Abstract
BACKGROUND AND PURPOSE: Using percutaneous cement injection to treat vertebral compression fractures (VCF) stemming from advanced malignancy, particularly those showing posterior cortical defect and epidural extension, is associated with higher risk of complications compared with treating benign osteoporotic VCF. The purpose of this study was to examine the clinical feasibility of a technique designed to improve control of cement placement.
MATERIALS AND METHODS: A prospective series of patients (n = 15) with metastatic lesions having epidural extension of tumor and/or cortical disruption were treated. The percutaneous procedure consisted of using a plasma-mediated radio-frequency-based device to etch a cavity within the affected vertebral body and filling the cavity and adjacent interstices with bone cement. Change in pain status was determined by asking the patient to grade back pain preoperatively and at the 2–4-week postprocedure examination using a visual analog scale.
RESULTS: An adequate amount of cement was injected in all cases. Extraosseous extension of cement was observed in 4 cases (anterior cortex, n = 3; through neural foramen, n = 1) but was clinically inconsequential. No thermal or neuronal insult was observed during the procedure in any case. Thirteen of the 15 (87%) patients reported decreased pain.
CONCLUSION: Dissolution of tissue rather than displacement to create a cavity before injecting bone cement permitted well-directed cement deposition into the compromised vertebral body, which may allow a safer procedure to be conducted in patients with advanced malignant VCF. Clinical benefits may include avoiding more extensive surgery and reducing the risk of complications associated with conventional bone cement injection procedures.
Percutaneous cement injection (vertebroplasty or kyphoplasty) is widely used to treat osteoporotic vertebral compression fractures (VCF), though it was originally described for application with painful VCF resulting from hemangioma of the spine.1 This approach was subsequently adopted in Europe, primarily in France, for treating painful VCF associated with spine osteolytic metastases and angiomas.2,3 Malignant vertebral body lesions are more challenging to treat than benign ones and carry a relatively higher risk of complications.2 They are often associated with epidural extensions or posterior cortical disruption, which may increase risk for leakage and subsequent compromise of the thecal sac during cement delivery.
The purpose of this study was to investigate clinical viability of a novel technique for treating VCF associated with advanced malignant tumors. Many of these types of cases are not even considered as candidates for a conventional percutaneous vertebroplasty or kyphoplasty procedure. We theorized that by creating a cavity, or void, inside the affected vertebral body before injecting cement, the etched pathway would aid in directing the injected cement away from the posterior portion of the vertebral body, often compromised because of the epidural extension or cortical disruption.
A cavity such as that required for this type of approach can be created using a plasma-mediated radio-frequency-based (coblation) device. This technology is used in several medical specialties for procedures requiring tissue ablation (ie, dissolution), including disk decompression,4 tonsillectomy,5 and tendon and cartilage debridement.6,7 It has 2 important features that make it suitable for use in the proposed application: 1) the ability to create a void in soft tissue via a precise molecular dissociation (ablation) process,8–10 rather than displacement of tissue such as that achieved by balloon-assisted kyphoplasty, and 2) deposition of minimal heat when used in the spine,11 in contrast to conventional electrosurgery used to perform tissue denaturation. The radio-frequency-based device used in this study was cleared by the US Food and Drug Administration for soft tissue ablation in the spine.
