Abstract
SUMMARY: Elevated intracranial intravenous pressure seems to be of importance in pseudotumor cerebri syndromes, either as a cause (secondary intracranial hypertension) or as a consequence (idiopathic intracranial hypertension) of increased intracranial pressure. We present 3 case reports in which diagnostic imaging before and after CSF diversion provided evidence that narrowing of the transverse sinuses is a secondary phenomenon. Stent angioplasty of the venous sinuses should not be considered a therapeutic approach in these cases.
The recent debate about the etiology of pseudotumor cerebri focuses on the role of elevated intracranial venous pressure.1,2 Bilateral transverse sinus (TS) narrowing in patients with idiopathic intracranial hypertension (IIH) can be found regularly on MR imaging and may cause venous outflow obstruction.3 Some patients benefit from stent treatment of these venous sinus obstructions,4 but others do not. The role of a TS stenosis remains to be evaluated.5 We present 3 patients with typical signs and symptoms of IIH in whom the reversibility and re-occurrence of venous sinus stenoses are emphasized.
Case Reports
Case 1
The 23-year-old woman (62 kg) presented with headache, diplopia, facial palsy, left auricular pain, and left-sided tinnitus. Clinical examination revealed bilateral papilledema, visual field reduction, and cranial nerve palsies of VI and VII. Findings of routine MR imaging were normal (Fig 1A). Lumbar CSF pressure was 41 cm H2O. Angiography depicted a stenotic left TS and aplasia of the right TS. No signs of acute sinus thrombosis were seen, and no fistula was found. Intravenous pressure-monitoring demonstrated elevated pressure in the superior sagittal sinus and a pressure gradient across the TS stenosis (Fig 1B), thus proving the hemodynamic significance of the stenosis. Stent angioplasty lead to reconstruction of the vessel lumen and normalization of intracranial venous pressure (Fig 1C, -D). Premedication with acetylsalicylic acid (2 × 100 mg), clopidogrel (1 × 75 mg), and intravenous heparin was given to prevent in-stent thrombosis.
Fig 1.
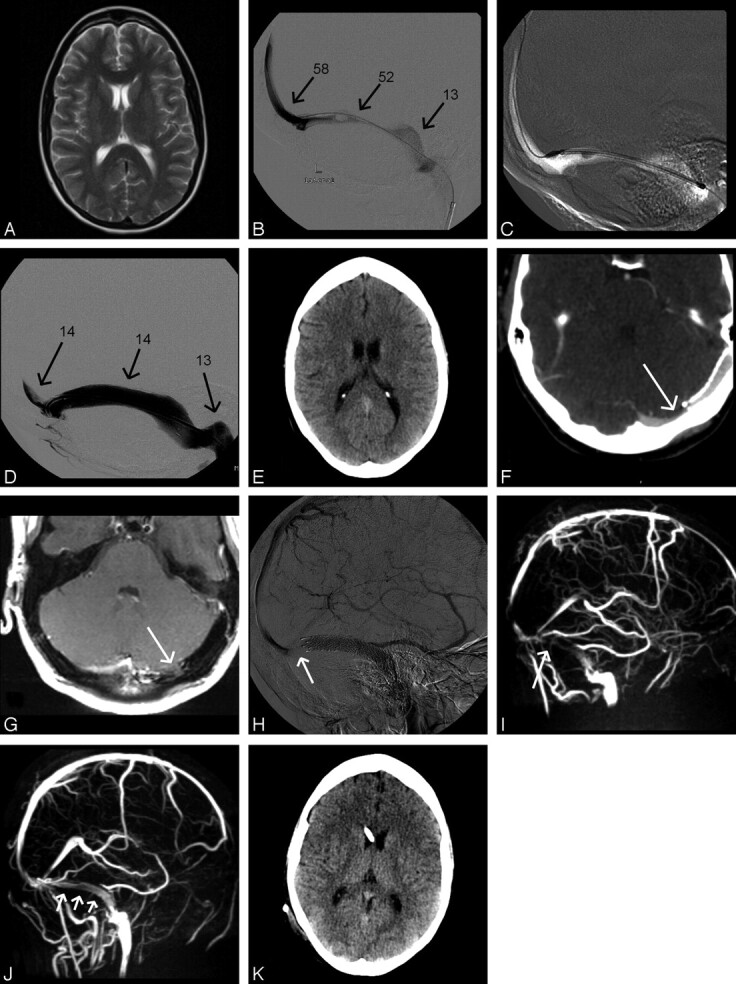
Case 1: A and B, Axial T2-weighted turbo spin-echo (A) and venography (B) using a microcatheter in the torcular herophili inserted through the left TS (arrows, known aplasia of the right TS). The left TS in the lateral projection shows high-grade narrowing distal to a filling defect assumed to be an arachnoid granulation. C, Pressure monitoring at different locations (in centimeters H2O). D, Placement of a self-expanding nitinol stent (Absolute, Guidant, Indianapolis, Ind; 8 mm in diameter, 6-cm in length). Stenosis is abolished after stent placement. E, Poststent placement on routine cranial CT. F and G, On follow-up, contrast-enhanced