Abstract
BACKGROUND AND PURPOSE: The purpose of this study was to determine the nature, incidence, and radiologic appearance of intracranial vascular anomalies that occur in association with periorbital lymphatic malformation (LM) and lymphaticovenous malformation (LVM).
MATERIALS AND METHODS: We retrospectively reviewed clinical records and imaging studies of 33 patients ranging in age from the neonatal period to 39 years (mean age, 5.1 years; median age, 1.0 year) who were evaluated for orbital LM or LVM at our institution between 1953 and 2002. Imaging studies, including CT, MR imaging, and cerebral angiograms, were evaluated by 2 radiologists to determine morphologic features of orbital LM and to identify associated noncontiguous intracranial vascular and parenchymal anomalies, including arteriovenous malformations (AVM), cerebral cavernous malformations (CCM), developmental venous anomalies (DVA), dural arteriovenous malformations (DAVM), and sinus pericranii (SP).
RESULTS: The malformation was left-sided in 70% of patients. Twenty-two patients (70%) had intracranial vascular anomalies: DVA (n = 20; 61%), CCM (n = 2; 6%), DAVM (n = 4; 12%), pial AVM (n = 1; 3%), and SP (n = 1; 3%). Arterial shunts were present in the soft tissues in 2 patients (6%). Three patients had jugular venous anomalies. Three patients (9%) had cerebral hemiatrophy, 2 (6%) had focal cerebral atrophy, and 2 had Chiari I malformation.
CONCLUSIONS: Intracranial vascular anomalies, some of which are potentially symptomatic and require treatment, are present in more than two thirds of patients with periorbital LM. Initial imaging of patients with orbital LM should include the brain as well as the orbit.
Lymphatic malformations (LMs), like other structural vascular anomalies, usually present in predictable anatomic locations and patterns. These slow-flow lesions are most commonly located, in order of frequency, in the head and neck, axilla, chest, and proximal upper extremity. These sites of predilection may be related to maldevelopment of primitive jugular, subclavian, and axillary lymphatic sacs. LM in the orbit is uncommon. It typically involves the subconjunctival and periocular tissues, and often extends into the adjacent frontotemporal region and cheek. The extensive lesions typically cause swelling, intraorbital hemorrhage, ocular proptosis, blepharoptosis, and cellulitis. Although abnormal lymphatic channels and spaces predominate, they may be combined with anomalous venous channels, in which case they are termed lymphaticovenous malformations (LVMs). Although lymphatic endothelium can now be identified histologically by applying special stains, these techniques were not available in the past; often, histologic examination of resected tissue could not distinguish between LM and LVM.
It is known that patients with extensive facial venous malformations have a high incidence of associated intracranial developmental venous anomalies (DVAs).1 Likewise, intracranial vascular anomalies (ICVAs) have been described in some patients with periorbital LVMs. We undertook this study to confirm the association of orbital LM (or LVM) and ICVA and to document the frequency of this association, as well as its clinical significance.
Materials and Methods
This is a retrospective review of 33 consecutive patients evaluated for periorbital LM or LVM at a single institution between 1953 and 2002. The diagnosis of LM or LVM was based on clinical presentation and radiologic findings and was confirmed by histopathologic examination in 15 patients.
After obtaining approval by the institutional review board, we searched the data base of the Vascular Anomalies Center and reviewed all available imaging studies (craniofacial MR imaging, CT, and angiography) and the medical records. Patients were excluded from the study if they did not have MR imaging or contrast-enhanced CT that included the orbits and brain. Of an initial series of 39 patients with a clinical diagnosis of periorbital LM or LVM, 6 were excluded because of inadequate imaging.
Diagnosis
The diagnosis of periorbital LM or LVM was made when imaging and clinical features (the latter assessed by a plastic surgeon with extensive experience in the field of vascular anomalies) were both consistent with this diagnosis. Clinical features included soft tissue swelling or mass, evident since early childhood and growing in proportion to the growth of the patient; normal temperature of overlying skin; orbital overgrowth; conjunctival vesicles; and absence of pulsatility or bruit. Imaging criteria for LM included the presence, in or in and adjacent to the orbit, of abnormal soft tissue with decreased attenuation on precontrast CT images and increased signal intensity on T2-weighted MR images, without or with discrete cysts with rim enhancement and bony overgrowth. Lesions containing identifiable cysts measuring ≥1 cm in diameter were termed “macrocystic” and those with smaller cysts or no recognizable cysts were termed “microcystic.” The diagnosis of LVM was made when phleboliths or areas of attenuated enhancement were seen in addition to the nonenhancing areas. Intracranial vascular anomalies were diagnosed in the presence of enlarged or abnormally positioned arteries, veins, and dural sinuses. Cerebral atrophy was diagnosed if brain structures were reduced in volume; hemitrophy is global volume loss of a cerebral hemisphere, whereas focal volume loss refers to volume loss involving one lobe or less.
Imaging
Because a number of patients were referred from other institutions, imaging studies were technically variable. Fifteen patients had both MR imaging and CT, 12 patients had MR imaging, 5 patients had contrast-enhanced CT, and 4 patients had selective internal and external carotid angiography.