Materials and Methods
Patients
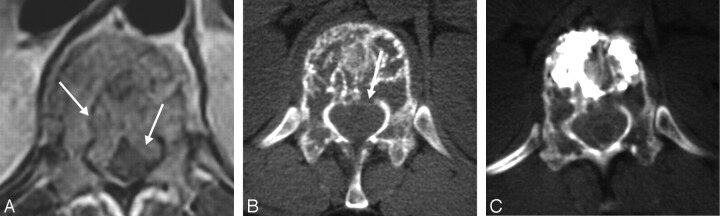
Fifteen patients (16 levels; 7 women and 8 men) ranging in age from 27 to 82 years (mean, 61 ± 17 years) were included in the study. Patients presented with severe pain associated with VCF as a result of metastasized malignancy. All patients but one underwent MR imaging before the procedure to determine the extent of the epidural disease. The patient who did not undergo MR imaging received a bone scan (MR imaging was contraindicated in this case). All patients underwent CT examination before and after the procedure. CT examination was performed before the procedure to assist in preprocedure planning and to determine the status of cortical bone. Postprocedure CT was used to examine the distribution of the cement deposition after the procedure. In all cases, the metastatic lesions showed evidence of epidural extension of tumor and/or cortical disruption (Fig 1A).
Fig 1.
A, Axial CT scans show a large degree of cortical disruption in a 70-year-old man with metastatic colon cancer.
B and C, The SpineWand device was inserted into the cannula to perform the tissue ablation; the clinician is capable of ablating tissue in a superior or inferior lateral direction.
D, In this patient, 2 10-mm Kyphon balloons were inserted to obtain hemostasis while preparing the bone cement. A myelogram was performed to clearly delineate the posterior cortical margin.
E, The axial CT scans collected immediately after the procedure showed that cement was cleanly deposited in the ablated tumor void with no posterior extraosseous extension of cement.
After determining candidacy for the proposed procedure, all viable treatment alternatives and risks and benefits associated with each were discussed with the prospective patients and their referring physicians. Informed consent was obtained according to our institutional requirements from those who opted to undergo the procedure. Formal review of the study protocol by the Institutional Review Board was not required because internal consent requirements for standard patient management were being followed, both the radio-frequency-based device and bone cement used in the procedure were used “on-label,” and patients were treated in an outpatient setting.
The Procedure
The procedure was performed under fluoroscopic imaging with the patient placed in the prone position under conscious sedation. All patients received intravenous antibiotics (1 g of cefazolin sodium). Access to the vertebral body was gained by using the KyphX introducer kit (Kyphon, Sunnyvale, Calif) in 12 cases or an 11-gauge bone marrow harvesting needle in 3 cases (Cook, Bloomington, Ind). After crossing the posterior cortex, the needle was positioned in the posterior portion of the vertebral body. At this point, a biopsy was collected to confirm malignancy of the lesion.
The bipolar radio-frequency-based device (CAVITY SpineWand; ArthroCare, Sunnyvale, Calif) was advanced into the delivery port (harvesting needle or introducer kit) until its tip protruded beyond the cannula tip. With the radio-frequency controller placed on a setting of “6,” the activated device was directed anteriorly through the malignant mass to ablate (ie, excise tissue) a small channel (Fig 1B, -C). The curve of the device allows it to ablate slightly beyond the trajectory of the access cannula. This maneuver was repeated along several clock positions (different orientations) to etch a cavity; ablation was ceased when a noticeable reduction in tactile resistance was detected. When inserting the device toward the anterior aspect of the vertebral body, the tissue dissolution (ie, coblation) setting was used; when retracting the device posteriorly, the coagulation setting was used to provide hemostasis. A total of 3–6 passes were made to complete the cavity, which took between 30 and 60 seconds depending on the size of the vertebral body and lesion. In 9 cases, a 10-mm KyphX inflation balloon (Kyphon) was inserted to assure hemostasis (Fig 1D); it was left in place while preparing the bone cement for insertion.
After completing the ablation portion of the procedure, a mixture of bone cement (Zimmer, Warsaw, Ind) and Biotrace sterile barium sulfate (Bryan, Woburn, Mass) was injected into the ablated cavity under fluoroscopic guidance. In 6 patients, a myelogram was performed immediately before the cement injection to further delineate the posterior cortical margin because it was so severely disrupted. On average, 3–6 mL of bone cement was found to be sufficient to fill the ablated vertebral body cavity. In all cases, a postprocedure CT scan was performed to examine the distribution of cement deposition (Fig 1E). All patients were admitted to the hospital and kept overnight to monitor postoperative recovery.
