Abstract
BACKGROUND AND PURPOSE: We report our experiences in the treatment of intracranial arteriovenous malformations (AVMs) with Onyx embolization before neuro- or radiosurgery, focusing on the embolization technique with Onyx.
METHODS: Ninety-three patients (40 women and 53 men, mean age 38 years) with 94 intracranial AVMs with a mean volume of 9.2 mL were embolized with Onyx. The following features of all AVMs were evaluated before the treatment: type of nidus and shunt, draining veins, and feeding arteries.
RESULTS: Complete obliteration rates were 20% at the end of all embolization steps and 53% after embolization and surgery. In 21% of patients the final control angiography is not yet available (after surgery 9%, after radiosurgery 12%). In 4% of patients, radiosurgery was planned due to a persistent arteriovenous shunt. The injection of Onyx resulted in high occlusion rates (volume reduction >90%) when the AVM was in a supratentorial and cortical location, the nidus was compact and plexiform, and when there was a small number of supplying (direct) feeders and one superficial draining vein. Access device–related complications (stuck catheter, vessel perforation) occurred during the embolization of 220 feeding arteries in 6% of patients, with all such instances having had no clinical consequences.
CONCLUSION: With knowledge of the morphologic characteristics of AVMs that are suitable for a treatment with Onyx, high occlusion rates and low complication rates in treating a small number of feeders are feasible. Superselective intranidal or perinidal catheter positions and slow, controlled injections that protect the draining veins make the therapy safe even in complex AVMs and critical locations.
Embolization of high-grade intracranial arteriovenous malformations (AVMs) before surgical resection is an accepted treatment technique to improve the outcome.1 The goal of embolization before surgery or radiosurgery is to reduce the AVM volume and to occlude critical feeders.2 In low-grade AVMs, surgical resection alone is considered without primary embolization.3 Radiosurgery is recommended as the first-line treatment technique for AVMs in anatomically difficult locations. The intention of embolization before radiosurgery is to increase the obliteration rate while reducing the AVM volume. However, long-term follow-up examinations after stereotactic radiosurgery resulted in obliteration rates of about 80% for low-grade AVMs4 and lower rates in high-grade AVMs.
We report our experiences in the treatment of intracranial AVMs with Onyx embolization before neuro- or radiosurgery. Our strategy was to embolize AVMs as much as possible before a secondary therapy was applied.
This article focuses on embolization techniques with Onyx based on our experience. Additionally, we report our retrospective analysis of the morphologic features of AVMs in which the injection of Onyx was successful and in which the technique failed.
Methods
Patients and AVMs
Ninety-three patients (40 women and 53 men, mean age 38 years, range 9–65 years) with 94 intracranial AVMs were embolized between July 2001 and November 2004. Eighty-four AVMs were treated by W.W. and D.K. and 10 by R.S. According to the Spetzler-Martin Grading Scale,5 48 of the AVMs were ranked as grades I–II, 24 as grade III, and 22 as grades IV–V. The average AVM volume following Pasqualin’s method6 was 9.2 mL (range 1.0–58.0 mL). Seizures represented initial symptoms in 45 cases, hemorrhages in 19, headaches in 16, and neurologic deficits in 4. Nine patients were asymptomatic. Localization of the AVM treated is listed in Table 1. All patients and their families were informed of the risks and benefits of the intended procedure by the treating neuroradiologist. Each of them signed a written consent form at least 24 hours before endovascular intervention.
Table 1:
Localization of 94 arteriovenous malformations
| Localization | N |
|---|---|
| Frontal | 21 |
| Temporal | 15 |
| Parietal | 8 |
| Occipital | 13 |
| Fronto-temporal | 1 |
| Fronto-parietal | 9 |
| Temporo-occipital | 3 |
| Temporo-parietal | 1 |
| Temporo-parieto-occipital | 1 |
| Parieto-occipital | 9 |
| Corpus callosum | 1 |
| Basal ganglia | 2 |
| Thalamus | 2 |
| Choroid plexus | 2 |
| Cerebellum | 5 |
| Brain stem | 1 |
Liquid Embolic Agent Onyx
The application of the ethylene-vinyl alcohol copolymer (EVOH) in the endovascular treatment of intracranial AVMs was first described by Taki et al7 and Terada et al8 in the early 1990s. A mixture of 60 parts of the solvent dimethyl-sulfoxide (DMSO), 5 parts of EVOH, and 35 parts of the contrast agent metrizamide was used.