Two radiologists evaluated all of the imaging studies. Clinical, CT, and/or MR imaging criteria were used to categorize the lesions by location: intraconal, extraconal, and periorbital (including eyelids and frontotemporal region). We also differentiated between isolated orbital LM and those with additional involvement outside the orbital cavity and periorbita. LMs were further characterized by the presence or absence of macrocysts (individual cysts measuring ≥1 cm in diameter), diffuse or rim enhancement, fluid-fluid levels, and phleboliths.
The intracranial vascular abnormalities were categorized by location as: infratentorial and/or supratentorial; deep or superficial; with reference to the LM, ipsilateral, contralateral, bilateral, or midline; and by channel type, including developmental venous anomaly (DVA; dilated parenchymal veins without arterial communication), sinus pericranii (SP; communication between dural sinus and scalp veins), dural arteriovenous malformation (DAVM; high-flow vascular malformation consisting of shunts between meningeal arteries and a dural sinus), cerebral cavernous malformation (CCM; focal low-flow lesion with hyperintense center and hemosiderin rim on T2-weighted sequence), abnormal dural enhancement, dural sinus and jugular venous anomalies (abnormal size or shape), and cerebral parenchymal anomalies, such as ipsilateral hemiatrophy, focal atrophy, infarction, or bony anomalies.
Clinical Analysis
The patients’ clinical records were reviewed with attention to age, sex, and history of prior therapeutic procedures.
Results
Clinical Findings
There was an equal sex distribution: 18 men and 15 women. Their ages ranged from the first day of life to 39.2 years at the time of the initial imaging (mean age, 5.1 years; median age, 1.0 year).
Six patients had an isolated orbital LM or LVM. Twenty-seven had adjacent facial involvement: frontal (n = 7), frontotemporal (n = 4), cranial base (n = 1), lower cervicofacial (n = 5), cheek (n = 4), cheek and frontal involvement (n = 3), and combined cervicofacial and frontotemporal (n = 1). Two patients had bilateral cervicofacial lesions; all other extraorbital extensions were ipsilateral to the orbital LM. The left orbit was involved in 69.7%.
Radiologic Findings
The periorbital lesion was macrocystic in 8 patients, microcystic in 20, and combined in 5. Eyelid involvement (usually upper eyelid) was noted in 29 patients. Imaging demonstrated the following anatomic involvement: intraconal location in 24 patients; extraconal in 23, periorbital (eyelid and/or forehead involvement) in 29, and diffuse orbital involvement (intraorbital, extraorbital, and periorbital) in 15. Four patients presented with isolated eyelid involvement.
A variable degree of LM enhancement was found after administration of contrast material. Five patients had rim enhancement of macrocysts. Twenty-two, including 6 isolated periorbital lesions, had diffuse mild enhancement. Four had heterogeneous enhancement, including intense enhancement in 1 (consistent with LVM), and 1 LM was nonenhancing.
Nine patients had bony involvement, including hyperostotic or lytic lesions involving the orbital roof, frontal and temporal bones, or cranial base on CT and/or increased signal intensity on T2-weighted MR imaging.
Intracranial Findings
Intracranial vascular anomalies were identified in 22 patients (67%) (Table 2). In 14 patients (60%), these were ipsilateral to the orbital involvement. Nine patients had bilateral intracranial vascular anomalies with the predominant abnormality on the same side as the orbit. The following intracranial vascular anomalies were identified: 20 DVAs (60.6%) (Figs 1to 3), 2 CCMs (6.1%) (Fig 4), 4 dural AVMs (12.1%) (Figs 5 and 6), 1 pial AVM (3%), and 1 SP (3%). Two patients had arterial shunts into the extracranial vascular malformation (Fig 7). There were also 3 jugular venous abnormalities (9%). Ipsilateral anomalies of the jugular vein included absent or occluded jugular veins associated with a hypoplastic or occluded transverse sinus in 2. A varix of the vein of Galen was seen in one of these patients. Dural enhancement in the middle cranial fossa, adjacent to the sphenoid bone, was seen in 5 patients (15.2%).
