Abstract
SUMMARY: Richter transformation is defined as a diffuse large cell lymphoma, occurring by transformation of chronic lymphocytic leukemia (CLL). We present a 64-year-old man with a history of CLL and a left parieto-occipital transtentorial extra-axial mass. The patient underwent CT and MR imaging, demonstrating a large dural-based mass with extracranial extension, which occluded the left transverse sinus. Biopsy of the mass proved a pathologic diagnosis of diffuse large cell non-Hodgkin lymphoma, consistent with Richter transformation of CLL.
Central nervous system lymphomatous involvement in the setting of chronic lymphocytic leukemia (CLL), known as Richter transformation, is rare. Thirteen cases have been reported,1–7 only 5 of which had isolated leptomeningeal involvement,4,5,8 and the other 8 demonstrated parenchymal involvement. We report a case of central nervous system Richter transformation, presenting as a large left occipital dural-based mass. It was investigated by means of conventional radiography, CT, MR imaging, and MR angiography and was surgically biopsied.
Case Report
A 64-year-old man presented to the emergency department with headaches, 2–3 days of vomiting, and a left occipital mass present for 3–4 weeks. He had a medical history of rheumatoid arthritis, immune thrombocytopenic purpura, and a 5-year history of chronic lymphocytic leukemia (CD5+, CD20+, and CD23+), treated through multiple chemotherapy courses (including 3 cycles of fludarabine 4 years prior). The patient described the headaches as “nagging” and running across his forehead, and he also stated that he had experienced some occasional blurry vision and “floaters” during the past weeks, but no visual loss. He had episodes of dizziness and balance difficulty, but no history of falls or loss of consciousness. Physical examination revealed an alert, well-nourished man, with a nontender mass in the left occipital region. A retinal hemorrhage was noted in the right eye on fundoscopic examination, and the left pupil appeared larger. An egg-sized lymph node was palpable in the right axilla. Findings of a neurologic examination were grossly nonfocal.
Skull radiographs did not show any osseous abnormality or soft-tissue calcification, but mild prominence of the left occipital soft tissues was noted. CT and MR imaging demonstrated a large 8.0 × 5.5 × 8.5 cm oval dural-based transtentorial mass with a dural tail in the left occipital-to-parietal region, with both extracranial and intracranial components. The dural tail extended along the left tentorium to the incisura. The intracranial component was 6.5 × 3.5 × 8.0 cm. The mass was hyperattenuated compared with normal brain parenchyma on unenhanced CT and enhanced homogeneously (Fig 1). There was localized effacement of sulci and ventricles, without parenchymal edema. The mass was abutting the confluence of dural sinuses and eroding the overlying left occipital and posterior parietal bones. MR imaging showed obliteration of the left transverse sinus, but the left sigmoid sinus was unaffected (Fig 2). CT studies of the chest, abdomen, and pelvis were unremarkable.
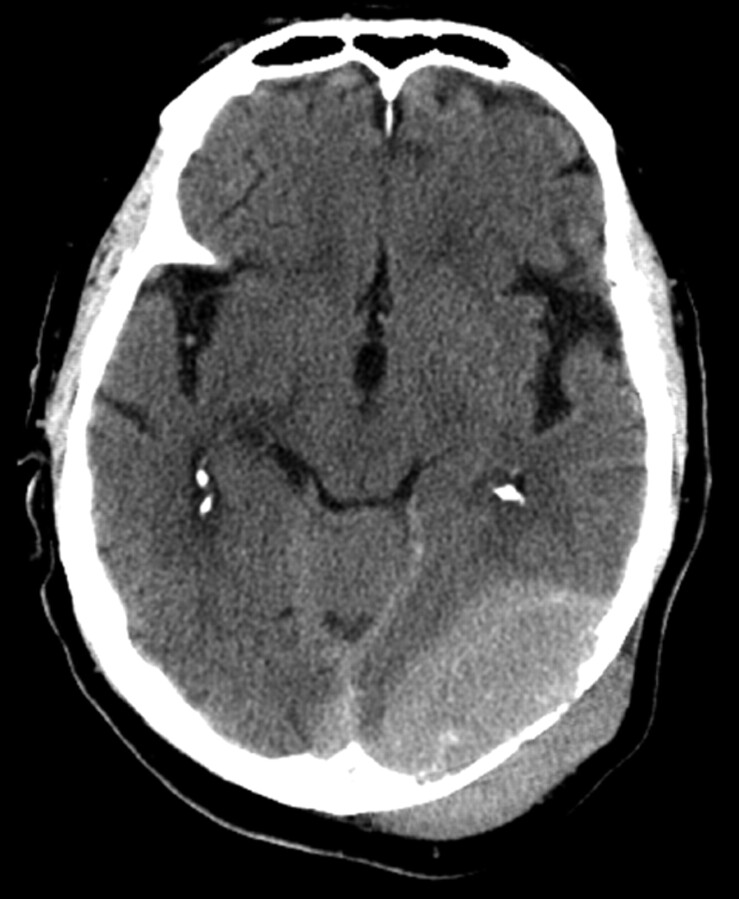
Fig 1.
Noncontrast CT scan of the brain shows a large heterogeneously attenuated dural-based mass extending across the tentorium and the calvaria.