Follow-Up and Patient Evaluation
Patients received a follow-up phone call within 2 days after the procedure as a safety measure. This was followed by an office visit 2–4 weeks after the procedure. At this visit, patients received a brief physical examination. In addition, they were also asked to rate the level of back pain they were currently experiencing using an 11-point scale, where 0 represented no pain and 10 represented the worst pain in their lives.
Results
Demographics, treatment level, type of pathologic condition, anatomy of the metastatic lesion, pain scores, and postprocedure CT findings for the treated patients are presented in the Table. In 12 of 16 levels, cement deposition was confined to the vertebral body, where CT imaging demonstrated no evidence of extraosseous extension of cement, even in cases with severe posterior cortical compromise and prominent epidural involvement (Figs 2 and 3). Postprocedure CT imaging showed minor extraosseous extension of cement in 4 cases; in 3 cases, cement was detected outside the anterior vertebral boundary, and in 1 case leakage was noted along a neural foramen. The extraosseous extension of bone cement in all of these cases was clinically inconsequential. In 3 cases, cement deposits were not detected inside the lytic lesion targeted for the augmentation, though evidence of cement placement was observed within the vertebral body.
Patient demographics
| Patient | Age | Sex | Treated Level(s) | Pathologic Conditions | Anatomy of Metastatic Lesion | Other Procedures | Pain Score (Before–After) | Postprocedure CT | |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | |||||||||
| 1* | 75 | F | L2 (Vp) | Plasmacytoma, history of breast cancer, lupus erythematosus, chronic steroid use | Posterior cortical defect | Vp, T6, T8, T9, T10 (benign compression fractures) | 9/10–2/10 | Cement confined to the lytic lesions | |
| 2* | 71 | M | L1 (Vp) | Lung cancer | Epidural extension and posterior cortical defect | 9/10–3/10 | Cement confined to the lytic lesions | ||
| 3 | 27 | M | T11 (Vp) | Undifferentiated testicular malignancy | Epidural extension | Vp, T12, L1 | 8/10–5/10, deceased | No postprocedure CT; no EE on plain film | |
| 4* | 82 | M | L5 (Vp) | Lung squamous cell carcinoma | Posterior cortical defect and epidural extension; tumor extension into right neural foramen | 9/10–5/10 | Cement on periphery of the metastatic lesion | ||
| 5 | 52 | F | T9 (Vp) | Breast cancer | Anterior cortical defect | Vp, T5, T7, T10 | 8/10–5/10 | Cement around the lytic lesions | |
| 6* | 70 | M | L2 (Kp) | Colon cancer | Posterior cortical defect | 9/10–5/10, deceased | Cement confined to the lytic lesions | ||
| 7* | 34 | F | T12 (Kp) | Multiple myeloma | Posterior cortical defect and epidural extension | Kp, T7,T8, T11 | Cannot tell difference | Cement confined to the lytic lesions | |
