Abstract
SUMMARY: As an essential part of the National Cancer Institute (NCI)-funded Pediatric Brain Tumor Consortium (PBTC), the Neuroimaging Center (NIC) is dedicated to infusing the study of pediatric brain tumors with imaging “best practice” by producing a correlative research plan that 1) resonates with novel therapeutic interventions being developed by the wider PBTC, 2) ensures that every PBTC protocol incorporates an imaging “end point” among its objectives, 3) promotes the widespread implementation of standardized technical protocols for neuroimaging, and 4) facilitates a quality assurance program that complies with the highest standards for image data transfer, diagnostic image quality, and data integrity. To accomplish these specific objectives, the NIC works with the various PBTC sites (10 in all, plus NCI/ National Institute of Neurological Diseases and Stroke representation) to ensure that the overarching mission of the consortium—to better understand tumor biology and develop new therapies for central nervous system tumors in children—is furthered by creating a uniform body of imaging techniques, technical protocols, and standards. Since the inception of the NIC in 2003, this broader mandate has been largely accomplished through a series of site visits and meetings aimed at assessing prevailing neuroimaging practices against NIC-recommended protocols, techniques, and strategies for achieving superior image quality and executing the secure transfer of data to the central PBTC. These ongoing evaluations periodically examine investigations into targeted drug therapies. In the future, the NIC will concentrate its efforts on improving image analysis for MR imaging and positron-emission tomography (PET) and on developing new ligands for PET; imaging markers for radiation therapy; and novel systemic, intrathecal, and intralesional therapeutic interventions.
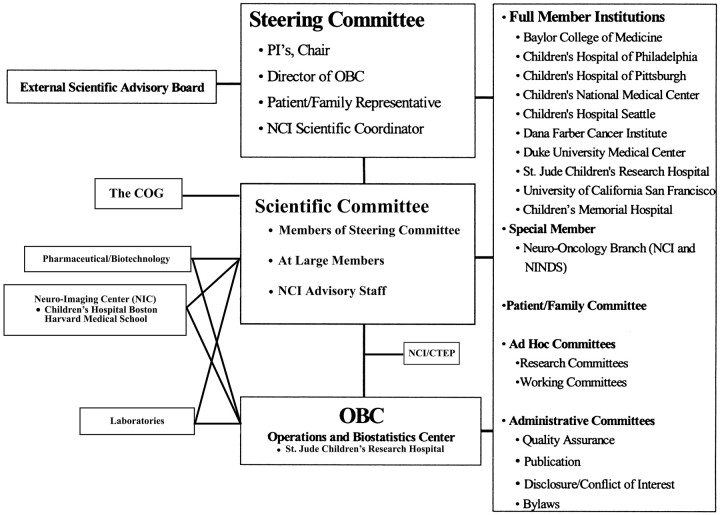
The Neuroimaging Center (NIC) is part of the Pediatric Brain Tumor Consortium (PBTC)—a multidisciplinary multicenter research organization dedicated to the study of correlative tumor biology and new therapies for primary central nervous system tumors of childhood.1–6 The PBTC was established in 1999 and is funded by the National Cancer Institute (NCI).7 It consists of 10 member institutions and 1 special institution (Fig 1). The NCI challenged the initial PBTC leaders to recognize the importance of neuroimaging to the success of the Consortium, and in 2000, PBTC sought partners from the pediatric brain tumor advocacy groups to fund the PBTC NIC. Funding was subsequently assumed by specific allocation of the NCI (Grant # NIH U01 CA 81456–08). The PBTC-NIC is the first NCI-funded cooperative imaging center in the United States. Since January 2003, the PBTC-NIC has been located in the Department of Radiology at Children's Hospital Boston (CHB).
Fig 1.
The PBTC organization. COG indicates Children's Oncology Group; CTEP, Cancer Therapy Evaluation Program.