CT (F) and proton-weighted MR imaging (G) in the same axial section position demonstrate a new stenosis in the medial aspect of the left transverse sinus (arrows) proximal to the stent. H and I, Venous phase of right internal carotid artery angiography in the lateral projection (H) and maximum intensity projection of MRV in the lateral projection (I) confirm the finding. Arrows indicate pre-stent stenosis. J, Following lumbar puncture, MRV shows significantly better flow signal intensity in the left transverse sinus proximal and within the stent (arrows) with albeit some signal-intensity reduction believed to be due to the stent material. K, Cranial CT after insertion of a ventriculoperitoneal shunt.
The patient reported significant improvement. However, headache and visual disturbances worsened 1 week later. Digital subtraction angiography, CT, and MR imaging showed a new stenosis of the TS just proximal to the stent (Fig 1F–I). Because no signs of acute thrombosis or any structure compressing the sinus such as hematoma were found, we believed that recurring elevation of CSF pressure led to restenosis. Color-coded transcranial duplex sonography confirmed this interpretation: An accelerated intrastenotic blood-flow velocity (150 cm/s) was found and could be reduced to almost normal levels (30 cm/s) by performing another lumbar puncture. In accordance with this finding, MR venography (MRV) depicted a marked reduction of the prestent stenosis after lumbar puncture (Fig 1J). MRV was performed in all our patients by using phase-contrast imaging on a 1.5T scanner (Achieva, Philips Medical Systems, Best, the Netherlands) with a uniform protocol (3D fast-field echo; TR/TE, 17/8; flip angle 10°; matrix, 256 × 256; S1, 1.6 mm; velocity encoding value/phase-contrast velocity, 15 cm/s), interpreting source images and maximum intensity projections.
Consequently, a ventriculoperitoneal shunt was inserted (Fig 1K), and the patient was discharged with only little residual head pain. Nine months later, she was free of pain and diplopia. Control MRV showed good venous drainage. Accordingly, blood velocity in the TS was normal as measured with transcranial color-coded duplex sonography (5–20 cm/s).
Case 2
A 16-year-old girl (87 kg) with headache, diplopia, reduced visual acuity, and hyposmia had papilledema and elevated lumbar CFS pressure (50 cm H2O). Bilateral TS stenoses were suspected on MRV (Fig 2A). Treatment with acetazolamide (2 × 250 mg) was unsuccessful in reducing her symptoms. MRV following lumbar puncture showed some improvement of the blood-flow signal intensity in the lateral TS (Fig 2B). Again, after a second lumbar puncture with hardly any increased pressure left, the stenoses almost completely resolved on MRV (Fig 1C) and vision improved.
Fig 2.