| 8 | 58 | F | T12 (Kp) | Breast cancer | Posterior cortical disruption and epidural extension | Cervical, sacral, and bilateral hip metastases | 9/10–5/10, deceased | Cement confined to the lytic lesions | |
| 9 | 80 | F | L4 (Kp) | Stomach & breast cancer | Epidural extension and complete absence of posterior cortex on CT | Kp, L2; Vp, L3; followed by Kp, T7, T8, T11 | 7/10–3/10 | Cement confined to the lytic lesions | |
| 10 | 76 | M | L1 (Kp) | Hepatoma | Posterior cortical disruption | Kp, L3 | 10/10–5/10, deceased | Cement confirmed in normal bone anterior to the lytic lesion | |
| 11* | 72 | M | L1 (Kp) | Multiple myeloma | Epidural extension; cortical defect | Vp, L2 | 6/10–2/10 | Cement confined to the lytic lesions | |
| Group 2 | |||||||||
| 12 | 56 | M | L1 (Vp) | Renal cell metastasis | Paravertebral extension and lateral cortical disruption | 2 stage coblation for vertebral & paravertebral components; arterial embolization | 8/10–2/10 | Cement confined to the lytic lesions; EE through anterolateral cortex | |
| 13 | 36 | F | L2 (Kp) | Cervical cancer | Anterior and posterior cortical defect; paraspinal mass extended into left L2–L3 NRB | Recurrent lesion after radiation; left L2–L3 NRB | 9/10–9/10 | Cement confined to the lytic lesions; some anterior EE | |
| 14 | 66 | F | L3 (Kp right; Vp left) | Lung cancer | Posterior cortical defect; epidural extension; extension into right neural foramen | Right NRB | 10/10–0/10 | Cement confined to the lytic lesions; EE anteriorly and into right neural foramen | |
| 15 | 68 | M | L5 (Kp) | Urethral cancer | Epidural extension and posterior cortical defect; extension into the right pedicle and neural foramen | S1 sacroplasty, NRB | 9/10–7/10 | Cement on periphery of lesion, extending into the right pedicle | |
Note:—EE indicates extraosseous extension of cement; Group 1, bone cement confined to lytic lesions and confined to vertebral body; Group 2, bone cement with extraosseous extension outside vertebral body; Vp, vertebroplasty; Kp, kyphoplasty; NRB, nerve root block.
Myelogram performed.
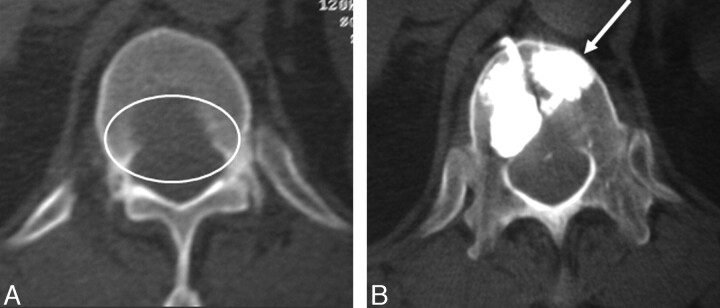
Fig 2.
A, An axial T1-weighted image through the T11 level showing prominent epidural involvement in a 34-year-old woman with multiple myeloma.
B, The axial CT image showed associated cortical disruption.
C, The axial CT images obtained after the procedure, showing adequate cement filling with no epidural extension. Note the tight thecal sac as evident by using myelographic contrast agent.
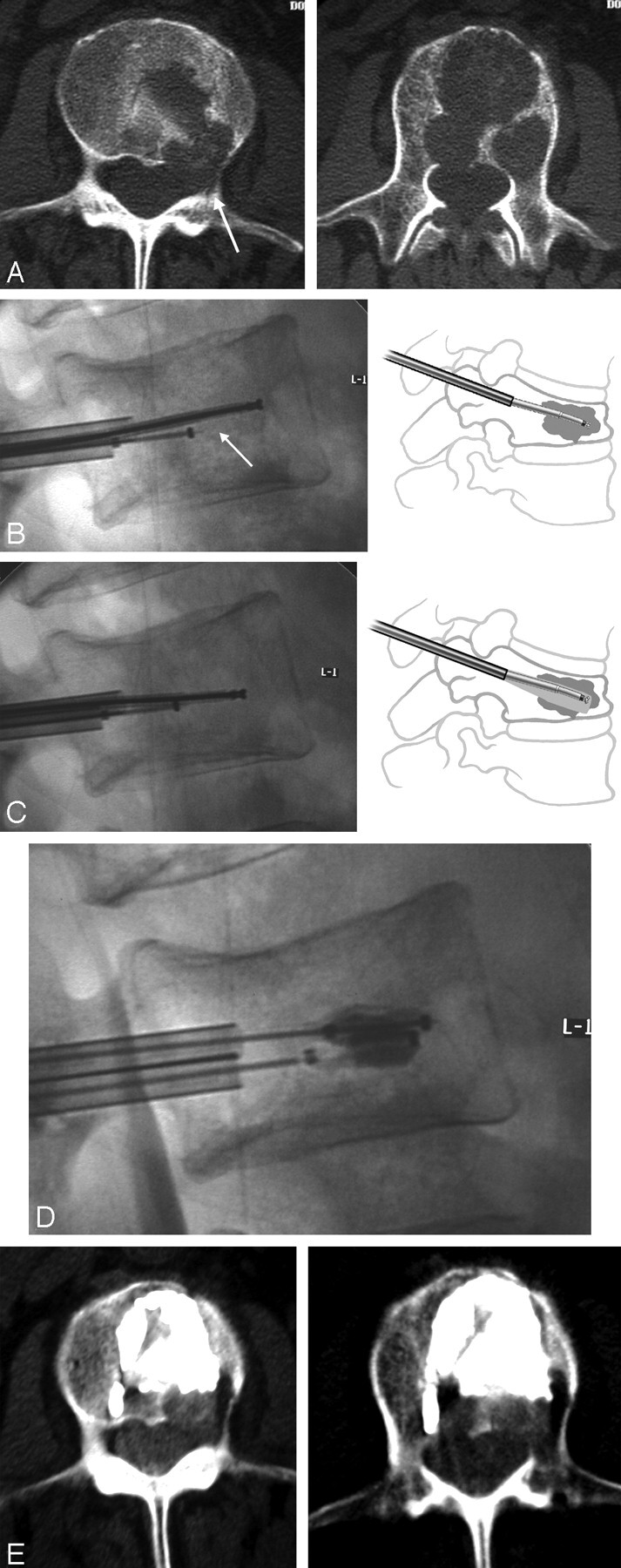
Fig 3.
A, The preprocedure axial CT examination showing almost complete absence of the posterior cortex at the L1 level in a 76-year-old man with metastatic hepatoma to the spine.
B, The postprocedural CT axial images showed well-bounded deposition of cement in the anterior part of the vertebral body with no extension into the compromised posterior aspect.