Mission and Goals
The mission of the NIC is to provide leadership in diagnostic imaging as a major component of the investigational studies of the PBTC. Imaging research is central to the core mission of the Consortium. The NIC coordinates development of imaging objectives, engaging imaging investigators in considering relevant research aims and taking the lead in fostering stewardship of Consortium and institutional resources. A major responsibility for the NIC is implementing and coordinating appropriate standardized imaging procedures. Imaging data have included digital data gathered electronically from protocol-specific conventional MR imaging, MR diffusion, MR perfusion, MR spectroscopy, and positron-emission tomography (PET) procedures.
The goals of the NIC are to develop a correlative imaging research plan related to the novel PBTC therapeutic interventions. Every PBTC protocol has incorporated imaging end points among its research objectives. Standardized acquisition protocols for institutional use have been promulgated, and a systematic program of quality assurance (QA) for neuroimaging equipment at member institutions and acquisition studies is in place. A system of compliance with image data transfer, diagnostic image quality, and data integrity is included in the QA program. The NIC also oversees cross-platform translations for comparative MR imaging (volumetric MR imaging, MR diffusion, MR perfusion, and MR spectroscopy) and PET analyses.
Organization
The director of the NIC is responsible for coordinating all aspects of neuroimaging evaluations and investigations in the PBTC (Fig 2). The director works closely with the principal investigators (PIs) of each site, the chair of the PBTC Steering Committee, and the executive director of the PBTC Operations and Biostatistics Center (OBC). The NIC director serves on the PBTC Scientific Committee, ensuring imaging input in strategic priorities for the Consortium and the incorporation of appropriate diagnostic neuroimaging aims and procedures for each protocol. A research team of individuals at the NIC supports the activities of the Center, with expertise in clinical research, data analysis of MR imaging and PET, QA, and information technology and transfer.
Fig 2.
The PBTC NIC organization. The Neuroimaging Committee consists of neuroradiologists with MR imaging expertise and PET physicians, 1 from each site. respectively.
Neuroimaging Committee
The Neuroimaging Committee (composed of neuroradiologists involved in MR imaging—1 or more from each participating institution) collaborates with the NIC in the development and conduct of imaging protocols and research objectives, thereby combining expertise across institutions. An initial organizational and consensus conference at CHB in March 2003 approved the MR imaging acquisition protocols for each imaging procedure and provided uniform institutional commitments for rigorous QA procedures for MR imaging and PET. MR imaging protocols that were in place as well as equipment specifications and contact information were updated for each site. In addition, the meeting included a consensus-based approach for identifying and prioritizing research projects. Biannual meetings of the Neuroimaging Committee occur at the PBTC meetings. Ongoing communication is achieved through e-mails and conference calls. Information about the NIC is maintained at a dedicated Website: www.childrenshospital.org/research/pbtcnic.
PET Investigator Committee
In March 2005, a PET Consensus Conference was held at the NIC to engage and enlist the PET investigators from each site. The objective of this meeting was to share information among PBTC PET sites regarding equipment, techniques, tools used for interpretation, and potential areas of research. During this meeting, committee members reviewed the ongoing PET protocols, the PET QA processes then used at the respective sites, and the process of PET data transfer from these sites to the OBC of the PBTC. The PET Investigator Committee comprises PET investigators, 1 from each site.
