasospasm severity assessed by DSA. The relationship between the PCT results and the decision to perform an endovascular treatment was also analyzed.
MSCTA Data Processing and Analysis
The review of MSCTA was performed on a workstation (Advantage Windows; GE Healthcare, Milwaukee, Wis) to allow interactive reconstruction and interpretation, which has proved to be more accurate than an isolated review of hardcopy images.28 Axial raw images, multiplanar 2D reconstructions, maximum intensity projection (MIP) reconstructions, and shaded surface display reconstructions with volume rendering technique were used for MSCTA review.29 At the skull base, MIP reconstructions were performed in an oblique and curved mode to depict the complete length of the distal ICA on the same image despite the large amount of surrounding bone. The review of the MSCTA typically lasted 10–15 minutes for each patient and was performed by 3 readers in agreement. Images were first reviewed individually and a consensus was reached thereafter. The same interpretation criteria were applied to both MSCTA and DSA, focusing on arterial stenosis indicating vasospasm.
The intracranial arterial tree was subdivided into 13 segments as follows: bilateral distal intracranial intradural carotid artery (extending from the region of the dural ring to the distal carotid bifurcation), bilateral A1 segment of the anterior cerebral artery (ACA), bilateral A2 segment of the ACA, bilateral M1 segment of the middle cerebral artery (MCA), bilateral M2 segment of the MCA, bilateral P1 and P2 segments (taken together) of the posterior cerebral artery, and the basilar artery. These 13 arterial segments were then grouped into 7 locations (6 bilateral segments and the basilar artery). In each segment, the presence of a vasospasm was investigated and characterized using a grading scale as follows: none, mild (<50% reduction), moderate (50%–75% reduction), and severe (>75% reduction).30 It was decided to group the vasospasm categories, putting the mild and moderate grade in the same class, producing 3 main categories: no vasospasm, mild and moderate vasospasm, and severe vasospasm. To correctly judge the degree of spasm and to avoid confusion with vessel hypoplasia, each spastic arterial segment in the MSCTA studies was subjectively compared with the same segments in the MSCTA studies done during the initial aneurysm diagnosis. No quantitative measure was performed because the disease process was often widespread and the vessels appeared relatively small compared with the measurement error.
DSA Data Analysis
Two interventional neuroradiologists performed the DSA studies and evaluated the results. The review of the DSA studies was performed according to the same criteria used for MSCTA on a separate workstation, and the angiographic transit time was visually assessed by evaluating the presence or the absence of a circulatory delay on the angiographic runs. The DSA studies were also subjectively compared with the initial DSA to correctly judge the severity of the spasm.
Statistical Analysis
The 2-by-2 tables were constructed from true-positive, false-positive, false-negative, and true-negative results for MSCTA compared with the results of the “gold standard” DSA method on a per-arterial-segment basis, concerning the identification of vasospasm and the characterization of vasospasm severity. Likewise, the results of PCT were tabulated to assess the relationship between the PCT map abnormalities and the degree of vasospasm severity assessed by DSA. Sensitivity, specificity (with their respective 95% confidence intervals), positive and negative predictive values, as well as the accuracy regarding the detection of an intracranial vasospasm were calculated. The same procedure was applied regarding the characterization of vasospasm severity at MSCTA as well as at PCT. To assess interobserver agreement for the evaluation of MSCTA and PCT, we calculated the κ statistic for multiple observers. Agreement between the observers is reported below in terms of κ values; those greater than 0 indicate positive correlations. κ values of less than 0.20 indicated positive but poor agreement, those of 0.21–0.40 indicated fair agreement, those of 0.41–0.60 indicated moderate agreement, those of 0.61–0.80 indicated good agreement, and those greater than 0.81 indicated excellent agreement.
Results
Patient Population
Among the 27 patients studied, the SAH grade was distributed as follows: Fisher grades 1, 2, 3, and 4 in 1, 2, 17, and 7 cases, respectively.31 Ruptured intracranial aneurysms were anatomically distributed as follows: 5 on the distal internal carotid artery, 12 on the anterior communicating artery, 5 on the MCA bifurcation, and 4 on the posterior communicating artery; in 1 case, there was no evidence of aneurysm. The median time between SAH onset and the appearance of symptoms and signs of vasospasm was 7.0 days, with a range of 4.0 to 15.0 days. As vasospasm was clinically detected, 22 patients underwent the MSCTA (median, 6 hours; range, 2–10 hours), and 23 underwent the PCT session of the protocol with the same timing. Eighteen patients underwent the complete vasospasm CT protocol, including MSCTA associated with PCT during the same session. MSCTA and PCT were well tolerated by all patients, without any reported side effects. Suboptimal enhancement of intracranial arteries as a result of improper injection timing was observed in 1 case, and mild motion artifacts were seen in another case. DSA was performed after MSCTA and/or PCT in all patients, with a median time between these 2 examinations of 4 hours (range, 1–12 hours). No procedure-related complications and no technical failures were encountered.