No operative complications were observed. In 6 cases, a myelogram was performed immediately before cement injection to improve visualization of the epidural component during cement injection; no displacement of the contrast column was detected in any case. Three patients received a selective nerve root block during the procedure to relieve radicular pain associated with extension of the tumor into a neural foramen. No patient required extended hospital care exceeding the 1-night planned admission.
No postoperative complications (eg, nerve root or spinal cord damage, new neurologic symptoms) occurred. Thirteen of 15 patients (87%) reported pain relief at the 2–4-week follow-up examination.
Discussion
We investigated a new technique that can be used to create a cavity inside the vertebral body before performing cement injection. It was hypothesized that this cavity would provide a clean space for bone cement placement, thereby improving precision and redirecting the cement away from the compromised posterior or lateral wall. In theory, this may reduce incidence of extraosseous extension of cement and decrease the risk of increasing size of the epidural extensions. Creating a cavity allowed us to perform cement augmentation in patients who would not be considered good candidates for conventional vertebroplasty or kyphoplasty, given the extent of their disease. A cavity like this can be created within a lytic lesion, which may help with deposition of cement inside the lesion for better augmentation, or it may be created in the anterior aspect of the vertebral body, in an effort to decrease risk of posterior extraosseous extension of cement or displacement of any epidural extension. The ability to perform precise cement augmentation to treat an anterior lesion may provide an alternative method to performing the more extensive anterior corpectomy and posterior fusion. Such metastatic lesions are usually unstable and cannot be treated conservatively (eg, radiation alone); in such cases, this procedure may be particularly valuable for the relatively healthy patient with cancer who simply requires stabilization.12
Metastatic spine disease is usually extremely painful, and most patients with this condition become disabled. Radiation therapy may increase the risk of fractures and cannot correct the anatomic abnormalities that result from the fracture itself.13 A considerable component of pain is due to spinal instability and thus stabilization is required for pain relief.12,14 Malignant lesions in the spine are often challenging to treat, and practitioners may shy away from these clinically and technically more difficult patients. Posterior cortical disruption and large epidural extensions, in particular, limit the ability to perform vertebroplasty or kyphoplasty in these cases because of the higher risk of potential extraosseous extension of cement into the thecal sac.