Data Transfer
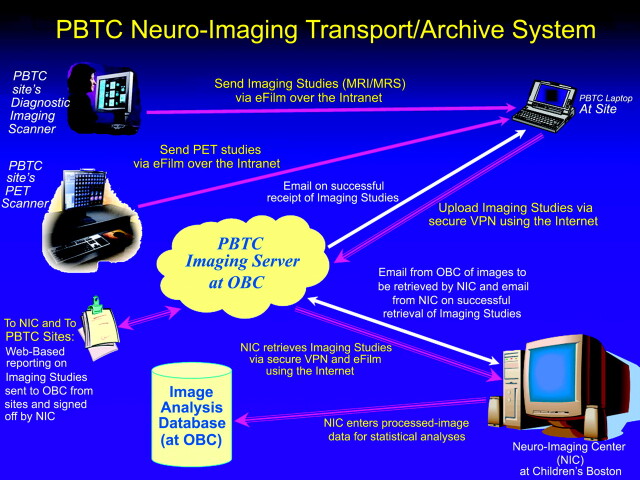
The PBTC neuroimaging studies are sent across a secure tunnel from the participating sites to the OBC at St. Jude Children's Research Hospital and then to the NIC at CHB (Fig 3). Software in the OBC replaces all patient identifiers with PBTC codes; as a centralized function within the OBC, this enables the NIC to match correct similarly coded patient data with the imaging studies. In the transmission process from the member institutions to the OBC and the NIC, a virtual private network (VPN) is used. The data are highly encrypted and encapsulated in a secure tunnel. The OBC staff conforms to the Health Insurance Portability and Accountability Act regulations in all work for the Consortium.
Fig 3.
PBTC neuroimaging data transfer for MR imaging and PET studies. MRI indicates MR imaging; MRS, MR spectroscopy.
MR imaging studies are uploaded by the clinical research associate at each site and are downloaded from the OBC after e-mail notification to the NIC. The components of each study are tabulated by the OBC and subsequently verified by the NIC after image download. The NIC completes a Web-based form to indicate that the download is complete and signs off on each designated series of the examination. The studies are sorted into the appropriate protocol folder on the NIC PC workstation, backed up on the PBTC NIC server, and archived daily on tape drive systems. The data are analyzed for image quality, data integrity, and compliance with the protocol requirements.
A system of transfer of the MR spectroscopy raw data requires specific steps based on the type of MR imaging scanner. Transfer of the MR spectroscopy raw data requires file transfer protocol (FTP) transfer to the OBC. Each site does a screen save of the MR spectroscopy spectrum with the ratios displayed on the image. A vendor-specific process for MR spectroscopy raw data file transfer is in place.
All PET image data acquired via a specific acquisition protocol are forwarded to the OBC and subsequently sent to the NIC for analysis by download or FTP. All PET image data, along with their corresponding MR imaging data, are uploaded to a multitechnique processing and review station (HERMES Multi Modality; Hermes Medical Solutions, Stockholm, Sweden) for image registration and fusion.
Protocol Development and Implementation
Acquisition protocol development through the NIC involves providing standardized descriptions of qualitative and quantitative neuroimaging procedures and their implementation in the participating institutions. Guidelines for imaging protocols are provided through consultation with the Scientific Committee and the Neuroimaging and PET Investigator Committees, who provide input into the proposed neuroimaging research objectives. We seek consensus in determining which acquisition protocols are appropriate and likely to be productive. Neuroimaging research questions relevant to the therapeutic aims of the protocol are incorporated. A specific neuroimaging objective relevant to the investigational intervention and disease types has been incorporated into most of the 21 PBTC protocols. Imaging guidelines for protocol-determined procedures and acquisition protocols are compiled in the procedure manual generated by the NIC and the clinical research program at CHB, which includes descriptions of parameters for clinical and research-related MR imaging, MR perfusion, MR diffusion, MR spectroscopy, and fluorodeoxyglucose (FDG)-PET scanning.
Image Analysis
Image analysis of MR imaging and PET studies is determined in accordance with the particular PBTC research protocol that is used. Region-of-interest, qualitative, and quantitative analyses are performed for MR imaging and PET examinations. Each MR imaging study is reviewed by pediatric neuroradiologists from the NIC. Before image analysis, imaging studies are evaluated for readability and compliance with the protocol.