Results of the DSA Sessions
In our clinical cohort, 3 patients were free of vasospasm (11.1%), 13 (48.2%) showed mild-moderate angiographic vasospasm, and 11 (40.7%) showed severe vasospasm. Vasospasm was investigated in 286 intradural arterial segments. According to the results of the “gold standard” DSA, 67 (23.4%) arterial segments demonstrating vasospasm were identified, among which 48 (72%) were classified as mild or moderate and 19 (28.3%) as severe. Arterial segments demonstrating vasospasm were located as follows: 18 (27.2%) on the A2 segment of the ACA, 16 (24.2%) on the A1 segment of the ACA, 13 (19.7%) on the M1 segment of the MCA, 11 (16.7%) on the intradural carotid siphon, 7 (10.6%) on the M2 segment of the MCA, one (1.5%) on the basilar artery, and none on the P1–P2 segments of the posterior cerebral artery.
Comparison of MSCTA and DSA in the Depiction of Intracranial Vasospasm
Initial agreement was reached at DSA and MSCTA for 279 (97.5%) and 265 (92.6%), respectively, of the 286 arterial segments analyzed, whereas secondary consensus was required for the remaining 7 and 21 segments, respectively. The κ values for DSA and MSCTA were 0.875 and 0.790, respectively, which indicated excellent interobserver agreement in the identification of arterial segments demonstrating vasospasm by DSA and good interobserver agreement by MSCTA. The overall results of sensitivity, specificity, and accuracy of MSCTA compared with DSA, with respect to vasospasm detection, are presented in Table 1. MSCTA failed to detect 6 of the 11 vasospasms located on the intradural carotid siphon; the 6 false-negative results concerned only the mild-moderate vasospasm category, whereas no false-negative results were obtained in the severe vasospasm category; therefore, all severe vasospasms were correctly identified by MSCTA.
Table 1:
Sensitivity, specificity (95% confidence limits in parentheses), positive predictive value, negative predictive value, and accuracy of MSCTA (compared with DSA) in the depiction of intracranial vasospasms, depending on anatomic location
| Location of Vasospasm | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Distal carotid artery | 45 (16–74) | 100 | 100 | 83 | 85 |
| A1 segment | 87.5 (71–100) | 100 | 100 | 92 | 95 |
| M1 segment | 100 | 96 (88–100) | 93 | 100 | 97 |
| A2 segment | 100 | 100 | 100 | 100 | 100 |
| M2 segment | 100 | 100 | 100 | 100 | 100 |
| Basilar artery | 100 | 100 | 100 | 100 | 100 |
| Total | 87.7 (79–96) | 99.2 (98–100) | 98.3 | 94.1 | 95.4 |
Note:—MSCTA indicates multisection CT angiography; DSA, digital substraction angiography. Posterior cerebral artery location was not reported because no vasospasm was found on this segment.
On the other hand, MSCTA identified all vasospasms located on the distal intracranial segments and no difference could be found regarding the sensitivity of MSCTA for vasospasm located on the ACA or MCA. For distal arterial segments, all MSCTA reconstruction techniques were in agreement in vasospasm identification, whereas at the skull base, shaded surface display reconstructions with volume rendering technique were not useful, and vasospasm diagnosis relied mainly on MIP reconstructions in the oblique and curved modes. Only a single vasospasm was detected at the basilar artery location, and no vasospasm was found at the posterior cerebral artery location, causing a lack of data in our study concerning the sensitivity of MSCTA to vasospasm in these particular segments.
Three patients with clipped anterior communicating artery aneurysms showed slight clip artifacts that prevented a precise anatomic depiction of the corresponding A1 segments. Five patients underwent endovascular treatment (3 at the level of the carotid siphon and 2 at the level of the anterior communicating artery) producing coils artifacts, which obscured the parent artery at MSCTA, thus preventing a complete analysis of all 13 arterial segments.
Comparison of MSCTA and DSA in the Characterization of Intracranial Vasospasm
Initial agreement was reached at DSA and MSCTA for 271 (94.8%) and 259 (90%), respectively, of the 286 arterial segments analyzed, whereas secondary consensus was required for the remaining 15 and 27 segments, respectively. The κ value for DSA and MSCTA was 0.820 and 0.760, respectively, which indicated an excellent interobserver agreement in the determination of vasospasm grade by DSA and a good interobserver agreement by MSCTA. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of MSCTA in characterizing vasospasm severity are summarized in Table 2. Results were subdivided into 3 main categories of vasospasm severity: none, mild-moderate, and severe. At the level of the distal carotid siphon, the sensitivity and the accuracy of MSCTA were significantly reduced in the detection of mild-moderate vasospasm, whereas they were much higher in the case of severe vasospasm (33.3% versus 100% and 86% versus 100%, respectively). Indeed, mild-moderate vasospasm of the distal carotid siphon was difficult to differentiate from a normal arterial diameter on axial images, and the narrow window width allowed by the bony carotid canal on 2D MIP segmentations prevented the adequate demonstration of short mildly stenotic segments of the distal ICA. The sensitivity to mild-moderate vasospasm on the ACA and MCA proved to be similar to that of severe vasospasm: if the 95% confidence intervals are taken into account, the values of 86.8% (76%–98%) versus 76.5% (57%–96%) are not significantly different. In the same way, the accuracy was observed to be similar for severe and mild-moderate vasospasm (97.5% versus 95.2%).