The bipolar plasma-mediated radio-frequency-based device used in these cases functions using a process in which radio-frequency energy is used to excite the electrolytes in a conductive medium, such as saline solution, to create a precisely focused plasma field. This plasma field is composed of highly ionized particles8–10 and forms immediately adjacent to the device tip (electrodes). The energized particles in the plasma have sufficient energy to break molecular bonds, excising or dissolving soft tissue at relatively low temperatures (typically 40°C to 70°C). This molecular dissociation process converts the ablated tissue into gases, which exit the treatment site through the cannula. With this ablation process, a cavity can be created at the treatment site. A plasma-mediated radio-frequency-based process results in much less heat generation in the surrounding tissue than the conventional diathermy process,15 which makes it safer for applications in the spine. Recent in vitro work showed that when this device was used in cancellous bone tissue, minimal thermal injury was observed adjacent to the plasma radio-frequency-treated sites (HM Do and MK Rippy, presented at a poster at the Congress of Neurologic Surgeons Annual Meeting, Boston, Mass, October 8–13, 2005). The maximum depth of necrotic injury attributed to the procedure (drill plus radio-frequency-based treatment) was less than 1.8 mm. We believe that advances in the design of the device used in this study and the technology it is based on will offer a means for performing a more accurate and targeted augmentation procedure in the future.
Creating a tissue cavity can be achieved using other means, such as the kyphoplasty system. However, we believe that producing a cavity via tissue displacement in this patient group may result in dislodged tumor tissue, which could stray into the thecal sac or even into the bloodstream. The advantage of using plasma-mediated radio-frequency-based technology is the ability to create such a cavity using a tissue dissolution process, as opposed to simply displacing it. This technique also provides the additional theoretic benefit of improving interdigitation of the cement, as adjacent interstices, which would normally be blocked by displaced tissue, are left open. Radio-frequency-based technology also allows quick and easy tissue coagulation, which helps to decrease bleeding resulting from vascular tumors. Nevertheless, in some cases, we found it necessary to use a small 10-mm KyphX inflation balloon to achieve additional homeostasis when radio-frequency-based coagulation was not sufficient.
Shimony et al12 described percutaneous vertebroplasty performed in patients who had compression fractures resulting from malignant lesions with epidural involvement. The patient population did not include cases with posterior cortical disruption. The investigators reported good success with the procedure for diminishing pain and improving mobility. Although our cases generally had more advanced epidural and cortical involvement, our experience was similar. Our results support the Shimony et al12 findings that vertebroplasty is safe when performed in patients with epidural involvement. It is noteworthy that Shimony et al12 used cement with very liquid composition, whereas we preferred a more viscous compound. We felt that increased viscosity reduces risk of extraosseous extension of cement and provides more control during injection.
Combining both radio-frequency-based ablation and vertebroplasty into a single procedure has been described previously in the literature.16,17 Gronemeyer et al16 treated 4 patients with unresectable spine metastases who demonstrated substantial reduction in pain and disability. Nakatsuka et al17 treated 17 spinal cases using a radio-frequency-based device consisting of internally cooled straight electrodes with vertebroplasty under CT fluoroscopy guidance. They reported serious complications (incomplete hemiplegia and radiculopathy) in 4 patients (23.5%). Interestingly, these were the patients in whom the tumor had invaded the posterior cortex and a pedicle. Use of the current technique allowed us to treat patients with this condition successfully, without any significant complications.