EVOH is now commercially available as the nonadhesive liquid embolic system under the names Onyx 18, Onyx 20, and Onyx 34 (ev3, Irvine, Calif) and is CE-marked for the treatment of intracranial AVMs in Europe. Since July 2005, Onyx 18 and Onyx 34 have been approved in the United States by the Food and Drug Administration. The numbers 18, 20, and 34 quantify the viscosity of Onyx in centipoise (cp). Onyx 18 contains 6% EVOH and 94% DMSO, Onyx 20 6.5% EVOH and 93.5% DMSO, and Onyx 34 8% EVOH and 92% DMSO. Tantal powder is added to the mixture for radiopacity. Onyx must therefore be shaken for at least 20 minutes before injection9 to achieve homogeneous radiopacity of the mixture. DMSO is potentially angiotoxic, but this effect is negligible if used with the recommended infusion rates.10 If the mixture comes into contact with aqueous solutions, precipitation of the polymer is initiated by diffusion of DMSO. This process begins on the surface while the core is still liquid, resulting in a soft, nonadherent mass. Therefore, Onyx has a lavalike flow pattern within blood vessels without any fragmentation during the injection. Due to these properties and because Onyx is not absorbable, it is capable of producing permanent vascular occlusion.11 Once the microcatheter is wedged into the Onyx cast around the tip of catheter, several compartments12 of the nidus can be embolized from a single catheter position. The embolized part of the nidus appears larger than the opacified part during selective contrast agent injections from the microcatheter (Fig 1). Because of the nonadhesive properties of Onyx, the injection can be interrupted to assess the progress of the embolization and can then be continued.
Fig 1.
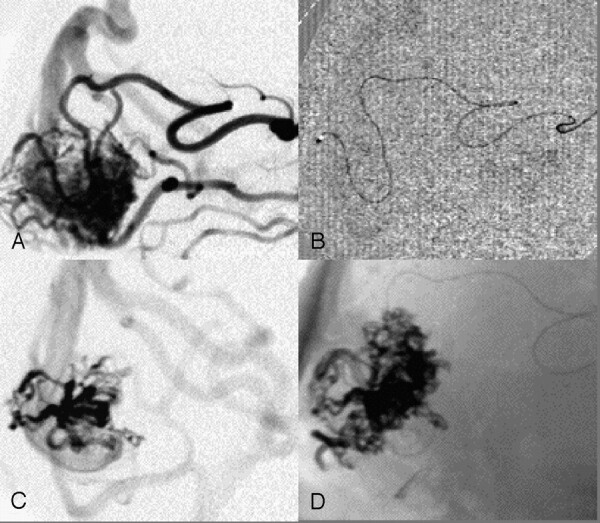
A, Parietooccipital AVM after contrast injection of the guiding catheter. B, Selective perinidal catheter position. C, Contrast injection from this position in panel B. D, Onyx cast after embolization from position in panel B demonstrating that the Onyx cast has a greater volume than the AVM nidus contrasted with the selective injection.
Assessment of AVMs and Peri-Interventional Management
All embolization sessions were performed under general anesthesia at a biplane angiography unit (Neurostar and Axiom Artis, Siemens, Erlangen, Germany). Vascular access was obtained with a 6F guiding catheter (Envoy, Cordis, Miami Lakes, Fla). Superselective angiography was carried out with a DMSO-compatible microcatheter (UltraFlow; ev3). For distal vascular access we used the Mirage 0.008-inch or the SilverSpeed 0.010-inch microwire (ev3). Clinical assessment of all patients was performed by a neurologist both pre- and postinterventionally. When necessary, the patient was monitored at an intensive or intermediate care unit. Angiographic and clinical follow-up examinations were scheduled in patients with complete AVM obliteration by embolization at discharge, after 3 months, and after 1 year, and in patients treated with embolization and surgery at discharge and after 1 year.
After the microcatheter tip was placed at an intra- or perinidal position, superselective angiography was performed to analyze the anatomy of the nidus segment. We assessed the course of the microcatheter within the vasculature (elongated or less elongated), the caliber of the feeders, the distance from the catheter tip to the nidus (in case of perinidal catheterization), and normal brain-supplying vessels. Following the Joint Writing Group of the Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology13 and the publication by Valavanis and Yaşargil,12 we collected the following angiomorphologic criteria of the embolized AVM: nidus: compact or diffuse type; shunt: plexiform, fistulous, or mixed type; draining veins: number, superficial, deep, or mixed; feeding arteries: number, direct, leptomenigeal, en-passant, or perforating. Following these criteria, the results in AVMs with complete occlusion or occlusion rates higher than 90% after embolization were compared with those in which the occlusion rate was lower than 70%.
Depending on the morphologic features of the superselective contrast injection described above, we selected the most appropriate Onyx type for embolization and estimated the maximum possible reflux to remove the microcatheter and to avoid embolization of normal brain vessels. Fistulous arteriovenous shunts were treated with Onyx 34 (due to its highest viscosity) or with n-butyl cyanoacrylate (n-BCA) (in some cases in combination with liquid coils). Plexiform arteriovenous shunts were embolized with Onyx after intra- or perinidal catheterization, starting with Onyx 20 (best compromise between easy penetration of the nidus and possibility of creating a short reflux; Onyx 34 is not suitable for easy penetration in our experience) to form a cast around the tip of the microcatheter with a short reflux, and continuing with Onyx 18 (with lower viscosity) to obtain a better secondary penetration into the nidus. Plexiform arteriovenous shunts were embolized with Onyx 18 (better primary penetration due to its lower viscosity) after intra- or perinidal catheterization in small feeders (less than 2-fold diameter of the microcatheter), but only for better primary penetration. Perforating arteries, leptomeningeal collaterals, and en-passant feeder and catheter positions far away from the nidus were embolized with n-BCA without reflux.