Table 2:
Sensitivity, specificity (95% confidence limits in parentheses), positive predictive value, negative predictive value, and accuracy of MSCTA in the characterization of intracranial vasospasms at the level of the distal internal carotid artery and the intracranial cerebral arteries
| Vasospasm Grade | True-Positive | False-Positive | False-Negative | True-Negative | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mild-Moderate | |||||||||
| Distal ICA | 3 | 0 | 6 | 34 | 33.3 (64–3) | 100 | 86 | 100 | 85 |
| Intracranial cerebral arteries | 33 | 6 | 5 | 183 | 86.8 (76–98) | 96.8 (94–99) | 95.2 | 84.6 | 97.3 |
| Severe | |||||||||
| Distal ICA | 2 | 0 | 0 | 34 | 100 | 100 | 100 | 100 | 100 |
| Intracranial cerebral arteries | 13 | 1 | 4 | 183 | 76.5 (57–96) | 99.5 (98–100) | 97.5 | 92.9 | 97.9 |
Note:—MSCTA indicates multisection CT angiography; ICA, internal carotid artery.
Perfusion CT Results
The individual review of the PCT data performed by the 3 readers showed an interobserver agreement of 100% at the initial analysis, and no secondary consensus was required thereafter. PCT revealed abnormalities in 11 of the 23 patients and normal results in the remaining 12. These abnormalities were related to severe vasospasm in 9 patients and to mild-moderate ones in 2. Each abnormality disclosed on the PCT maps corresponded precisely to the vascular territory of the arterial segment demonstrating vasospasm. In 1 patient harboring severe vasospasm located on the distal segments of the left ACA, PCT results were normal, constituting the only false-negative result of PCT in the case of severe vasospasm. In this case, the parenchymal perfusion abnormalities were probably located outside the spatial coverage of the PCT. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of PCT in characterizing vasospasm severity are summarized in Table 3. Perfusion CT was able to detect severe vasospasm in all but one patient, showing high values of sensitivity and accuracy (90% versus 92.3%), whereas it proved to be much less reliable in the case of mild-moderate vasospasm, as demonstrated by the very low values of sensitivity and accuracy in this setting (20% versus 38.5%). PCT featured severe vasospasm in 6 patients as areas with increased MTT, normal CBF, and increased CBV (Fig 1), in 2 patients as areas with increased MTT, decreased CBF, and increased CBV (Fig 2), and in 1 patient as areas with increased MTT, decreased CBF, and decreased CBV (Fig 3). This last case was considered as a vasospasm-related irreversible ischemic lesion that was confirmed on a follow-up CT scan. The other 8 patients with severe vasospasm showed no ischemic abnormalities on initial, intercurrent, and follow-up brain CT scans. The 2 cases showing increased MTT, decreased CBF, and increased CBV were considered vasospasm-related reversible ischemic lesions according to PCT studies on cerebral ischemia.32,33 PCT featured the 2 cases of mild-moderate vasospasm as areas with increased MTT alone. We analyzed the correlation of the PCT maps with the angiographic transit time (ATT) determined subjectively at the DSA in patients showing severe vasospasm. We observed a delayed filling of the vascular territory on DSA related to vasospasm in all 3 cases, showing an increased MTT with reduced rCBF, whereas among the 6 patients showing an increased MTT alone, only the 2 patients with a diffuse spasm showed a delayed filling on DSA.
Table 3:
Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of perfusion CT in the characterization of intracranial vasospasms
| Vasospasm Grade | Sensitivity (%) | Specificity (%) | Accuracy (%) | Positive Predictive Value (%) | Negative Predictive Value (%) |
|---|---|---|---|---|---|
| Mild-Moderate | 20 | 100 | 38.5 | 100 | 27.3 |
| Severe | 90 | 100 | 92.3 | 100 | 75 |
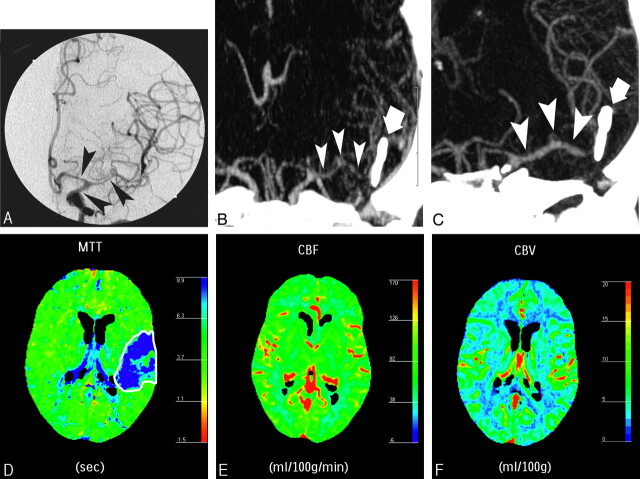
Fig 1.