A technical limitation of the study lies in the inability to visualize the presumed cavity or void created by ablation. This was difficult to see in postprocedure CT examinations 1) because in most cases, the cement was presumably deposited at the site of the created void or 2) because of beam-hardening artifacts. We believe that there were some indirect indicators of the tissue void created, including visualization of gas in the needle tract stemming from tissue dissolution in some cases and the pattern of cement deposition outside the lytic lesion (group 2 in the Table). In some situations, the SpineWand device was placed outside, rather than inside, the lytic lesions as a result of geometric factors related to the location of the lesion (ie, being in the center of the vertebral body) away from the needle trajectory because a transpedicular approach was used for all the cases. The cement was deposited at the presumed ablated site rather than into the lytic lesion. However, the possibility of not creating a cavity at all, with the cement deposited in a manner similar to the standard kyphoplasty or vertebroplasty procedure, still exists.
Some clinicians have advocated placing an angiographic wire through the access cannula after ablation to identify the created void before depositing the cement (G. Anselmetti, personal communication). This maneuver was not performed in this study. Evidence of creating a void has been described in the literature, where Chen et al11 showed that a cavity is created in disk tissue with no evident damage to adjacent structures such as the spinal cord or nerve roots. Nevertheless, the extent to which ablation of a cavity contributed to the successful completion of these cement injection procedures remains unclear. Additional in vitro work is necessary to clearly characterize the cavity created using this approach. Research study using CT fluoroscopy or 3D imaging techniques may be necessary to better demonstrate the dynamics of cement flow inside the malignant vertebral lesions after creation of a tissue void.
Another limitation of this study was the lack of prospective, longer-term postoperative follow-up. The current study was not designed for this purpose but rather was primarily fashioned to explore the clinical feasibility of this new technique. Such a patient population is inheritably difficult to follow because a large proportion of those patients is terminally ill. In addition, most of these patients do not have isolated spine disease but usually have other metastatic lesions in different levels of the spine and the rest of the body as well. This makes it difficult for the patient to separate symptoms and allow us to obtain clean feedback in regard to the problem being treated. For these reasons, use of an analgesic diary or mobility outcome is impossible to implement. Furthermore, a prospective study designed to compare this technique with a conventional vertebroplasty or kyphoplasty procedure may be needed in the future to validate these results. However, it may prove difficult, because most clinicians are usually reluctant to perform this type of procedure in advanced cases demonstrating presence of epidural extension or posterior cortical disruption.
In summary, we found that this new technique, combining percutaneous plasma-mediated radio-frequency-based ablation with percutaneous cement augmentation, seems to provide a valuable addition to our surgical armamentarium for treating painful malignant VCF. It seems to be particularly promising for patients with advanced lesions showing evidence of posterior cortical defect or epidural extension. We believe that creating a cavity through tissue dissolution, rather than tissue displacement alone, may aid in redirecting the cement away from the posterior aspect of the vertebral body and hence decrease the risk for perioperative complications. In addition, this radio-frequency-based approach is associated with minimal heat production. This technique allowed us to perform cement augmentation successfully in patients with more advanced disease or in those who would not have been candidates for the more conventional bone augmentation procedures. Success using a technique such as that presented here may potentially alter conventional surgical intervention by allowing precise placement of the cement in lytic anterior vertebral body lesions to provide stability and eliminate the need for extensive anterior approach surgery. Nevertheless, further, longer-term experience is necessary to validate these preliminary results and to further delineate the clinical benefits that might be associated with its use in preference to other bone augmentation procedures.
Conclusion
Finely directed tissue dissolution in conjunction with bone cement injection in patients with vertebral body fractures occurring with advanced malignancy relieved the severe pain associated with this pathologic condition in most patients treated in this series. This technique may be safer than conventional vertebroplasty or kyphoplasty by providing a space for bone cement placement, particularly in cases with posterior cortical defect and epidural extension, which is known to be associated with higher risk of extraosseous extension of cement. Potential clinical benefits of this procedure for this population of patients include avoiding more extensive surgery and reducing the risk of complications associated with conventional bone cement injection procedures.