Table 2:
Patient data on orbital LM and ICVA
| Patient | Gender | OLM Side | Facial LM | ICVA |
SP | Hemiatrophy | Focal Atrophy | AVM | Cavernoma | Local DVA | Dural Enhancement | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infratentorial | Supratentorial | Ipsilateral | |||||||||||
| 1 | M | L | C | − | − | − | − | − | − | − | − | − | − |
| 2 | F | R | F | − | − | − | − | − | − | − | − | − | − |
| 3 | M | L | F + T | ++ | + | − | − | − | + | + | − | + | + |
| 4 | F | L | CF bil | − | + | + | − | ++ | + | + | − | + | − |
| 5 | F | L | C + F | ++ | + | − | + | + | − | + | + | + | + |
| 6 | M | L | C + F | − | − | − | − | − | − | − | − | − | − |
| 7 | F | L | C | − | + | + | − | − | − | − | − | + | − |
| 8 | F | L | − | − | + | + | − | − | − | − | + | + | − |
| 9 | M | L | C +F + T | − | − | − | − | − | − | − | − | − | + |
| 10 | M | L | F | − | − | + | − | − | + | − | − | − | + |
| 11 | F | L | F | + | − | + | − | − | − | − | − | + | − |
| 12 | M | L | F + T | − | + | + | − | − | − | − | − | + | + |
| 13 | F | R | CF bil | ++ | + | − | − | − | − | − | − | + | − |
| 14 | M | R | CF | − | − | − | − | − | − | − | − | − | − |
| 15 | M | R | CF + T | + | − | − | − | − | − | − | − | + | − |
| 16 | F | L | CF | − | + | + | − | − | − | − | − | + | − |
| 17 | M | L | T | ++ | − | + | − | ++ | − | − | − | + | − |
| 18 | F | L | − | − | + | + | − | − | + | + | − | − | − |
| 19 | M | R | F + T | + | + | + | − | − | − | + | − | + | − |
| 20 | F | L | CF | − | − | − | − | − | − | − | − | − | − |
| 21 | M | L | CF | + | − | + | − | − | + | − | − | + | − |
| 22 | M | L | F | − | + | + | − | − | − | − | − | + | − |
| 23 | F | R | − | + | − | + | − | − | − | − | − | + | − |
| 24 | M | L | F + T | + | − | + | − | − | − | − | − | + | − |
| 25 | M | L | − | − | − | − | − | − | − | − | − | − | − |
| 26 | F | R | F | − | − | − | − | − | − | − | − | − | − |
| 27 | M | R | F + C | − | − | − | − | − | − | − | − | − | − |
| 28 | M | R | − | + | + | − | − | − | − | − | − | + | − |
| 29 | M | L | C | − | − | − | − | − | − | − | − | − | − |
| 30 | M | L | F | − | − | − | − | − | − | − | − | − | − |
| 31 | F | R | F | − | + | − | − | − | − | − | − | + | − |
| 32 | F | L | − | − | + | − | − | − | − | − | − | + | − |
| 33 | F | L | C | − | + | + | − | − | − | − | − | + | − |
| Total | 18 M, 15F | 23L, 10R | 4 bil, 7 uni | 14 | 15 | 1 | 2 bil, 1 uni | 5 | 5 | 2 | 20 | 5 | |
Note:—C indicates cheek; bil, bilateral; CF, cervicofacial region; F, frontal; L, left; OLM, orbital lymphatic malformation; R, right; T, temporal; uni, unilateral; −, none; +, present or unilateral; ++, bilateral.
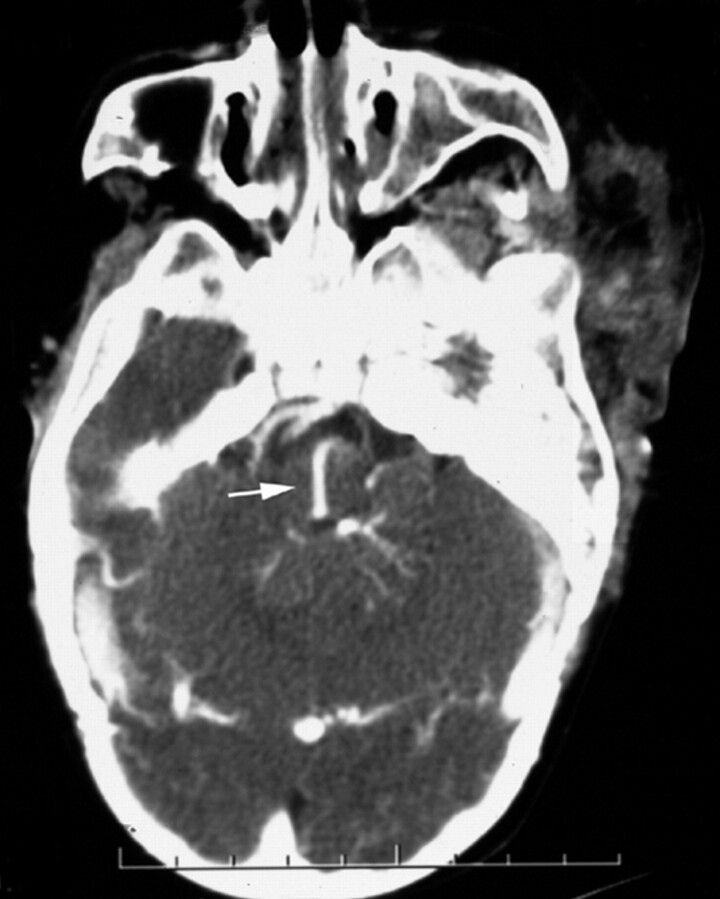
Fig 1.
Postcontrast axial CT scan in a patient with a left orbitofrontal lymphatic malformation demonstrates a posterior fossa DVA draining into a transpontine collector (arrow).
Fig 3.
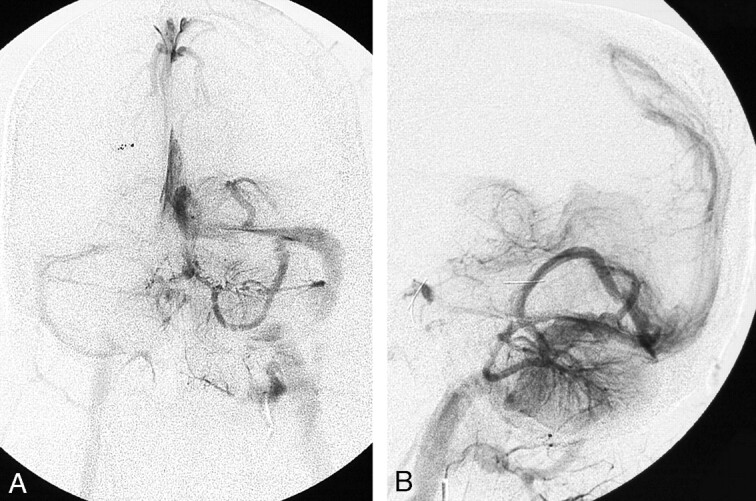
A and B, Posterior fossa DVA in another patient is confirmed angiographically. Frontal (A) and lateral (B) venous phase of left vertebral angiogram shows the left cerebellar and left occipital DVAs draining into the left transverse sinus.