A 44-year-old woman presenting with weakness of the right arm and leg, clinically attributed to cerebrovascular vasospasm 6 days after SAH related to a ruptured saccular aneurysm of the left MCA bifurcation, which was clipped (bold arrow). DSA showed moderate vasospasm on the distal carotid segment and severe vasospasm on the A1 segment of the left ACA and the M1 and proximal M2 segments of the left MCA (A, black arrowheads). Maximum intensity projection (MIP) MSCTA image before (B) and after (C) intra-arterial infusion of nimodipine showing resolution of the vasospasm (white arrowheads), and followed by the resolution of the symptoms. At pretreatment PCT, MTT was increased in the left MCA territory (D), CBF was normal (E), and a slight increase in CBV (F) was observed, representing vasospasm related auto regulation mechanisms.
Fig 2.
A 44-year-old man with right-sided hemiparesis attributed to cerebrovascular vasospasm occurring 9 days after SAH consecutive to a ruptured aneurysm of the anterior communicating artery, treated by surgical clipping. DSA (A) showed moderate vasospasm on M1 segment of left MCA and focal severe vasospasm on the M2 segment (arrowheads). These findings were confirmed by MIP MSCTA reconstruction (B) and volume-rendered MSCTA reconstruction (C). Perfusion CT performed during the same CT session revealed an increase in MTT (D) and a decrease in rCBF (E), with slight increase of rCBV (F). This pattern of perfusion alterations corresponds to a reversible ischemic lesion consecutive to vasospasm. The patient was then treated by a local intra-arterial nimodipine infusion and a balloon angioplasty of the left M1 segment.
Fig 3.
A 52-year-old man with symptoms of cerebrovascular vasospasm 5 days after SAH. He was treated with a surgical clipping of a ruptured aneurysm of the anterior communicating artery (AcomA). A first postoperative angiogram showed no abnormalities. Actual DSA (A) showed an absence of the distal segments of the right AcomA (black arrowheads), interpreted as secondary to a very tight vasospasm. Posteroanterior (B) MIP reconstructions of the AcomA at MSCTA confirmed the lack of enhancement of the right AcomA. A nonenhanced cerebral CT (not shown) disclosed a vague hypoattenuation in the territory of the right AcomA. Perfusion CT results confirmed an irreversible ischemic lesion in the territory of the right AcomA, characterized by an increased MTT (C), a decreased rCBF (D), and a decreased rCBV (E). Thus, no specific endovascular treatment of the right AcomA was undertaken.
Dosimetry
The overall effective dose equivalent required for the global CT protocol was 6.0 mSv with the following distribution: 2.5 mSv for routine nonenhanced CT scan, 0.9 mSv for MSCTA, and 2.6 mSv for the 2 levels of PCT. It seems that the effective dose for PCT was only slightly higher than for a routine CT scan and that the effective dose concerning the MSCTA was only a small fraction of a conventional brain CT. Thus, the overall effective dose equivalent for the complete CT protocol was only slightly higher than a conventional brain CT study, including both nonenhanced and enhanced series.
Treatment of Vasospasm
All 27 patients underwent hypertension-hypervolemia-hemodilution therapy34 in the intensive care unit. The criterion used to decide whether patients with spasm needed endovascular therapy was clinical deterioration despite hypertension-hypervolemia-hemodilution therapy. Sixteen patients were treated by an endovascular procedure that consisted in all cases of an intra-arterial infusion of nimodipine coupled with balloon angioplasty in 10 cases. Among the 16 patients treated, 3 had no PCT study but had severe vasospasm at MSCTA, 4 had a normal PCT study and mild-moderate vasospasm at MSCTA, 7 showed an increased MTT at PCT (corresponding to 1 mild-moderate and 6 severe vasospasms), and 2 showed an increased MTT with decreased rCBF at PCT corresponding to severe vasospasm. In the 9 patients in whom abnormalities were observed in the PCT maps, the PCT findings strongly influenced the decision to treat. In the 4 patients with normal PCT and mild-to-moderate vasospasm, endovascular treatment was undertaken according to a specific demand by the neurosurgeon, who wanted a preventive treatment for unconscious patients in whom a clinical evaluation was impossible. Among the 11 patients who were not treated by endovascular therapy, one had no PCT study and mild-to-moderate vasospasm, 8 showed normal PCT studies, and mild-to-moderate vasospasm, and 1 showed a reduced rCBV at PCT in addition to increased MTT and decreased rCBF, consisting of a completed infarct and representing thus a relative contraindication to endovascular therapy (Fig 3).