For MR imaging, volumetric analyses are performed by using the Vitrea workstation (Vital Images, Plymouth, Minn) with the perimeter technique.8 Automated segmentation software defines the regions of interest. The perfusion, diffusion, and MR spectroscopy components of each study are then transferred from the server to a Sun workstation (Sun Microsystems, Santa Clara, Calif) for image analysis. From the apparent coefficient diffusion map, a region of interest is obtained within the solid part of the tumor and divided by the value from a region of interest obtained in the frontal white matter. For perfusion imaging analysis of the dynamic susceptibility contrast-enhanced technique, a region of interest is obtained in the tumor from a generated cerebral blood volume map (by using the technique developed at Massachusetts General Hospital)9,10; this value is divided by a region of interest obtained from the frontal white matter.11 Calculation of MR spectroscopy ratios including N-acetylaspartate otal creatine, choline otal creatine, and lipid otal creatine values is done from the single voxel MR spectroscopy data.
All PET image data, along with the corresponding MR imaging data, are uploaded to a multitechnique processing and review station (see previous description). The presence and readability of the image data are determined. If readable, the PET data are registered to the MR imaging data and are reviewed in conjunction with the MR imaging data. Subjective and objective ratings of FDG tumor uptake are performed by joint analyses of a pediatric neuroradiologist, PET physicist, and PET physician.
After the postanalysis MR imaging and PET data are reviewed, the information is backed up on the NIC workstation, the NIC server, and a dedicated tape drive system.
QA
QA and quality control procedures have been implemented for MR imaging, PET, and CT as they relate to PET/CT.
MR Imaging
Quarterly scanning of the American College of Radiology MR imaging phantom is performed with a QA form completed for each site scanner used for PBTC imaging studies. A video of the MR imaging QA procedure has been made for the MR imaging technologists at each site and is posted on the Website. QA reports, generated by the Clinical Research Program, are reviewed quarterly by the NIC and submitted to the Neuroimaging Committee and the OBC.
PET
Each site completes a quarterly PET QA form that is part of the PET quality control program. Each site has submitted a uniform PET phantom for a 1-time submission. QA reports are generated quarterly and submitted to the PET Investigator Committee. A PET phantom report has been generated.
Data Management
At the time of image analysis, data are generated by the NIC data analysts, NIC nuclear medicine physicians, physicists, and neuroradiologists. Data are recorded on paper forms, collected and reviewed under the supervision of the NIC research coordinator, and then entered into data entry screens with built-in variable range checks for on-line editing by using a relational data base, Microsoft Access (Microsoft, Redmond, Wash). Data entry status reports and frequency distributions of study variables are generated to provide timely and relevant feedback to the NIC regarding image quality and compliance with the protocols. Data obtained from imaging studies are entered into a Microsoft Access data base and subsequently transferred by secure tunnel to the PBTC OBC. The NIC collaborates with the OBC to facilitate statistical analysis of imaging studies and to correlate imaging findings with clinical and biologic data from the respective PBTC therapeutic protocols.
Site Visits
Site visits have been conducted at each of the member institutions to review acquisition protocol development and QA. An evaluation checklist was completed and suggestions tabulated from the site for inclusion in a site visit report shared with the PBTC Neuroimaging Committee and PBTC Steering Committee.
Neuroimaging Research Development
An imaging research plan related to the novel therapeutic trials of the Consortium has been developed. Consensus on research objectives occurs in concert with the study chair and working group, is considered within the Neuroimaging and PET Committees, and is ultimately presented to the Scientific Committee. Routine assessments of tumor response and neurotoxicity are augmented by image-based research objectives to identify potential imaging surrogates for tumor response. This has been particularly challenging in the new molecularly targeted and antiangiogenesis agents, where “response” may be defined by parameters other than simply tumor volume. Imaging research objectives occur within protocols, and ultimately imaging research results will be pooled across protocols by tumor type and similar therapies to determine if there are potential imaging surrogates of tumor response. In addition, correlative imaging and clinical and biologic study data will be incorporated as appropriate.
Preliminary Data
PBTC-004: Phase I Trial of Intrathecal Spartaject-Busulfan in Children with Neoplastic Meningitis.5
Quantitative and qualitative MR imaging analysis was performed, including correlation with clinical response. Compliance, patterns of MR imaging involvement (linear versus nodular) and number of sites of involvement were collated. Progressive disease was found in 14 patients; stable disease, in 8 patients. Those with progressive disease had more sites of involvement on MR imaging.