Injection Procedure
The microcatheter was flushed with saline while the DMSO and Onyx were prepared in 2 different 1-mL luerlock syringes. The deadspace of the microcatheter was then filled with DMSO. The Onyx injection started at a rate of 0.1 mL/min. The embolic agent released the tip of the microcatheter under free-flow conditions and filled the directly dependent nidus compartment antegradely and later retrogradely until it flowed to the tip of the microcatheter (first penetration, Figs 2, 3). The goal was to form an attenuated cast of Onyx around the tip of the microcatheter over a short distance (cast or precipitation time, Figs 2–4). The injection procedure was then interrupted while waiting 2 minutes for precipitation and injecting small volumes of the Onyx per cycle until there was enough reflux and attenuated cast for a second penetration of the nidus (Figs 2–4). The injections were stopped if the draining veins or leptomeningeal collaterals were embolized or if the reflux was larger than determined earlier. The entire injection procedure was terminated if the reflux distance was exceeded or if the opening segment of draining veins was embolized several times. The embolization procedure was stopped immediately when the injection resistance increased significantly to prevent rupture of the microcatheter or vessels. We think that this phenomenon is an indicator of the complete filling of the embolized nidus compartment. After aspirating with the Onyx syringe, the microcatheter was removed by slowly increasing and then decreasing the tension on the tip and repeating this maneuver several times until the microcatheter released the cast.
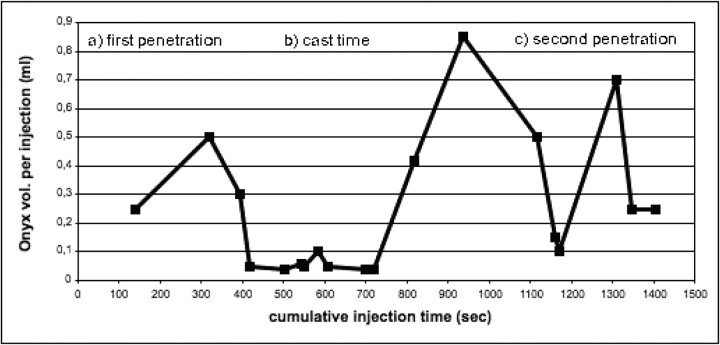
Fig 2.
Typical injection curve of one feeder treated with 4.77 mL Onyx (feeder 4 from Table 2) demonstrating the typical injection phases: A, first penetration (<400 seconds); B, cast time or precipitation time (400–700 seconds); and C, second penetration (>700 seconds).
Fig 3.
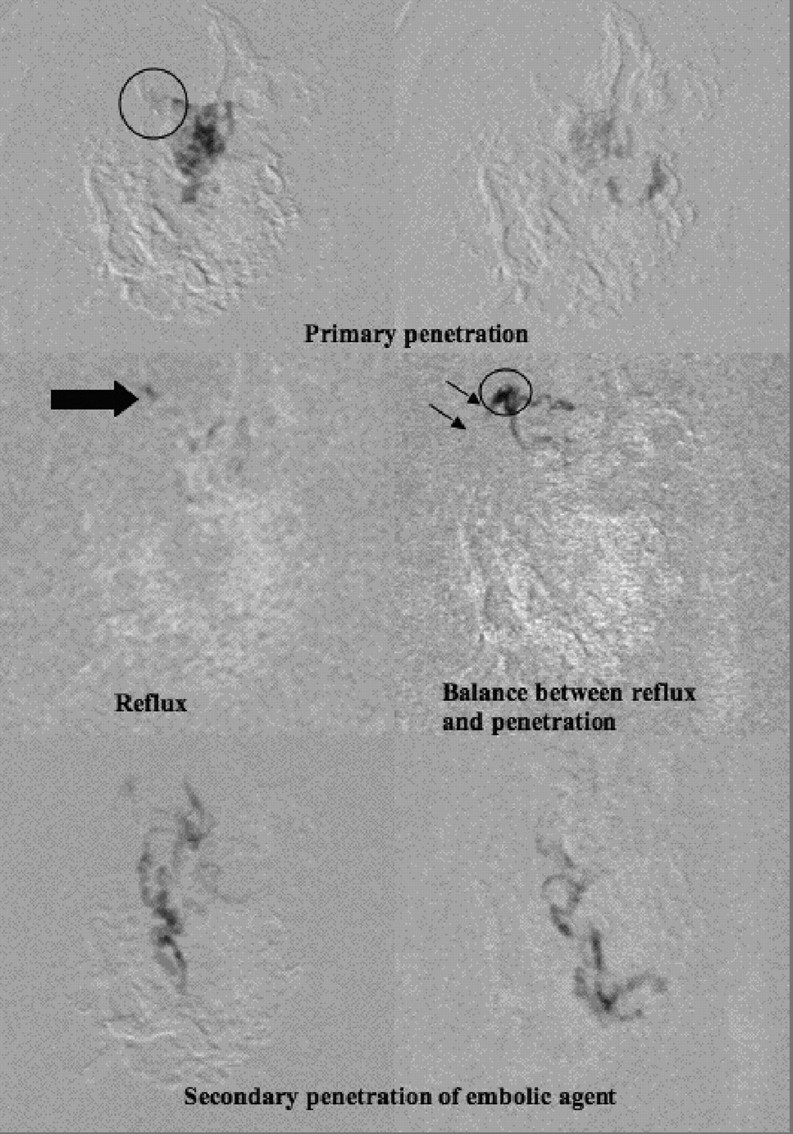
Injection situations (feeder 4 from Table 2 and Fig 2) demonstrating the injection phases: primary penetration, and cast time where reflux begins until the secondary penetration.