Discussion
Practical Feasibility of MSCTA and PCT in Cerebrovascular Vasospasm
This study confirms the feasibility of MSCTA coupled with PCT to diagnose hemodynamically significant vasospasm after SAH. The combined MSCTA/PCT study was available on an emergency basis 24 hours per day, and the procedure typically lasted 15–20 minutes. Because of the risk of toxicity, the total amount of contrast material was kept below 3 mg/kg, and intravenous hydration was increased when the CT protocol was followed by DSA.
Value of MSCTA in Cerebrovascular Vasospasm
CT is routinely used to investigate common reasons for neurologic deterioration after SAH, such as hydrocephalus, rebleeding, or progressing intracranial hypertension.35 MSCTA can easily be added to brain CT scan to assess cerebrovascular vasospasm during the same session. In this study, mild and moderate vasospasm were grouped together because of their similar hemodynamic impact. Two groups were considered (mild-moderate and severe) in the decision for an invasive treatment. A previous study conducted by Anderson et al36 showed a similar MSCTA accuracy for mild and moderate spasm (57% and 64%, respectively), compared with the accuracy values obtained for absent spasm and severe spasm (92% and 100%, respectively). Our results indicate that MSCTA can accurately depict distally located vasospasm, whereas its sensitivity and accuracy was significantly reduced for proximal mild-to-moderate vasospasm because of limitations of 2D reformatting and 3D segmentation near the skull base, even with the use of our latest version of the CT postprocessing software. We also tried automated segmentation of bone, but this technique could correctly highlight the distal ICA over the bony landmarks in only a minority of patients (5/22, 22.7%) and mostly resulted in erosion and degradation of the segmented ICA. Nevertheless, MSCTA was able to detect all severe vasospasms located at the distal carotid siphon, and the false-negative results concerned exclusively the category of mild-moderate vasospasm, which theoretically would have little influence on cerebral perfusion impairment.37 At distal locations, MSCTA was highly accurate in the characterization of all types of vasospasm (95.2% for mild-moderate vasospasm and 97.5% for severe vasospasm). After endovascular treatment, the intracranial arteries were incompletely depicted as a result of platinum coil-related artifacts. However, MSCTA correctly identified vasospasm located outside the arterial segment harboring the coil mass, and PCT data added substantial information about the hemodynamic impact of vasospasm on cerebral perfusion.
Value of PCT in Cerebrovascular Vasospasm
Brain perfusion measurements after SAH are justified by the fact that CBF reduction in this particular setting is closely related to the presence and severity of cerebral vasospasm and is a significant predictor of clinical outcome as well.38–40 PCT allows the assessment of cerebral auto regulation, and some reports have already discussed the use of perfusion studies in cerebrovascular vasospasm.41–43 In our study, PCT detected severe vasospasm in all but one patient; perfusion abnormalities were probably located outside the PCT spatial coverage in that case. Nevertheless, PCT showed good results in terms of specificity and accuracy in detecting severe vasospasm (100% versus 92.3%). In case of mild-moderate vasospasm, PCT was characterized by normal parameters in 8 cases and an increased MTT in only 2 cases, indicating that in this category of spasm the perfusion abnormalities are mild and sporadic. A normal rCBV and rCBF in the 2 cases of mild-moderate vasospasm showing an increased MTT suggests that MTT prolongation alone may suggest mild-moderate vasospasm, whereas an MTT prolongation associated with rCBF and/or rCBV abnormalities may suggest severe vasospasm. PCT was always normal when vasospasm was absent, suggesting that an abnormal PCT is compatible with a vasospasm until proved otherwise. As described by Wintermark et al,44 MTT maps are highly sensitive in screening patients for acute brain ischemia, whereas rCBF and rCBV maps are the most specific PCT maps to diagnose infarction. Among the possible vasospasm-related perfusion abnormalities, we found 3 distinct patterns of PCT, according to previous PCT studies on cerebral ischemia.17,32 The first consisted of a hemodynamic hypoperfusion without a true ischemic lesion and was characterized by an increased MTT and rCBV in response to the activation of the autoregulation related to ischemia (Fig 1). The second represented a reversible ischemic lesion characterized by an increased MTT in association with a decreased rCBF; rCBV was normal or increased (Fig 2). The third pattern represented a definitive and irreversible ischemic lesion characterized by an increased MTT in association with a decreased rCBF and a decreased rCBV (Fig 3). The comparison of PCT maps with the ATT at DSA showed that in the case of severe vasospasm, an increase in MTT at PCT does not always correspond to an increase in ATT at DSA, particularly when the vasospasm is focal, whereas a diffuse severe vasospasm always leads to an increase in ATT and MTT. Moreover, an increase in ATT was also observed when PCT maps indicated a reduced rCBF with increase in MTT, corresponding to the maximal severity of vasospasm.