Brain Stem Glioma Trials.
Brain stem tumors can be categorized as diffuse or focal. Diffuse brain stem gliomas constitute 80% of all brain stem gliomas, and despite multiple treatment approaches, the prognosis is poor with long-term survival of <10%.12 Thus, children with this tumor have been the focus of novel therapies in clinical trials to determine if there is an effective therapy.13 MR imaging, including advanced imaging techniques such as MR diffusion, perfusion, spectroscopy, and PET, have been used for assessment of the physiologic characteristics of the tumor with time within drug therapy PBTC trials. The analyses of these imaging studies as well as a prospective analysis of intratumoral hemorrhage are ongoing and will be reported separately. The NIC has served a role in providing central review for MR imaging studies in a recent ongoing study of a molecularly targeted agent, in which intratumoral hemorrhage occurred within brain stem gliomas. Further study of the association of intratumoral hemorrhage in brain stem glioma is ongoing within the PBTC mechanism.14
Future Directions
Future research directions for the NIC will include developing improved analysis techniques for MR imaging and PET as well as new PET ligands. In addition, the correlation of neuroimaging parameters to response and toxicity may produce imaging markers for radiation therapy as well as novel systemic, intrathecal, and intralesional therapeutic interventions. We are currently exploring early MR imaging signs of apparent response based on imaging perfusion changes. We hope to incorporate early perhaps dose-determining assessments in a unique exploration of imaging-based end points.
With advances in the molecular analysis of tumors, the correlation of pathology and biology studies with imaging studies will aid in the development of the image-based and molecular classification of tumors. Novel molecular imaging techniques may be incorporated into PBTC future trials. Funding for independent research projects that analyze acquired data across protocols, grouped by disease type or similar drug mechanisms, will be essential. In addition, data base sharing across techniques should be available to other imaging researchers through the PBTC mechanism. Such research efforts are aimed at improving our understanding of the physiologic and metabolic profile of brain tumors in children.
Conclusion
Of all the solid tumors, pediatric brain tumors are the most lethal and are second only to leukemia as the most commonly found malignancy in children. As with any cancer, particularly those that arise in childhood, early detection is essential to achieving a positive outcome. Among children 0–19 years of age, the prevalence rate for all primary brain and central nervous system tumors was roughly 9.5 per 100,000, with an estimated 26,000 children living with this diagnosis in the United States in 200015; the incidence rate was 29.1 cases per 1,000,000 from 1996–2003.16
Against this ominous backdrop, the drive to find a cure is enormous. Fortunately, the medical community has made impressive strides in the last decade in developing enabling technologies aimed at improving the detection, diagnosis, and treatment of many cancers, including pediatric brain tumors. Nowhere has this been more the case than in the rapid deployment of extremely powerful imaging techniques such as conventional proton MR imaging, MR diffusion, MR spectroscopy, MR perfusion, and PET, which are variously able to define and differentiate tumor with incredible precision.
In 2003, the NIC was established as an essential arm of the NCI-funded PBTC, whose charge is to better understand tumor biology, including the genetic, metabolic, and physiologic factors underlying this insidious disease. The NIC is specifically dedicated to 1) developing a set of standardized imaging protocols that are uniformly applied across the 10 member sites, 2) devising a correlative imaging research plan that responds to the therapeutic interventions being advanced by the PBTC, 3) ensuring that every PBTC protocol has incorporated imaging end points, and 4) facilitating a QA plan that addresses the secure transfer of data, superior image quality, and data integrity. The work of the NIC is ongoing; future objectives are aimed at enhancing current techniques for MR imaging and PET image analysis as well as identifying new PET ligands for the development of novel PET radiotracers in cancer imaging.