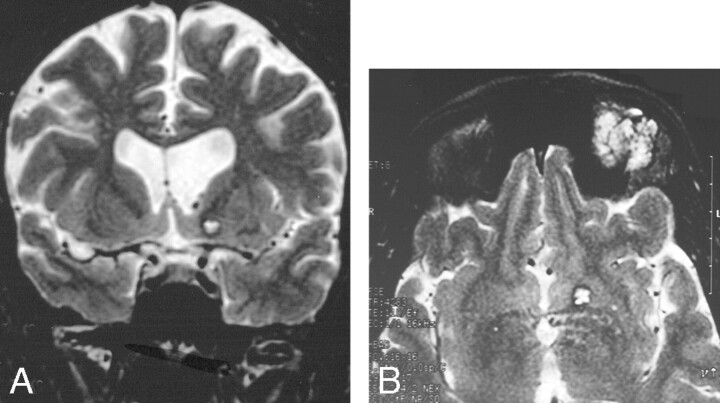
Fig 4.
A and B, Cavernoma-like lesion in a patient with a left orbital lymphatic malformation.
A, Coronal T2-weighted MR imaging shows a focal T2-hyperintense area in the left basal ganglia, associated with ventricular dilation.
B, Axial image showing the orbital and basal ganglia lesions.
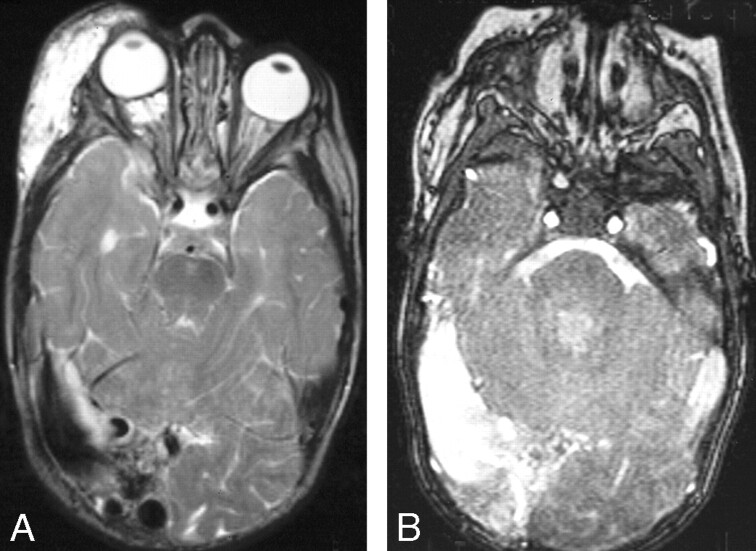
Fig 5.
A and B, Right orbital/periorbital lymphatic malformation with right posterior dural AVM.
A, Axial T2-weighted image shows the orbital and periorbital low-flow vascular malformation as dilation of the right transverse sinus which has flow voids in its wall. Associated right hemispheric atrophy is indicated by dilation of the temporal horn of the right lateral ventricle.
B, Gradient recalled-echo sequence confirms the high-flow nature of this dural sinus lesion.
Fig 6.
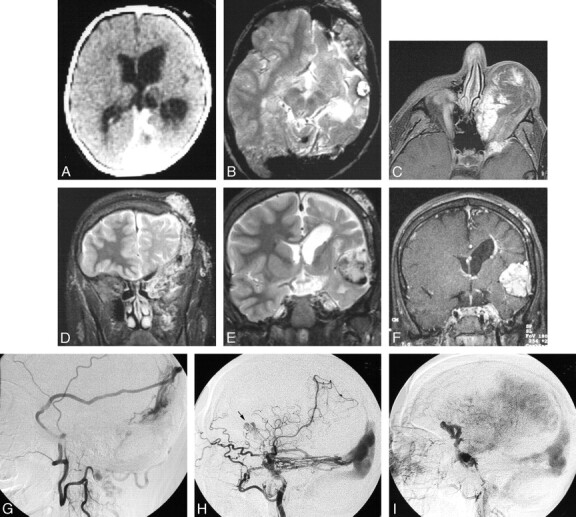
Serial imaging of a girl with an extensive left orbitofrontal lymphatic malformation associated with a left posterior dural AV fistula, dural sinus enlargement, left cerebral hemiatrophy, sinus pericranii, and progressive formation of cavernoma-like lesions. The patient presented at birth in high output cardiac failure and underwent ligation of numerous extracranial arteries with clinical improvement. She subsequently underwent numerous surgical procedures to treat the orbital LM, which was complicated by recurrent hemorrhage. At age 17, she presented with severe orbital chemosmosis and underwent angiography. It is possible that some of the anterior vascular abnormalities are secondary to the craniotomies performed to debulk the left orbit.