Fig 4.
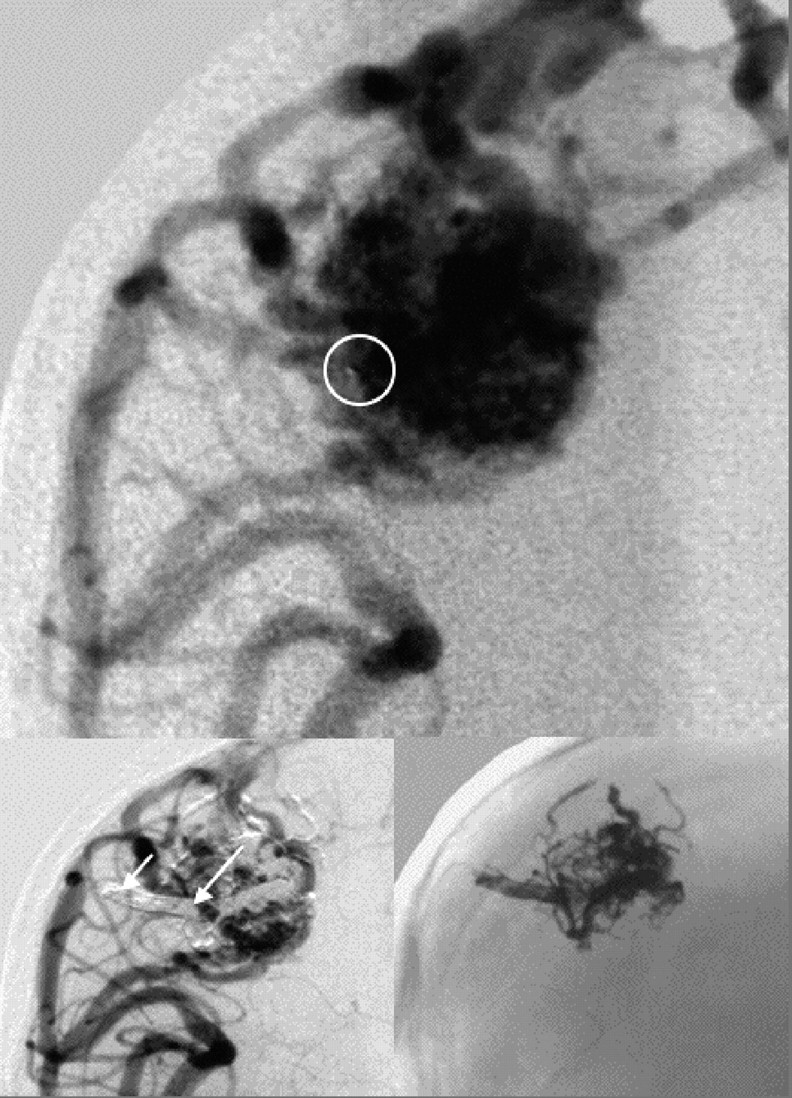
Intranidal position of the microcatheter tip is marked by circle, arrows demonstrate the reflux distance, and final embolization result in an unsubtracted angiogram is shown at bottom right.
Results
A total of 220 feeders in 93 patients with 94 AVMs were embolized with Onyx 18, 20, or 34 (Onyx 18, 57 feeders; Onyx 20, 80 feeders; Onyx 34, 11 feeders; Onyx 18 and 20, 64 feeders; Onyx 18 and 34, 1 feeder; Onyx 20 and 34, 3 feeders; Onyx 18 and 20 and 34, 4 feeders). As a result of our increased knowledge and experience, we started the injections with an Onyx type of higher viscosity and continued with lower viscosity. Most of the injections with a combination of different Onyx types were performed beginning with Onyx 20 (or on rare occasions with Onyx 34) and ending with Onyx 18. Onyx 20 was used as the first injected type in 124 feeders. The volume of Onyx injected per AVM was 3.3 mL on average (range 0.1–14.8 mL). The volume injected per feeder was 1.4 mL on average (range 0.1–7.0 mL). The average reduction in volume after embolization was 79.5% (<50%, 7; 50%–69%, 11; 70%–79%, 12; 80%–89%, 22; 90%–99%, 23; 100%, 19). This reduction resulted from embolizing 2.3 feeders per AVM. These data refer to results of substraction angiographies done after the last endovascular treatment (available in all patients) and to follow-up examinations (average follow-up time 9.5 months) with the same technique (available in 61 patients).