Comparison with Previous Studies and Limitations
Anderson et al36 and Otawara et al,45 reported a higher MSCTA accuracy for proximal spasm compared with distal spasm; Yoon et al46 recently reported a location-independent sensitivity of 97.5% of MSCTA in the detection of intracranial vasospasm using a volume-rendering algorithm and an automated segmentation technique. Our results, in contrast, indicate that a correct detection of mild-moderate vasospasm at the level of the distal ICA remains challenging, even when using the latest versions of CT postprocessing software.
Wintermark et al47 recently conducted a very similar study and reported that vasospasm was most accurately diagnosed by MSCTA coupled with a PCT-derived MTT threshold at 6.4 seconds; moreover, an rCBF value ≤39.3 mL/100 g/min was the most accurate indicator for endovascular therapy.47 Our study, though very similar, focused more on visual inspection of PCT maps rather than on threshold values to characterize vasospasm. Indeed, our goal was to define the possible patterns of vasospasm-related perfusion abnormalities48 to assess the ischemic risk for the patient. Our data corroborate those of Wintermark et al47 and Harrigan et al,43 showing that the MSCTA/PCT study was able to identify severe vasospasm and delayed cerebral ischemia to guide the appropriate treatment; it could also identify a definitive brain infarction secondary to vasospasm.
We acknowledge several limitations of our study. The main drawback of PCT in assessing acute brain perfusion disorders was a limited spatial coverage when using the 16-row CT as in this study, enabling the assessment of only a 20-mm thickness of brain parenchyma for each bolus of contrast material. Thus, the pathologic findings could perhaps be located outside this space, as has been reported for other dynamic PCT techniques.49,50 The second limitation consisted of beam-hardening artifacts caused by aneurysm coils, preventing a complete visualization of all arterial segments. As was the case with other previous studies,47 we did not evaluate the vertebrobasilar system because of the lower frequency of vasospasm occurrence at that location and because PCT has important known limitations in the posterior fossa circulation. Finally, despite the promising results, it is premature to conclude that MSCTA alone can completely replace DSA in SAH patients with clinical suspicion of vasospasm because its negative predictive value still does not reach the value of 100%, leaving the possibility of a negative MSCTA with a positive DSA, especially in the case of symptomatic mild-moderate vasospasm.
Use of MSCTA and PCT Data in Cerebrovascular Vasospasm and Clinical Applications
In patients with clinical signs of cerebrovascular vasospasm, MSCTA/PCT data were of great help not only in defining the anatomic location and the severity of vasospasm but also in assessing the related risk of cerebral ischemia. Our results indicate that this technique offers enough accuracy to be used at any time in clinical practice. In our study, no clinical correlation was performed, and there was no controlled study showing the relationship between the detection of spasm using MSCTA and PCT and the clinical outcome, so we do not recommend that the decision to treat should depend only on MSCTA/PCT parameters; it must be guided by the clinical findings. The MSCTA/PCT data can be considered a combined or an alternative way to the clinical approach to cerebrovascular vasospasm, particularly in unconscious patients where clinical examination is of little value. In patients with critical systemic conditions, the use of nimodipine related to the vasospasm treatment may be particularly delicate, so that MSCTA/PCT data can influence the decision to treat. Because clinical symptoms of vasospasm can change or relapse on a daily basis, a potential disadvantage of this CT protocol is the cumulative radiation dose when repeated evaluations are needed. Thus, we recommend the use of the MSCTA/PCT in a patient when DSA has to be performed according to the clinical and the transcranial Doppler sonography data. In these circumstances, DSA becomes more a therapeutic option than a diagnostic one, particularly in view of an endovascular treatment of the CT-assessed cerebral vasospasm.
Acknowledgments
We gratefully acknowledge Keith Brooks, PhD, for his help in editing the manuscript.