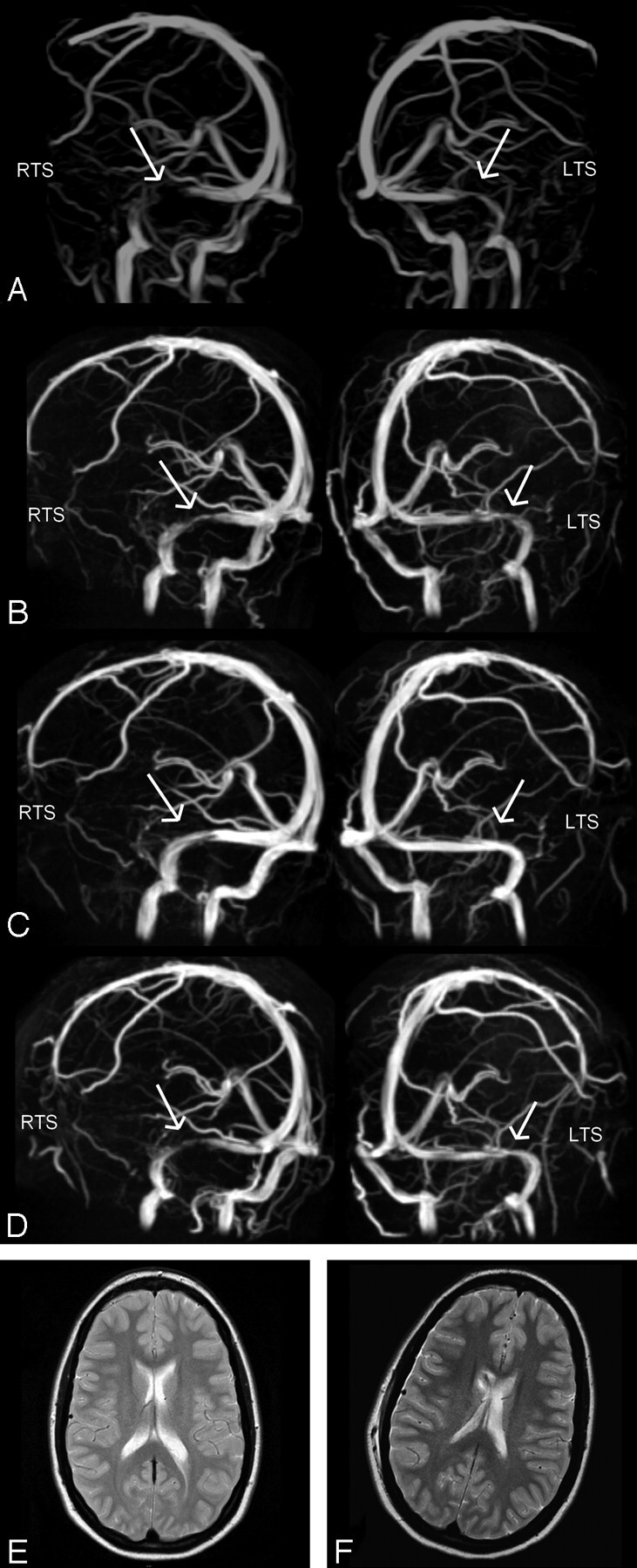
Case 2: MIP of MRV in oblique anteroposterior left and right views obtained at a lumbar CSF pressures of 50 cm H2O (A), after lumbar puncture (B), after a 2nd lumbar puncture with no residual pressure being monitored (C), and after placement of a ventriculoperitoneal shunt (D). Stenoses in both the left and right transverse sinuses (LTS, RTS), as marked with arrows, are reduced in accordance with a lowered CSF pressure, with complete resolution in C. Probably as a result of a relatively high opening pressure of the valve of the ventriculoperitoneal shunt, transverse sinuses show some residual narrowing (D). T2-weighted axial images before (E) and after (F) insertion of a ventriculoperitoneal shunt into the right ventricle show no difference in ventricle size.
The effect of lumbar puncture vanished 3 days later when the patient again had visual disturbances, and Doppler sonography showed elevated blood-flow velocities in the TS (40 cm/s). After ventriculoperitoneal shunt surgery, headache and papilledema did not reoccur (8-month follow-up) and MRV showed continuous blood-flow signal intensity with some residual stenosis in the right TS (Fig 2D).