The details of the injection procedure of 20 feeders with an injection volume ≥2 mL are presented in Table 2. The demonstrated injections show that different volumes of Onyx were injected with different numbers of injection cycles (9–19) and different time periods (14–25 minutes) for the entire treatment of one feeder. Note that all of these injection details are related to the angiomorphology of the AVM. Importantly, successful Onyx injections usually have a long precipitation time of up to 20 minutes for the second penetration. This requires several cycles of injecting and waiting. Figure 2 demonstrates a typical injection curve with Onyx as well as a double peak with second penetration.
Table 2:
Injection details from 20 feeders with an injection volume of 2 mL or more
| No. | Onyx Volume (mL) | Onyx Type | Total No. of Injections | Total Precipitation Time (min) | Total Injection Time (min) | No. of Injections for SP | Precipitation Time for SP (min) |
|---|---|---|---|---|---|---|---|
| 1 | 2.32 | 20 | 16 | 30 | 21 | 4 | 10 |
| 2 | 2.27 | 18 | 16 | 30 | 13 | 9 | 20 |
| 3 | 2.02 | 20 | 12 | 22 | 10 | 7 | 16 |
| 4 | 4.77 | 20/18 | 19 | 36 | 25 | 7 | 16 |
| 5 | 4.14 | 20/18 | 15 | 28 | 25 | 5 | 12 |
| 6 | 3.19 | 20/18 | 15 | 28 | 17 | 6 | 14 |
| 7 | 3.95 | 20/18 | 13 | 24 | 12 | 5 | 12 |
| 8 | 4.95 | 20/18 | 12 | 22 | 23 | 3 | 8 |
| 9 | 3.00 | 20/18 | 13 | 24 | 20 | 4 | 10 |
| 10 | 3.50 | 18 | 11 | 20 | 18 | 3 | 8 |
| 11 | 4.14 | 20/18 | 15 | 28 | 25 | 5 | 12 |
| 12 | 2.30 | 20/18 | 16 | 30 | 14 | 6 | 14 |
| 13 | 3.15 | 20/18 | 16 | 30 | 17 | 3 | 8 |
| 14 | 2.68 | 18 | 16 | 30 | 24 | 6 | 14 |
| 15 | 2.59 | 20/18 | 12 | 22 | 17 | 2 | 6 |
| 16 | 2.00 | 20/18 | 9 | 16 | 21 | 4 | 10 |
| 17 | 2.05 | 20/18 | 10 | 18 | 17 | 3 | 8 |
| 18 | 2.25 | 20/18 | 14 | 26 | 20 | 3 | 8 |
| 19 | 2.86 | 20/18 | 14 | 26 | 19 | 8 | 18 |
| 20 | 3.05 | 20/18 | 12 | 22 | 19 | 2 | 6 |
Note:—SP indicates secondary penetration.
Injecting Onyx to embolize intracranial AVMs requires patience. A slow injection of several volumes of Onyx is required for a successful embolization.
It is beyond the focus of this paper to present all angiomorphologic criteria related to AVMs, which we have collected in detail. We emphasize instead important results concerning the relationship between the aforementioned anatomic features13 of AVMs and the technique of Onyx injection. The injection of Onyx resulted in high occlusion rates (volume reduction >90%) when the AVM was in a supratentorial and cortical location, the nidus was compact and plexiform, and when there was a small number of supplying (direct) feeders and one superficial draining vein. There was no high occlusion rate (<70%) for Onyx in AVMs with multiple compartmental draining veins, multiple supplying arteries (especially leptomenigeal, en-passant, or perforating feeders) and when the nidus was diffuse. We hypothesize that there must be some communication between the AVM compartments to deal with the potential advantage of Onyx.
Device-related complications occurred during embolization of 220 feeding arteries in 13 cases (6%). We postulate that these complications are directly related to the reported techniques for working with the aforementioned catheters, microguidewires, and Onyx. We recorded 9 (4%) stuck catheters that could not be completely removed from the AVM and 4 (2%) distal perforations by the microcatheter or microguidewire with a local subarachnoid hemorrhage. Vessel perforations were treated immediately by injection of Onyx. All of these complications occurred without clinical consequences.
A clinically significant deficit (>2 on the modified Rankin Scale) after embolization was detected in 5 patients (5%). Additionally, quadrantopia or hemianopia (≤2 on the modified Rankin Scale) occurred in 4 patients (4%) with occipital AVMs after embolization. An acute intracranial hemorrhage occurred in 2 more patients (2%) after embolization. This needed an emergency evacuation and resection of the AVM but was without detectable neurologic deficit after postoperative restitution. The overall complication rate related to embolization procedures was 12% (11 cases).
The angiographic rate of complete obliteration of AVMs at the end of all embolization procedures was 20% (19 AVMs). Two angiographic recurrences were evident at 3 months’ follow-up, resulting in a complete obliteration rate of 18%. These relapses were operated on successfully. However, scheduled follow-up after 1 year was not been carried out in one patient. Fifty AVMs (53%) were cured by embolization and surgery. Four AVMs (4%) demonstrated a persistent arteriovenous shunt after embolization and surgery. Radiosurgery was planned in these cases. Control angiography was not performed on 8 patients (9%) because of unknown addresses in 6 cases and because 2 patients were nonresidents. Two AVMs were not occluded after embolization and radiosurgery. In one patient, control angiography carried out 31 months after radiation revealed a persistent arteriovenous shunt. Rebleeding occurred 5 months later, which was within the waiting time for further scheduled embolization attempts. Final resection of the AVM was performed in January 2006. Radiation of the AVM in the second patient was started in May 2005 after presurgical embolization, partial resection, and postoperative embolization. In this case, control angiography was scheduled after a minimum of 12 months after radiation. In summary, final control angiography after radiosurgery was not available for 11 patients. Therefore, there was an overall cure rate of 71% for all AVMs and a cure rate of 83% for patients who underwent embolization or embolization and surgery.