Acknowledgments
This work was supported in part by NIH grant U01 CA81457 for the Pediatric Brain Tumor Consortium (PBTC), The Pediatric Brain Tumor Foundation of the United States (PBTFUS) and American Lebanese Syrian Associated Charities. We acknowledge the site neuroradiologists including Dr. Robert Zimmerman (Children's Hospital of Philadelphia), Dr. Soonmee Cha (University of California San Francisco), Dr. Charles Fitz (Children's Hospital of Pittsburgh), Dr. Jill Hunter (Texas Children's Hospital), Dr. James Provenzale (Duke University), Dr. Dennis Shaw (Seattle Children's Hospital), Dr. L. Gilbert Vezina (Children's National), Dr. Delilah Burrowes (Children's Memorial), Dr. John Butman (NIH), and Dr. Fred Laningham (St. Jude Children's Research Hospital). We thank Christie O'Neill for figure preparation, Nancy Drinan for editorial assistance, and Virginia Grove for manuscript preparation.
References
- 1.Barkovich J, Beigel J, Boyett J, et al. The Pediatric Brain Tumor Consortium: strategies for the design and conduct of innovative phase I and phase II clinical trials for childhood brain tumors. Paper presented at: 13th International Conference on Brain Tumor Research and Therapy; October 3–6,1999; Sapporo, Japan
- 2.Blaney SM, Boyett J, Friedman H, et al. Phase I clinical trial of mafosfamide in infants and children aged 3 years or younger with newly diagnosed embryonal tumors: a pediatric brain tumor consortium study (PBTC-001). J Clin Oncol 2005;23:525–31 [DOI] [PubMed] [Google Scholar]
- 3.Chopra A, Brown KM, Rood BR, et al. The use of gene expression analysis to gain insights into signaling mechanisms of metastatic medulloblastoma. Pediatr Neurosurg 2003;39:68–74 [DOI] [PubMed] [Google Scholar]
- 4.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res 2003;9:3620–24 [PubMed] [Google Scholar]
- 5.Gururangan S, Petros WP, Poussaint TY, et al. Phase I trial of intrathecal spartaject busulfan in children with neoplastic meningitis: a Pediatric Brain Tumor Consortium Study (PBTC-004). Clin Cancer Res 2006;12:1540–46 [DOI] [PubMed] [Google Scholar]
- 6.Pediatric Brain Tumor Consortium website. Available at: www.pbtc.org. Accessed December 3,2006
- 7.[No authors listed] National Cancer Institute Research on Childhood Cancers. Bethesda, Md: National Cancer Institute. Available at www.cancer.gov/cancertopics/factsheet/sites-type/childhood. Accessed on December 3,2006
- 8.Sorensen AG, Patel S, Harmath C, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol 2001;19:551–57 [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard L, Sorensen AG, Chesler DA, et al. Combined diffusion-weighted and perfusion-weighted flow heterogeneity magnetic resonance imaging in acute stroke. Stroke 2000;31:1097–103 [DOI] [PubMed] [Google Scholar]
- 10.Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology 1999;210:519–27 [DOI] [PubMed] [Google Scholar]
- 11.Wetzel SG, Cha S, Johnson G, et al. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 2002;224:797–803 [DOI] [PubMed] [Google Scholar]
- 12.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys 1998;40:265–71 [DOI] [PubMed] [Google Scholar]
- 13.Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol 2006;24:1266–72 [DOI] [PubMed] [Google Scholar]
- 14.Broniscer A, Laningham FH, Kocak M, et al. Intratumoral hemorrhage among children with newly diagnosed, diffuse brainstem glioma. Cancer 2006;106:1364–71 [DOI] [PubMed] [Google Scholar]
- 15.CBTRUS(2005). Statistical Report: Primary Brain Tumors in the United States, 1998–2002. Chicago: Central Brain Tumor Registry of the United States;2005
- 16.Ries L, Harkins D, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975–2003. Bethesda, Md: National Cancer Institute;2006