Case 3
Bilateral papilledema and elevated lumbar CSF pressure (30 cm H2O) were observed in a 31-year-old woman (143 kg) with almost complete loss of vision in both eyes and worsening of headaches. MR imaging showed dilation of both optic sheaths and a flattened pituitary (Fig 3A), consistent with increased intracranial pressure. TS stenoses were suspected on MRV. Headache almost resolved after lumbar puncture, and vision improved. A lumbar drainage was used lowering CSF pressure to 11 cm H2O. MR imaging/MRV showed signs of intracranial pressure reduction (Fig 3B) and a decrease of venous stenoses. Venography was performed to exclude relevant hemodynamic stenoses (Fig 3B). We clinically monitored the natural course of the disease for 7 days, during which vision remained stable. Despite the benign clinical course, follow-up MR imaging showed re-occurrence of pressure signs (Fig 3C). Finally, ventriculoperitoneal shunt surgery was performed. Six months after dismissal, the patient was still free of headache. Vision had improved slightly.
Fig 3.
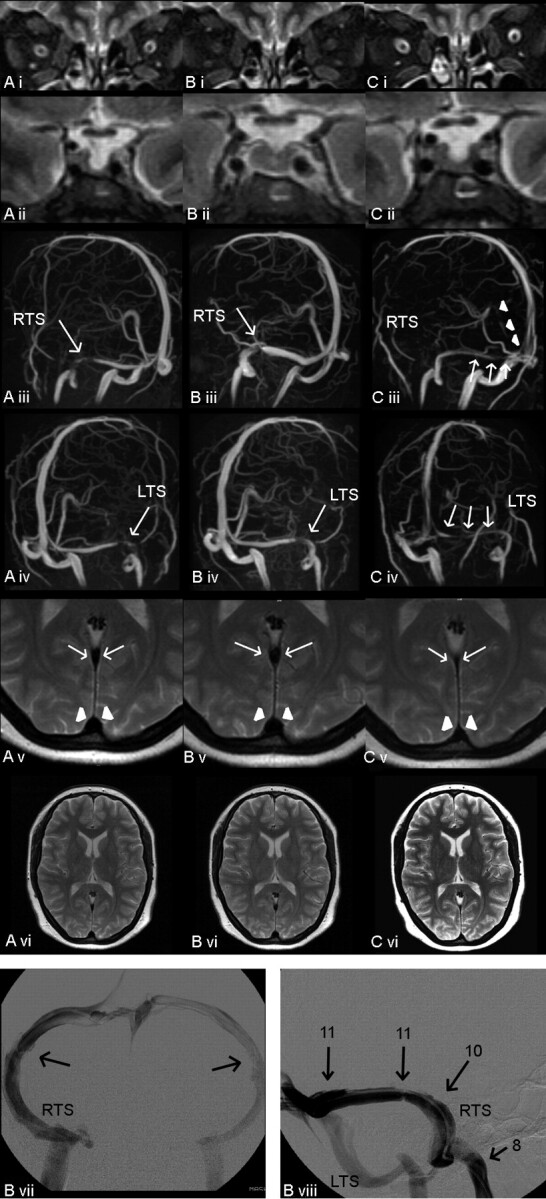
A–C, Case 3: MR imaging, MRV, and conventional venography at different points of time. CSF pressure 30 cm H2O (A); after CSF diversion, pressure of 11 cm H2O (B); and 7 days later (C). Fat-saturated T2-weighted turbo spin-echo inversion-recovery images in the coronal plane (TR/TE, 2650/180; Sl, 3 mm) (i), showing widening of the optic sheathes in A and C (arrows) and partially empty sella in A (ii) and C (ii). MIP of MRV in oblique anteroposterior views demonstrating narrowing of the RTS (iii) and the LTS (iv) (arrows) as well as in the straight sinus (arrowheads). T2-weighted turbo spin-echo images in the axial plane at the level of the straight sinus (arrows) and superior sagittal sinus (arrowheads, v), both being compressed in C. T2-weighted axial images showing normal ventricle size (vi). Conventional venography with the catheter placed in the RTS close to the torcular herophili was performed after CSF diversion (pressure of 11 cm H2O). B vii, Anteroposterior view shows bilateral low-grade TS stenoses. B viii, Lateral tilted view shows intravenous pressure monitoring in the RTS at different locations (arrows, pressure in centimeters H2O).