Discussion
Onyx is a nonadhesive embolic agent with lavalike flow patterns. The microcatheter tip is not glued within the vessel, and thus it is possible to interrupt the injection and analyze the actual Onyx casting. For both of these reasons, it is possible to inject large volumes from one catheter position in a very controlled manner and thus to embolize a large part of the AVM without filling the draining veins or leptomeningeal collaterals. There is an advantage for the use of Onyx in treatment conditions where it is possible to work with reflux and to form an attenuated cast around the catheter tip. n-BCA should be preferred in fistulous arteriovenous shunts, perforating arteries, leptomeningeal collaterals, en-passant feeders, and catheter positions far away from the nidus. With the knowledge of morphologic characteristics of AVMs that are suitable for treatment with Onyx, high occlusion rates and low complication rates are feasible in treating a small number of feeders.
Concerning our reported complications with removal of the UltraFlow microcatheter, most of these complications occurred during treatment of the first patients (7 stuck catheters within the first 10 patients). The difficulty in removing the catheter depends on the tortuosity of the catheterized vessels, the duration of the precipitation, the distance of the reflux, and the amount of experience that the investigator has who pulls on the catheter. With experience, and because of the manufacturer’s improvements to the catheter coating, removing the microcatheter is no longer a problem. The same is true of the technical complications with distal wire or catheter perforations. All 4 vessel perforations occurred within treatments of the first 25 patients. With some experience in handling the devices, perforations are extremely rare. We encountered hemorrhages in 2 cases of AVMs. One bleeding occurred 2 hours after an embolization when this AVM was nearly complete obliterated. We think that this bleeding was probably caused by overloading of draining veins with embolic agent. The second hemorrhage occurred in a patient 2 weeks after the last embolization step. The cause for this delayed complication remains speculative.
According to Akin et al,14 there is an advantage for Onyx in intraoperative handling because of its physical characteristics after precipitation. Onyx is a soft, spongelike mass that is easy to handle during surgery. The embolized vessels are completely filled by the embolic agent and are less fragile because of the lower inflammatory reaction and the absence of polymerization heat compared with n-BCA-embolized AVMs. Akin et al found less intraoperative blood loss and a shorter duration of surgical procedures for Onyx compared with n-BCA in an animal model.
We reviewed the complete AVM literature from 1995 up to 200312,15–30 and found nearly 7000 AVMs that were mainly treated with n-BCA. Details of the embolization sessions that were described and analyzed refer only to the number of sessions per AVM. Unfortunately, the number of embolized feeders and the volume of embolic agent that was injected were only mentioned in certain papers. On this point the publication by Valavanis and Yaşargil,12 which contains a detailed angiomorphologic analysis, is an exception. They embolized 7.7 feeders per AVM with a resulting cure rate of 40%. Debrun et al15 injected on average 1 mL n-BCA per feeder and Tokunaga et al16 injected 0.26 mL per feeder. In reviewing the aforementioned literature, we found a complication rate for a severe deficit of 5.5% and a mortality rate of 2.3%.
In comparison with our series, the average number of treatment sessions is similar, but we embolized fewer feeders. The injected Onyx volume per feeder is much higher compared with n-BCA. With the exception of the Valavanis and Yaşargil series,12 the overall cure rate and occlusion rate are higher in our Onyx series. The morbidity rate after embolization is similar in both groups, but there were fewer deaths due to postembolization hemorrhage in our group.
As mentioned earlier, embolization of intracranial AVMs with EVOH-containing embolic agents was first described by Taki et al7 and Terada et al8 in the early 1990s. A comparison with the reported results is not possible because of the low number of AVMs treated, the different mixtures of the embolic materials, and some technical difficulties with the access devices concerning the stiffness of the microwires and microcatheters.
Simonetti et al31 used Onyx, but they only reported the treatment of 2 AVMs. One AVM was completely occluded and one was embolized with an occlusion rate of 80%. Florio et al32 reported the treatment of 10 AVMs with Onyx. The cure rate after embolization was 20%.
Unfortunately, publications by Cekirge et al33 and Niemann et al34 are abstracts from oral presentations that do not include data concerning the treated patients and AVMs. Cekirge et al33 reported a cure rate of embolizations of about 38% in a series of patients mainly with AVMs of Spetzler-Martin grades I and II. This corresponds to our results.
The articles by Jahan et al35 and Hamada et al36 include detailed information about the treatment results, thus permitting suitable comparisons with our results.