References
- 1.Kassell NF, Sasaki T, Colohan AR, et al. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke 1985;16:562–72 [DOI] [PubMed] [Google Scholar]
- 2.Treggiari-Venzi MM, Suter PM, Romand JA. Review of medical prevention of vasospasm after aneurysmal subarachnoid hemorrhage: a problem of neurointensive care. Neurosurgery 2001;48:249–61 [DOI] [PubMed] [Google Scholar]
- 3.Proust F, Hannequin D, Langlois O, et al. Causes of morbidity and mortality after ruptured aneurysm surgery in a series of 230 patients. The importance of control angiography. Stroke 1995;26:1553–57 [DOI] [PubMed] [Google Scholar]
- 4.Kassel NF, Peerless SJ, Durward QT, et al. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery 1982;11:337–47 [DOI] [PubMed] [Google Scholar]
- 5.Shimoda M, Oda S, Tsugane R, et al. Intracranial complications of hypervolemic therapy in patients with a delayed ischemic deficit attributed to vasospasm. J Neurosurg 1993;78:423–29 [DOI] [PubMed] [Google Scholar]
- 6.Janjua N, Mayer SA. Cerebral vasospasm after subarachnoid hemorrhage. Curr Opin Crit Care 2003;9:113–19 [DOI] [PubMed] [Google Scholar]
- 7.Sloan M, Haley E, Kassell NF, et al. Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology 1989;39:1514–18 [DOI] [PubMed] [Google Scholar]
- 8.Grolimund P, Seiler RW, Aaslid R, et al. Evaluation of cerebrovascular disease by combined extracranial and transcranial Doppler sonography. Stroke 1984;18:1018–24 [DOI] [PubMed] [Google Scholar]
- 9.Lysakowski C, Walder B, Costanza MC, et al. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke 2001;32:2292–98 [DOI] [PubMed] [Google Scholar]
- 10.Okada Y, Shima T, Nishida M, et al. Comparison of transcranial Doppler investigation of aneurysmal vasospasm with digital subtraction angiography and clinical findings. Neurosurgery 1999;45:443–50 [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Offin R, Teasdale GM, et al. Is routine transcranial Doppler ultrasound monitoring useful in the management of subarachnoid hemorrhage? J Neurosurg 1998;88:272–76 [DOI] [PubMed] [Google Scholar]
- 12.Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm after subarachnoid hemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir 1988;42:81–84 [DOI] [PubMed] [Google Scholar]
- 13.Laumer R, Steinmeier R, Gonner F, et al. Cerebral hemodynamics in subarachnoid hemorrhage evaluated by transcranial Doppler sonography; I: reliability of flow velocities in clinical management. Neurosurgery 1993;33:1–7 [DOI] [PubMed] [Google Scholar]
- 14.Heiserman JE, Dean BL, Hodack JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 1994;15:1401–07 [PMC free article] [PubMed] [Google Scholar]
- 15.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm and arteriovenous malformation: a meta-analysis. Stroke 1999;30:317–20 [DOI] [PubMed] [Google Scholar]
- 16.Dillon EH, Van Leeuen MS, Fernandez MAA, et al. Spiral CT angiography. AJR Am J Roentgenol 1993;160:1273–78 [DOI] [PubMed] [Google Scholar]
- 17.Wintermark M, Reichhart M, Thiran JPH, et al. Prognostic accuracy of cerebral blood flow measurements by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol 2002;51:417–32 [DOI] [PubMed] [Google Scholar]
- 18.Miles KA. Brain perfusion: computed tomography applications. Neuroradiology 2004;46:194–200 [DOI] [PubMed] [Google Scholar]
- 19.Hatazawa J, Iida H, Shimosegawa E, et al. Regional cerebral blood flow measurement with iodine-123-IMP autoradiography: normal values, reproducibility and sensitivity to hypoperfusion. J Nucl Med 1997;38:1102–08 [PubMed] [Google Scholar]
- 20.Voldby B, Enevoldsen EM, Jensen FT. Regional CBF, intraventricular pressure, and cerebral metabolism in patients with ruptured intracranial aneurysms. J Neurosurg 1985;62:48–58 [DOI] [PubMed] [Google Scholar]
- 21.Wintermark M, Maeder P, Verdun FR, et al. Using 80 kVp versus 120 kVp in perfusion CT measurements of regional cerebral blood flows. AJNR Am J Neuroradiol 2000;21:1881–84 [PMC free article] [PubMed] [Google Scholar]
- 22.Wintermark M, Maeder P, Thiran JP, et al. Quantitative assessment of regional blood flows by perfusion CT studies at low injection rates: a critical review of the underlying theoretical models. Eur Radiol 2001;11:1220–30 [DOI] [PubMed] [Google Scholar]
- 23.Axel L. Cerebral blood flow determination by rapid-sequence computed tomography. Radiology 1980;137:679–86 [DOI] [PubMed] [Google Scholar]
- 24.Axel L. A method of calculating brain blood flow with a CT dynamic scanner. Adv Neurol 1981;30:67–71 [PubMed] [Google Scholar]
- 25.Axel L. Tissue mean transit time from dynamic computed tomography by a simple deconvolution technique. Invest Radiol 1983;18:94–99 [DOI] [PubMed] [Google Scholar]