A, Axial CT image after intravenous contrast administration in the first week of life shows the large straight and left transverse sinuses and left hemiatrophy. Note the absence of a focal mass lesion in the left middle cranial fossa.
B, Axial T2-weighted MR imaging of the brain at 10 years of age shows persistent enlargement of the left transverse sinus, and a cavenomalike lesion in the left middle fossa.
C, Axial T1-weighted postgadolinium MR image at 17 years of age, after numerous orbital debulking procedures. There are enhancing channels within the orbital LM and adjacent sphenoid bone. These may represent small arterial lymphatic communications or a pure venous component.
D, Coronal T2-weighted image from the same study as (C) shows LM involvement of the left infratemporal space, left orbit, left sphenoid and frontal bones, and the scalp.
E, Coronal T2-weighted and MR image at the level of the frontal horns demonstrates enlargement of the cavenomalike lesion in the Sylvian fissure.
F, Postgadolinium T1-weighed image shows attenuated enhancement of the extra-axial mass. A periventricular DVA is also present.
G, Left middle meningeal angiogram, lateral projection, (via cervical collateral) shows supply of the dural AVM by the parietal branch of the middle meningeal artery.
H, Left internal carotid angiogram, lateral projection, shows additional supply to the posterior dural AVM by the tentorial branches of the internal carotid artery. Note a second, more anterior lesion (arrow).
I, Venous phase of the left internal carotid angiogram shows opacification of anomalous veins probably constituting part of the vascular mass in the Sylvian fissure.
Fig 7.
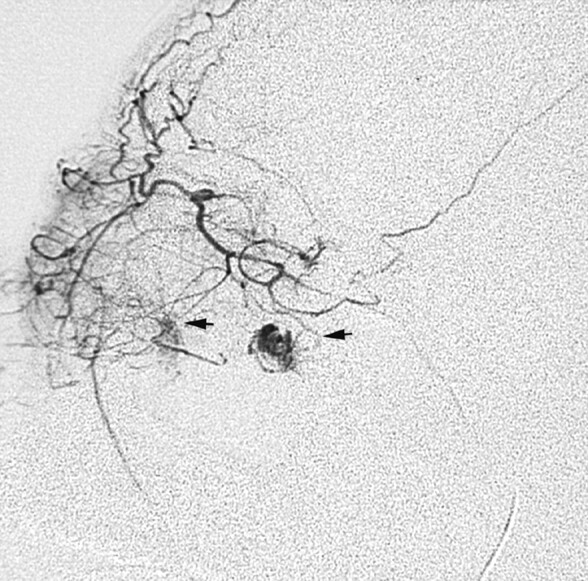
Selective ophthalmic arteriogram in a patient with recurrent bleeding into a postseptal orbital lymphatic malformation demonstrates tiny arterial communications with the soft issue malformation (arrows).
Parenchymal abnormalities included ipsilateral cerebral hemiatrophy in 3 patients (Fig 6) (bilateral in 2), and focal volume loss in 5. Two patients had Chiari I malformation.
The ICVAs were infratentorial in 6 patients, supratentorial in 9, and both infratentorial and supratentorial in 5 patients. The DVAs drained via dilated transpontine (n = 2), perimesencephalic (n = 5), peripontine (n = 4), periventricular (n = 1), and deep hemispheric (n = 2) collector veins. Two drained into the petrosal sinuses, 1 into the sigmoid sinus, and the others ultimately drained into the transverse sinus. One patient with isolated orbital LM had bilateral infratentorial DVAs.
Discussion
Periorbital LMs are highly symptomatic low-flow vascular anomalies. Lymphatic anomalies are present at birth, but can be inconspicuous until expansion occurs. Most are evident by 2 years of age.3 They typically fluctuate in size with upper respiratory infections, and expand suddenly due to intralesional hemorrhage and infection.3–7 Ocular complications include astigmatism, corneal exposure, hyperopia secondary to pressure on the posterior globe, strabismus, glaucoma, and compressive optic neuropathy.3, 4, 8 These complications typically lead to amblyopia or blindness. Excision is the traditional therapy.3 However, the lymphatic anomaly permeates the extraconal and intraconal compartments, making complete resection impossible. Thus, postoperative recurrence is common and the situation may actually be worse than before surgical intervention. Sclerotherapy of intraconal LM was infrequently attempted until recently.3 Early reports using OK-432 and sodium tetradecyl have shown good results,9, 10 though there is a risk of loss of vision due to orbital compartment syndrome.
The clinical and radiologic findings regarding the orbital LMs in this series are similar to those reported previously, with a few additional findings.11 Intralesional hemorrhage occurred, either spontaneously or with trauma, in many of our patients. Potential sources of bleeding include small nutrient vessels within the fragile cyst walls and adjacent veins, particularly after surgical intervention.6, 12 Alternatively, some lesions may represent primary combined LVM. Both situations could explain the presence of phleboliths (calcified thrombi) and contrast enhancement, which may be seen in orbital lesions. Selective arteriograms in 2 patients in our series with acute proptosis and chemosis showed direct filling of irregularly shaped cystic spaces from tiny branches of the ophthalmic artery (Fig 7) at the sites where MR imaging revealed intensely enhancing components. These spaces had no venous drainage and thus were assumed to represent lymphatic spaces. We have termed these “arterio-lymphatic” communications, because they occurred within the lymphatic malformation. They could also be termed sites of arterial extravasation. Because arteries are present normally in the walls or septae of LMs, it is not surprising that such a communication could develop; to our knowledge, however, this has not been reported previously.