Hamada et al36 described the embolization of 57 AVMs with EVOH mixed with alcohol as a solvent (30%) and with a contrast media (Iopamiron, Nihon Schering, Osaka, Japan). They embolized 185 feeders in 87 sessions (2.1 feeders per session und 3.2 feeders per AVM). Thirty-three AVMs (58%) were located in eloquent brain regions. According to the Spetzler-Martin Grading Scale, the AVMs were graded as I in 2 cases, II in 16 cases, III in 27 cases, and IV in 12 cases. The average injection volume per feeder was between 0.7 and 0.9 mL, and the average injection time was 2–3 minutes. The cure rate after embolization was zero; for definitive therapy, 3 patients were treated with radiosurgery and 54 with open surgery. The mortality rate was zero and the morbidity rate for a permanent deficit was 5.3%.
Jahan et al35 presented their experiences treating 23 AVMs with Onyx, having embolized 129 feeding arteries in 33 sessions (3.9 feeders per session, 5.6 feeders per patient). The average volume of the AVM treated was 14.5 mL. The maximum injection time was 12 minutes. The average obliteration rate of AVMs was 63%. The cure rate after embolization was zero; for definitive therapy, 11 patients were treated with radiosurgery and 12 with open surgery. The mortality rate was zero and the morbidity rate for a permanent deficit was 4%.
In comparison with the series of Jahan et al35 and Hamada et al,36 we embolized fewer feeders to achieve the reported occlusion rates. We injected a higher volume of Onyx per feeder, and the injections times were even longer. The occlusion rate of AVMs treated and the cure rate after embolization are higher in our group. Our clinical complication rate is comparable with both series and is acceptable compared with the natural course of the disease.
When compared with other liquid embolic agents, Onyx has some distinguishing features that broaden the therapeutic options when treating intracranial AVMs. By using the described reflux technique, it is possible to embolize large AVM parts from one catheter position and to achieve high occlusion rates while embolizing only a few feeders. Superselective intranidal or perinidal catheter positions and slow, controlled injections, which protect the draining veins, make the therapy safer even in complex AVMs and in critical locations. Onyx is an additive liquid embolic agent with advantages in selected AVMs, but there are still arguments for the use of n-BCA.
Conclusion
With the knowledge of angioarchitectural characteristics of AVMs that are suitable for treatment with Onyx, high occlusion rates and low complication rates in treating a small number of feeders are feasible. Superselective intranidal or perinidal catheter positions and slow, controlled injections that protect the draining veins make the therapy safer even in complex AVMs and critical locations.
References
- 1.Ogilvy CS, Stieg PE, Awad I, et al. AHA Scientific Statement: Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke 2001;32:1458–71 [DOI] [PubMed] [Google Scholar]
- 2.TerBrugge KG. Brain AVM. Interventional Neuroradiol 2003;9(suppl 2):107–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaller C, Schramm J, Haun D. Significance of factors contributing to surgical complications and to late outcome after elective surgery of cerebral arteriovenous malformations. J Neurol Neurosurg Psychiatry 1998;65:547–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollock BE, Gorman DA, Coffey RJ. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery 2003;52:1291–96 [DOI] [PubMed] [Google Scholar]
- 5.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986;65:476–83 [DOI] [PubMed] [Google Scholar]
- 6.Pasqualin A, Barone G, Cioffi F, et al. The relevance of anatomic and hemodynamic factors to a classification of cerebral arteriovenous malformations. Neurosurgery 1991;28:370–79 [DOI] [PubMed] [Google Scholar]
- 7.Taki W, Yonekawa Y, Iwata H, et al. A new liquid material for embolization of arteriovenous malformations. AJNR Am J Neuroradiol 1991;11:163–68 [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T, Nakamura Y, Nakai K, et al. Embolization of arteriovenous malformations with peripheral aneurysms using ethylene vinyl alcohol copolymer: report of three cases. J Neurosurg 1991;75:655–60 [DOI] [PubMed] [Google Scholar]
- 9.Molyneux AJ, Coley S. Embolization of spinal cord arteriovenous malformations with ethylene vinyl alcohol copolymer dissolved in dimethyl sulfoxide (Onyx liquid embolic system). J Neurosurg 2000;93:304–08 [DOI] [PubMed] [Google Scholar]
- 10.Chaloupka JC, Huddle DC, Alderman J, et al. A reexamination of the angiotoxicity of superselective injection of DMSO in the swine rete embolization model. AJNR Am J Neuroradiol 1999;20:401–10 [PMC free article] [PubMed] [Google Scholar]
- 11.Muramaya Y, Vinuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery 1998;43:1164–75 [DOI] [PubMed] [Google Scholar]
- 12.Valavanis A, Yaşargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg 1998;24:131–214 [DOI] [PubMed] [Google Scholar]