Discussion
By definition, in secondary intracranial hypertension (SIH), there is an underlying medical condition, whereas in IIH, the cause is not known. In many cases of SIH, an increased cerebral venous pressure raises the intracranial pressure as in dural venous fistulas, venous sinus thrombosis, or venous sinus compression.1 However, in almost all patients with IIH (and also in our patients), neuroimaging shows narrowing of the transverse sinuses,2,3,5–7 not reflecting acute thrombosis. The important question is whether those venous abnormalities are cause or consequence of increased intracranial pressure. In the former situation, fixed stenoses (for example postthrombotic fibrotic changes) could obstruct the venous outflow, increase intracranial venous pressure proximal to the stenosis, and lead to increased CSF pressure as a result of a reduction in CSF absorption via the arachnoid granulations. In this setting, a pressure gradient across the stenosis should be measured and reconstruction of the venous lumen with endovascular stents would be effective in lowering elevated CSF pressure. This procedure was successfully used several times4,6 but seems not to be efficient in all patients.6 In the latter (ie, venous abnormalities as a consequence of intracranial pressure), elevated intracranial CSF pressure could lead to a secondary narrowing of the sinus lumen by compression, which can be reversed by lumbar puncture or shunt surgery procedures.5,7,8 In fixed stenoses (the 1st model), therapeutic reduction of CSF pressure should have no effect on sinus diameter.
Restenosis of the TS despite prior stent angioplasty in our 1st patient taught us that increased intracranial pressure was the primary problem leading to compression of the venous sinus and not venous sinus stenosis leading to increased intracranial pressure. Consequently, in all our patients, venous sinus stenoses could be reduced or eliminated by CSF diversion procedures. Although reversibility of TS stenoses by CSF diversion is known,5,7,8 this case, for the first time, illustrates how sinus stent placement fails to have a lasting effect and that a high-grade stenosis with a pressure gradient can very well be a secondary phenomenon.
We, therefore, propose that MRV before and after maximal CSF diversion should be performed in all patients with suspected IIH to distinguish reversible and fixed stenoses of the transverse sinuses, aiding in the choice of therapy (ventriculoperitoneal shunt surgery versus stent placement). Only in cases in which no or incomplete restoration of the sinuses occurs with CSF diversion should conventional venography and manometry be used additionally (as in our 3rd patient). Moreover, rapid changes in the shape of the pituitary and in the dilation of the optic nerve sheathes can be visualized in good accordance with the degree of the intracranial pressure. Also, it was shown that generalized narrowing of the venous sinuses can sometimes be seen even on routine axial T2-weighted MR images.
References
- 1.Binder DK, Horton JC, Lawton MT, et al. Idiopathic intracranial hypertension. Neurosurgery 2004;54:538–52 [DOI] [PubMed] [Google Scholar]
- 2.Karahalios DG, Rekate HL, Khayata MH, et al. Elevated intracranial pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 1996;46:198–202 [DOI] [PubMed] [Google Scholar]
- 3.Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 2003;60:1418–24 [DOI] [PubMed] [Google Scholar]
- 4.Owler BK, Parker G, Halmagyi M, et al. Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg 2003;98:1045–55 [DOI] [PubMed] [Google Scholar]
- 5.King JO, Mitchell PJ, Thomson KR, et al. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 2002;58:26–30 [DOI] [PubMed] [Google Scholar]
- 6.Higgins JNP, Cousins C, Owler BK, et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 2003;74:1662–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JNP, Pickard JD. Lateral sinus stenoses in idiopathic intracranial hypertension resolving after CSF diversion. Neurology 2004;62:1907–08 [DOI] [PubMed] [Google Scholar]
- 8.Baryshnik DB, Farb RI. Changes in the appearance of venous sinuses after treatment of disordered intracranial pressure. Neurology 2004;62:1445–46 [DOI] [PubMed] [Google Scholar]