This large series of patients with periorbital LM had a high incidence of associated ICVAs, most commonly DVA and DAVM, but also including CCM and pial AVM. Most of the intracranial vascular anomalies were left-sided, ipsilateral to the periorbital LM. Most of the DVAs drained primarily through the perimesencephalic and deep cerebral veins. Seventy-three percent of our patients with periorbital LM and ICVAs had diffuse LM involving both deep and superficial orbital compartments. Although DVAs have been reported to have an association with orbital LM2, high-flow vascular anomalies have not been noted. DVAs in our series and in other reports were generally asymptomatic, but high-flow lesions can have devastating clinical effects and require early diagnosis and treatment. In our series, 4 of 5 patients with documented AVM had cerebral hemiatrophy or focal volume loss. AVM can lead to increased intracranial venous pressure that, in return, results in decreased cerebral perfusion, ischemic tissue loss, and developmental delay. Increased venous pressure also predisposes to intraorbital hemorrhage, thus accounting for the rapid onset of proptosis and lid swelling seen in 2 of our patients. One patient with bilateral cerebral hemiatrophy had CT findings of bilateral infratentorial DVA. This patient did not undergo angiography, so a high-flow lesion such as a DAVM could have been missed.
Katz et al2 reported coexistence of ICVAs and “combined venous-lymphatic vascular malformations” of the orbit. Before that report, a few patients with facial LM and complex DVA had been described, but the relationship between the 2 anomalies was thought to be “fortuitous.”13 Coll et al4 reported an ipsilateral DAVM in a patient with a right orbital LM and surmised that one would expect to find other vascular malformations coexisting in the same patient if the origin of such aberration occurred during vascular embryogenesis. Scavone et al14 reported a case of facial LM and concomitant large intracranial AVM. Our series confirms the relationship between periorbital LM and cerebral venous and arteriovenous anomalies. One patient in our series had a periorbital LM, a DAVM, a focal lesion resembling a CCM, and anomalous intracranial venous drainage (including SP), showing that vascular development of the orbit, scalp, calvaria, dura, and brain can be affected simultaneously, presumably by a common genetic defect. Other examples of vascular anomalies involving the orbit and brain include Wyburn-Mason syndrome (Bonnet-Dechaume-Blanc syndrome), in which facial and retinal vascular malformations are associated with ipsilateral intracranial AVM along the optic pathway15 and other “metameric” high flow vascular malformations,16 Sturge-Weber Syndrome, which includes facial and ocular venular malformations and leptomeningeal venular and venous malformations,17–19 and possibly even the PHACE (posterior fossa/hemangioma/arterial defect/cardiac problems/eye problems) association of facial hemangioma and intracranial arterial anomalies.20, 21 Patients with vascular anomalies simultaneously involving the face, limbs, and brain have also been reported.22, 23
Embryology of Lymphatic Malformations
It was generally believed that there are no lymphatic channels in the orbit, excepting the subconjunctiva. However, there is controversy regarding this anatomic point in the older ophthalmologic literature.24, 25 Duke-Elder described interlobular spaces in the orbital fat lined by an endothelial membrane,26 but others have argued that this endothelial lining is not characteristic of the lymphatic system.27
However, there is evidence for a lymphatic drainage system from the ocular globe that travels along the perivenous spaces passing through the superior and inferior fissures to the internal maxillary nodes and to the upper deep cervical groups.25, 27 This might explain why some orbital LMs extend through and enlarge the superior and/or inferior orbital fissures.5 Lymphatic communications may also exist between the orbital system and the nasal cavity.27
Recent studies on the vascular endothelial growth factors (VEGFs) and their specific cell surface receptors have shed light on the origin of the lymphatic system and its relationship to the venous system, and ultimately on abnormalities of vasculogenesis.28–32 Kaipainen et al discovered a gene (human fms-like tyrosine kinase 4 gene [FLT4]) whose protein product is related to 2 VEGFs. This gene is thought to be important in the development and maintenance of lymphatic vessels and is prominent in LMs.33
DVA and Embryogenesis
DVAs are medullary or cortical veins draining normal cerebral parenchyma. They usually represent an adaptation related to absence of a superficial or deep collector. DVAs (previously termed “venous angiomas”) are said to be the most common anomaly of the intracranial vasculature.34 DVA is usually an incidental finding but rarely can be associated with hemorrhage, headaches, or other neurologic symptoms. An association between CCM and DVA has been described, accounting for most of the cases of cerebral hemorrhage related to DVA.35 DVA, generally asymptomatic, is present in approximately 20% of patients with cervicofacial venous malformations.1
The embryogenesis of DVA is unclear. They are hypothesized to be malformations or anomalies of venous drainage developing in fetal life between 8 and 11 weeks,36, 37 possibly due to interruption of the developing medullary veins or their tributaries with formation of collateral pathways. Padget’s work on the development of the circulatory system demonstrated that abnormal vasculogenesis at an appropriate gestational stage could result in an anomalous draining vein supplementing the usual drainage.36, 38
Although DVAs are common and are usually asymptomatic, DAVMs and other high-flow intracranial vascular malformations often result in significant morbidity, including dural sinus thrombosis, venous ischemia, hydrocephalus, and hemorrhage. Identification of these anomalies before the onset of symptoms is desirable.