- 13.Joint Writing Group of the Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Joint Section on Cerebrovascular Neurosurgery a Section of the American Association of Neurological Surgeons and Congress of Neurological Surgeons; Section of Stroke and the Section of Interventional Neurology of the American Academy of Neurology. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke 2001;32:1430–42 [DOI] [PubMed] [Google Scholar]
- 14.Akin ED, Perkins E, Ross IB. Surgical handling characteristics of an ethylene vinyl alcohol copolymer compared with n-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurg 2003;98:366–70 [DOI] [PubMed] [Google Scholar]
- 15.Debrun GM, Aletich V, Ausman JI, et al. Embolization of nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery 1997;40:112–21 [PubMed] [Google Scholar]
- 16.Tokunaga K, Kinugasa K, Meguro T, et al. Curative treatment of cerebral arteriovenous malformations by embolisation using cellulose acetate polymer followed by surgical resection. J Clin Neurosci 2000;7(suppl 1):1–5 [DOI] [PubMed] [Google Scholar]
- 17.DeMeritt JS, Pile-Spellman, Mast H. Outcome analysis of preoperative embolization with n-butyl cyanoacrylate in cerebral arteriovenous malformations. AJNR Am J Neuroradiol 1995;16:1801–07 [PMC free article] [PubMed] [Google Scholar]
- 18.Frizzel RT, Fisher WS 3rd. Cure, morbidity and mortality associated with embolization of brain arteriovenous malformations: a review of 1246 patients in 32 series over a 35-year period. Neurosurgery 1995;37:1039–40 [DOI] [PubMed] [Google Scholar]
- 19.Wallace RC, Flom RA, Khayata MH, et al. The safety and effectiveness of brain arteriovenous malformation embolization using acrylic and particles: the experience of a single institution. Neurosurgery 1995;37:606–18 [DOI] [PubMed] [Google Scholar]
- 20.Grzyska U, Neumaier Probst E, et al. Differentialtherapie zerebraler Angiome. Wien Med Wochenschr 1997;7/8:186–93 [PubMed] [Google Scholar]
- 21.Wikholm G, Lundquist C, Svedsen P. Transarterial embolization of cerebral arteriovenous malformations. How few can you do? Interventional Neuroradiol 1997;3:119–23 [DOI] [PubMed] [Google Scholar]
- 22.Yakes WF, Krauth L, Ecklund J, et al. Ethanol endovascular management of brain arteriovenous malformations: initial results. Neurosurgery 1997;49:1145–52 [DOI] [PubMed] [Google Scholar]
- 23.Sorimachi T, Koike T, Takeuchi S, et al. Embolization of cerebral arteriovenous malformations achieved with polyvinyl alcohol particles: angiographic reappearance and complications. AJNR Am J Neuroradiol 1999;20:1323–28 [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenwasser RH, Armonda RA. Embolization as palliative or definitive therapy. In: Jafar JJ, Awad IA, Rosenwasser RH (eds), Vascular Malformations of the Central Nervous System. FPhiladelphia: Lippincott, Williams & Wilkins,2000. :405–12
- 25.Liu HM, Huang YC, Wang YH. Embolization of cerebral arteriovenous malformations with n-butyl-2-cyanoacrylate. J Formos Med Assoc 2000;99:906–13 [PubMed] [Google Scholar]
- 26.Song JK, Eskridge JM, Chung EC, et al. Preoperative embolization of cerebral arteriovenous malformations with silk sutures: analysis and clinical correlation of complications revealed on computerized tomography scanning. J Neurosurg 2000;92:955–60 [DOI] [PubMed] [Google Scholar]
- 27.Li T, Duan C, Wang Q, et al. Endovascular embolization of cerebral arteriovenous malformation. Zhonghua Yi Xue Za Zhi 2002;82:654–56 [PubMed] [Google Scholar]
- 28.Meisel HJ, Mansmann U, Alvarez H, et al. Effect of partial targeted N-butyl-cyano-acrylate embolization in brain AVM. Acta Neurochir (Wien) 2002;144:879–87 [DOI] [PubMed] [Google Scholar]
- 29.n-BCA Trail Investigators. N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol 2002;23:748–55 [PMC free article] [PubMed] [Google Scholar]
- 30.TerBrugge KG. Endovascular treatment of brain AVM. Interventional Neuroradiol 2003;9(suppl 2):109–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonetti L, Cenni P, de Santis F, et al. Prime esperienze di embolizzazione di MAV cerebrali con Onyx. Rivista di Neuroradiologica 2001;14(suppl 3):251–56 [Google Scholar]
- 32.Florio F, Lauriola W, Nardella M, et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx embolization: preliminary experience. Radiol Med Torino 2003;106:512–20 [PubMed] [Google Scholar]
- 33.Cekirge S, Saatci I, Arat A. Long-term intranidal Onyx injection in the endovascular treatment of pial brain AVMs: description of a new technique and philosophy aimed at cure. J Neurosurg 2002;96:17312450280 [Google Scholar]
- 34.Niemann DB, Molyneux AJ. Embolization of brain AVMs with ethylene-vinyl-alcohol copolymer dissolved in DMSO (Onyx liquid embolic system): evidence in a prospective series of 31 patients. J Neurosurg 2002;96:171 [Google Scholar]
- 35.Jahan R, Murayama Y, Gobin YP. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery 2001;48:984–95 [DOI] [PubMed] [Google Scholar]
- 36.Hamada J, Kai Y, Morioka M, et al. A mixture of ethylene vinyl alcohol copolymer and ethanol yielding a nonadhesive liquid embolic agent to treat cerebral arteriovenous malformations: initial clinical experience. J Neurosurg 2002;97:881–88 [DOI] [PubMed] [Google Scholar]