- 26.Ladurner G, Zilkha E, Iliff D, et al. Measurement of regional cerebral blood volume by computerized axial tomography. J Neurol Neurosurg Psychiatry 1976;39:152–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeffner EG, Case I, Jain R, et al. Cerebral perfusion CT: technique and clinical applications. Radiology 2004;231:632–44 [DOI] [PubMed] [Google Scholar]
- 28.Young N, Dorsch NW, Kingston RJ, et al. Spiral CT scanning in the detection and evaluation of aneurysms of the circle of Willis. Surg Neurol 1998;50:50–61 [DOI] [PubMed] [Google Scholar]
- 29.Binaghi S, Maeder P, Uské A, et al. Three-dimensional computed tomography angiography and magnetic resonance angiography of carotid bifurcation stenosis. Eur Neurol 2001;46:25–34 [DOI] [PubMed] [Google Scholar]
- 30.Kassel NF, Helm G, Simmons N, et al. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg 1992;77:848–52 [DOI] [PubMed] [Google Scholar]
- 31.Fisher CM, Kistel JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980;6:1–9 [DOI] [PubMed] [Google Scholar]
- 32.Wintermark M, Reichhart M, Maeder P, et al. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke 2002;33:2025–31 [DOI] [PubMed] [Google Scholar]
- 33.Wintermark M, Reichhart M, Thiran JP, et al. Prognostic accuracy of cerebral blood flows measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol 2002;51:417–32 [DOI] [PubMed] [Google Scholar]
- 34.Topcuoglu MA, Pryor JC, Ogilvy CS, et al. Cerebral vasospasm following subarachnoid hemorrhage. Curr Treat Options Cardiovasc Med 2002;4:373–84 [DOI] [PubMed] [Google Scholar]
- 35.Mayberg MR, Batjer HH, Dacey R, et al. Guideline for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation 1994;90:2592–95 [DOI] [PubMed] [Google Scholar]
- 36.Anderson GB, Ashforth R, Steinke DE, et al. CT Angiography for the detection of cerebral vasospasm in patients with acute subarachnoid hemorrhage. AJNR Am J Neuroradiol 2000;21:1011–15 [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkuma H, Manabe H, Tanaka M, et al. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2000;31:1621–27 [DOI] [PubMed] [Google Scholar]
- 38.Ferguson GG, Harper AM, Fitch W, et al. Cerebral blood flow measurements after spontaneous subarachnoid hemorrhage. Eur Neurol 1972;8:15–22 [DOI] [PubMed] [Google Scholar]
- 39.Geraud G, Tremoulet M, Guell A, et al. The prognostic value of non-invasive CBF measurements in subarachnoid hemorrhage. Stroke 1984;15:301–05 [DOI] [PubMed] [Google Scholar]
- 40.Knuckey NW, Fox RA, Surveyor I, et al. Early cerebral blood flow and computerized tomography in predicting ischemia after cerebral aneurysm rupture. J Neurosurg 1985;62:850–55 [DOI] [PubMed] [Google Scholar]
- 41.Grubb RL, Raichle ME, Eichling JO, et al. Effects of subarachnoid hemorrhage on cerebral blood volume, blood flow, and oxygen utilization in humans. J Neurosurg 1977;46:446–53 [DOI] [PubMed] [Google Scholar]
- 42.Hertel F, Walter C, Bettag M, et al. Perfusion-weighted magnetic resonance imaging in patients with vasospasm: a useful new tool in the management of patients with subarachnoid hemorrhage. Neurosurgery 2005;56:28–35 [DOI] [PubMed] [Google Scholar]
- 43.Harrigan MR, Magnano CR, Guterman LR, et al. Computed tomographic perfusion in the management of aneurysmal subarachnoid hemorrhage: new application of an existent technique. Nurosurgery 2005;56:304–17 [DOI] [PubMed] [Google Scholar]
- 44.Wintermark M, Fischbein NJ, Smith WS, et al. Accuracy of dynamic perfusion CT with deconvolution in detecting acute hemispheric stroke. AJNR Am J Neuroradiol 2005;26:104–12 [PMC free article] [PubMed] [Google Scholar]
- 45.Otawara Y, Ogasawara K, Ogawa A, et al. Evaluation of vasospasm after subarachnoid hemorrhage by use of multislice computed tomographic angiography. Neurosurgery 2002;51:939–42 [DOI] [PubMed] [Google Scholar]
- 46.Yoon DY, Choi CS, Kim KH, et al. Multidetector-row CT angiography of cerebral vasospasm after aneurysmal subarachnoid hemorrhage: comparison of volume-rendered images and digital subtraction angiography. AJNR Am J Neuroradiol 2006;27:370–77 [PMC free article] [PubMed] [Google Scholar]
- 47.Wintermark M, Ko NU, Smith WS, et al. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol 2006;27:26–34 [PMC free article] [PubMed] [Google Scholar]
- 48.Yundt KD, Grubb RL, Diringer MN, et al. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab 1998;18:419–24 [DOI] [PubMed] [Google Scholar]
- 49.Rother J, Jonetz-Mentzel L, Fiala A, et al. Hemodynamic assessment of acute stroke using dynamic single-slice computed tomographic perfusion imaging. Arch Neurol 2000;57:1161–66 [DOI] [PubMed] [Google Scholar]
- 50.König M, Klotz E, Luka B, et al. Perfusion CT of the brain: diagnostic approach for the early detection of ischemic stroke. Radiology 1998;209:85–93 [DOI] [PubMed] [Google Scholar]