Conclusions
This retrospective review confirmed a strong association between periorbital LMs (and LVMs) and ICVAs, including DVA, DAVM, occlusion or absence of dural sinuses and jugular veins, CCM, and pial AVM. Cerebral parenchymal volume loss may coexist with some of these, especially high-flow vascular malformations. We recommend that infants with periorbital LM undergo contrast-enhanced MR imaging of the orbits and brain, soon after clinical diagnosis, to identify those patients who require additional treatment or observation. Because orbital LM is a rare anomaly, the financial cost of such additional imaging is justified.
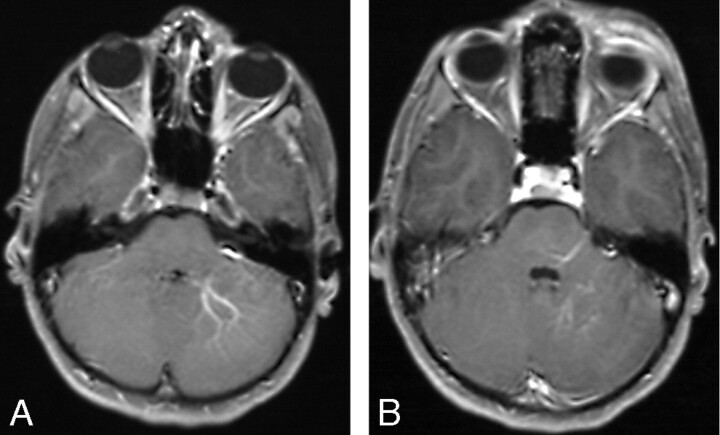
Fig 2.
A and B, Postgadolinium axial T1-weighted MR images of a patient with a left preseptal orbitofrontal lymphatic malformation shows ipsilateral posterior fossa DVAs.
Table 1:
Orbital LM Characteristics
| Location | No. | Macro | Micro | Combined | Intraconal* | Extraconal* | Periorbital* | Diffuse |
|---|---|---|---|---|---|---|---|---|
| Frontal | 7 | 1 | 5 | 1 | 1 | 3 | 4 | 3 |
| Frontotemporal | 4 | 1 | 2 | 1 | 1 | 1 | 2 | 2 |
| Cranial base | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Lower cervicofacial | 5 | 1 | 4 | 0 | 0 | 0 | 2 | 3 |
| Cheek & frontal | 3 | 1 | 2 | 0 | 1 | 1 | 2 | 1 |
| Cervicofacial & frontotemporal | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Cheek | 4 | 1 | 2 | 1 | 1 | 0 | 1 | 3 |
| Bilateral cervicofacial | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 1 |
| Isolated orbital | 6 | 2 | 3 | 1 | 3 | 2 | 2 | 2 |
| Total | 33 | 8 | 20 | 5 | 9 | 8 | 14 | 15 |
Note:—LM indicates lymphatic malformation; diffuse, intraconal + extraconal + periorbital; macro, macrocystic; micro, microcystic; combined, macrocystic and microcystic components.
Alone or associated with intraconal or extraconal or periorbital lesions.
References
- 1.Boukobza M, Enjolras O, Guichard JP, et al Cerebral developmental venous anomalies associated with head and neck venous malformations. AJNR Am J Neuroradiol 1996;17:987–94 [PMC free article] [PubMed] [Google Scholar]
- 2.Katz SE, Rootman J, Vangveeravong S, et al. Combined venous lymphatic malformations of the orbit (so-called lymphangiomas). Association with noncontiguous intracranial vascular anomalies. Ophthalmology 1998;105:176–84 [DOI] [PubMed] [Google Scholar]
- 3.Greene AK, Burrows PE, Smith L, et al. Periorbital lymphatic malformation: clinical course and management in 42 patients. Plast Reconstr Surg 2005;115:22–30 [PubMed] [Google Scholar]
- 4.Coll GE, Goldberg RA, Krauss H, et al. Concomitant lymphangioma and arteriovenous malformation of the orbit. Am J Ophthalmol 1991;112:200–05 [DOI] [PubMed] [Google Scholar]
- 5.Graeb DA, Rootman J, Robertson WD, et al. Orbital lymphangiomas: clinical, radiologic, and pathologic characteristics. Radiology 1990;175:417–21 [DOI] [PubMed] [Google Scholar]
- 6.Iliff WJ, Green WR. Orbital lymphangiomas. Ophthalmology 1979;86:914–29 [DOI] [PubMed] [Google Scholar]
- 7.Kennerdell JS, Maroon JC, Garrity JA, et al. Surgical management of orbital lymphangioma with the carbon dioxide laser. Am J Ophthalmol 1986;102:308–14 [DOI] [PubMed] [Google Scholar]
- 8.Eiferman RA, Gushard RH. Chocolate cysts of the orbit. Ann Ophthalmol 1986;18:156–57 [PubMed] [Google Scholar]
- 9.Pitz S, Dittrich M. Orbital lymphangioma. Br J Ophthalmol 2000;84:124–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, Obana A, Gohto Y, et al. Management of orbital lymphangioma using intralesional injection of OK-432. Br J Ophthalmol 2000;84:614–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows PE, Laor T, Paltiel H, et al. Diagnostic imaging in the evaluation of vascular birthmarks. Dermatol Clin 1998;16:455–88 [DOI] [PubMed] [Google Scholar]