Acknowledgments
We thank Debby Holmes-Higgin, MS, MPH, from ArthroCare Corporation for technical assistance with the manuscript.
Footnotes
Previously presented at: Annual Meeting of the American Society of Neuroradiology, April 29–May 5, 2006; in San Diego, Calif.
Disclosure: B.A.G. and W.W. participate in the speaker's bureau for ArthroCare Corporation, Austin, Tex.
References
- 1.Galibert P, Deramond H, Rosat P, et al. [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty]. Neurochirurgie 1987;33:166–68 [PubMed] [Google Scholar]
- 2.Chiras J, Depriester C, Weill A, et al. [Percutaneous vertebral surgery. Technics and indications]. J Neuroradiol 1997;24:45–59 [PubMed] [Google Scholar]
- 3.Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology 1996;200:525–30 [DOI] [PubMed] [Google Scholar]
- 4.Gerszten PC, Welch WC, King JT. Quality of life assessment in patients undergoing nucleoplasty-based percutaneous discectomy. J Neurosurg Spine 2006;4:36–42 [DOI] [PubMed] [Google Scholar]
- 5.Chan KH, Friedman NR, Allen GC, et al. Randomized, controlled, multisite study of intracapsular tonsillectomy using low-temperature plasma excision. Arch Otolaryngol Head Neck Surg 2004;130:1303–07 [DOI] [PubMed] [Google Scholar]
- 6.Tasto JP, Cummings J, Medlock V, et al. Microtenotomy using a radiofrequency probe to treat lateral epicondylitis. Arthroscopy 2005;21:851–60 [DOI] [PubMed] [Google Scholar]
- 7.Uribe JW. The use of radiofrequency devices for chondral debridement. Sports Med Arthrosc Rev 2003;11:214–21 [Google Scholar]
- 8.Stalder KR, Woloszko J, Brown IG, et al. Repetitive plasma discharges in saline solutions. Appl Phys Lett 2001;79:4503–05 [Google Scholar]
- 9.Woloszko J, Stalder KR, Brown IG. Plasma characteristics of repetitively-pulsed electrical discharges in saline solutions used for surgical procedures. IEEE transactions on plasma science 2002;30:1376–83 [Google Scholar]
- 10.Stalder KR, McMillen DF, Woloszko J Electrosurgical plasmas. J Phys D: Appl Phys 2005;38:1728–38 [Google Scholar]
- 11.Chen YC, Lee SH, Saenz Y, et al. Histologic findings of disc, end plate and neural elements after coblation of nucleus pulposus: an experimental nucleoplasty study. Spine J 2003;3:466–70 [DOI] [PubMed] [Google Scholar]
- 12.Shimony JS, Gilula LA, Zeller AJ, et al. Percutaneous vertebroplasty for malignant compression fractures with epidural involvement. Radiology 2004;232:846–53 [DOI] [PubMed] [Google Scholar]
- 13.Patel B, DeGroot H, III. Evaluation of the risk of pathologic fractures secondary to metastatic bone disease. Orthopedics 2001;24:612–17 [DOI] [PubMed] [Google Scholar]
- 14.Krishnaney AA, Steinmetz MP, Benzel EC. Biomechanics of metastatic spine cancer. Neurosurg Clin N Am 2004;15:375–80 [DOI] [PubMed] [Google Scholar]
- 15.Pearce JA. Electrosurgery. New York: Wiley Medical;1986. .
- 16.Gronemeyer DH, Schirp S, Gevargez A. Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J 2002;8:33–39 [DOI] [PubMed] [Google Scholar]
- 17.Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol 2004;15:707–12 [DOI] [PubMed] [Google Scholar]