- 12.Jones I. Lymphangiomas of ocular adnexa: an analysis of 62 cases. Am J Ophthalmol 1961;51:481–501 [DOI] [PubMed] [Google Scholar]
- 13.Lasjaunias P. Vascular Diseases in Neonates, Infants and Children. New York: Springer-Verlag,1997
- 14.Scavone C, Pascual Castroviejo I, Tendero A, et al. [Giant intracranial arteriovenous malformation and facial lymphangioma (author’s transl)]. An Esp Pediatr 1980;13:589–92 [PubMed] [Google Scholar]
- 15.Patel U, Gupta SC. Wyburn-Mason syndrome. A case report and review of the literature. Neuroradiology 1990;31:544–46 [DOI] [PubMed] [Google Scholar]
- 16.Luo C, Bhattacharya J, Ferreira M, et al. Cerebrofacial vascular disease. Orbit 2003;22:89–102 [DOI] [PubMed] [Google Scholar]
- 17.Griffiths PD. Sturge-Weber syndrome revisited: the role of neuroradiology. Neuropediatrics 1996;27:284–94 [DOI] [PubMed] [Google Scholar]
- 18.Rocco CD. Sturge-Weber disease. In: Cerebrovascular Diseases in Children. New York: Springer-Verlag,1992. :168–87
- 19.Pascual-Castroviejo I, Diaz-Gonzalez C, Garcia-Melian RM, et al. Sturge-Weber syndrome: study of 40 patients. Pediatr Neurol 1993;9:283–88 [DOI] [PubMed] [Google Scholar]
- 20.Poetke M, Frommeld T, Berlien HP. PHACE syndrome: new views on diagnostic criteria. Eur J Pediatr Surg 2002;12:366–74 [DOI] [PubMed] [Google Scholar]
- 21.Metry DW, Dowd CF, Barkovich AJ, et al. The many faces of PHACE syndrome. J Pediatr 2001;139:117–23 [DOI] [PubMed] [Google Scholar]
- 22.Williams DW 3rd, Elster AD. Cranial CT and MR in the Klippel-Trenaunay-Weber syndrome. AJNR Am J Neuroradiol 1992;13:291–94 [PMC free article] [PubMed] [Google Scholar]
- 23.Deutsch J, Weissenbacher G, Widhalm K, et al. [Combination of the syndrome of Sturge-Weber and the syndrome of Klippel-Trenaunay (author’s transl)]. Klin Padiatr 1976;188:464–71 [PubMed] [Google Scholar]
- 24.Birch-Hirschfeld A. Graefe-Saemisch, Handb. d. ges. Augenheilk., 2nd ed. Liepzig,1909
- 25.Bartels P. Das Lymphgefaesystem Jena: Gustav Fischer;1909
- 26.Duke-Elder S. Lymphangioma. In: System of Ophthalmology. St. Louis: Mosby,1974. :1102–04
- 27.Whitnall SE. Lymphatics of the human orbit. In: Anatomy of the Human Orbit and Accessory Organs of Vision. Oxford Medical Publications. London: Frowde and Hodder & Stoughton,1921. :321
- 28.Auguste P, Javerzat S, Bikfalvi A. Regulation of vascular development by fibroblast growth factors. Cell Tissue Res 2003 [DOI] [PubMed]
- 29.Baldwin ME, Stacker SA, Achen MG. Molecular control of lymphangiogenesis. Bioessays 2002;24:1030–40 [DOI] [PubMed] [Google Scholar]
- 30.Chang L, Kaipainen A, Folkman J. Lymphangiogenesis new mechanisms. Ann NY Acad Sci 2002;979:111–19 [DOI] [PubMed] [Google Scholar]
- 31.Lohela M, Saaristo A, Veikkola T, et al. Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost 2003;90:167–84 [DOI] [PubMed] [Google Scholar]
- 32.Thurston G. Role of angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res 2003;314:61–68 [DOI] [PubMed] [Google Scholar]
- 33.Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A 1995;92:3566–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truwit CL. Venous angioma of the brain: history, significance, and imaging findings. AJR Am J Roentgenol 1992;159:1299–307 [DOI] [PubMed] [Google Scholar]
- 35.Lasjaunias P, Berenstein A, Ter Brugge KT. Intracranial venous system. In: Surgical Neuroangiography, 2nd ed. New York: Springer-Verlag,2001. :631–713
- 36.Garner TB, Del Curling O Jr, Kelly DL Jr, et al. The natural history of intracranial venous angiomas. J Neurosurg 1991;75:715–22 [DOI] [PubMed] [Google Scholar]
- 37.Saito Y, Kobayashi N. Cerebral venous angiomas: clinical evaluation and possible etiology. Radiology 1981;139:87–94 [DOI] [PubMed] [Google Scholar]
- 38.Padget DH. The development of the cranial venous system in man from the viewpoint of comparative anatomy. Contrib Embryol 1957;36:79–140 [Google Scholar]