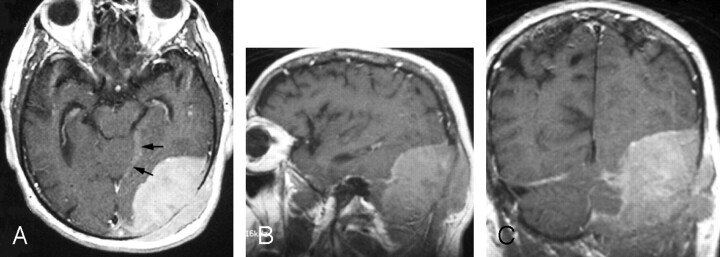
Fig 2.
Gadolinium-enhanced axial (A), sagittal (B), and coronal (C) fast spin-echo T1-weighted images (400/18/1 [TR/TE/excitations]) through the left occipital region show a large enhanced dural-based mass measuring 8.0 × 5.5 × 8.5 cm. A part of the dural tail is seen along the incisura (arrows). Laterally, the mass erodes the occipital bone and demonstrates a mass effect on the adjacent occipital lobe and cerebellum without invasion.
The images presented a differential consideration of metastatic disease, meningeal hemangiopericytoma, malignant meningioma, and plasmacytoma.
The mass was surgically biopsied. An incision of the left posterior scalp revealed a grayish tumor under the fascia, from which multiple biopsy specimens were obtained and sent for frozen and permanent histologic section and flow cytometry. The pathology report indicated diffuse large cell non-Hodgkin lymphoma, consistent with Richter transformation involving the dura, skull, and brain, as well as the extracranial soft tissue (Fig 3).
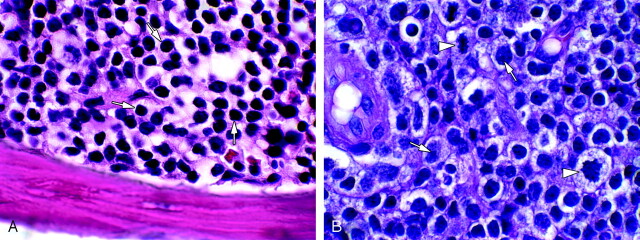
Fig 3.
A, Bone marrow hematoxylin-eosin (H&E) 1000× (oil), bone marrow in CLL, shows replacement of normal marrow elements by sheets of small mature lymphocytes (arrows). B, Brain H&E 1000× (oil), large cell non-Hodgkin lymphoma (Richter) transformation of CLL. Note nucleoli (arrows) and mitotic figures (arrowheads).
The patient was placed on chemotherapy (rituximab, cyclophosphamide [Cytoxan], doxorubicin hydrochloride [Adriamycin], vincristine, and prednisone) and radiation therapy regimens, after which the mass markedly decreased in size, with only 2-cm dural thickness on a follow-up MR imaging study, but with persistent occlusion of the left transverse sinus and lymphomatous involvement of the left mastoid bone (Fig 4). The patient died 3 months after the initial presentation to the emergency department for the cranial mass.
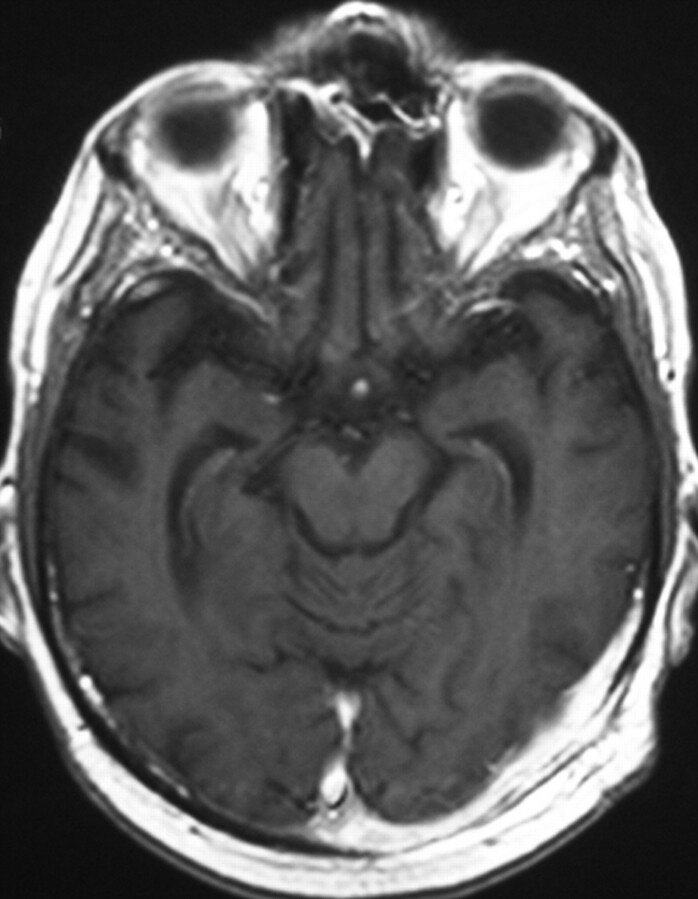
Fig 4.
Post-therapy gadolinium-enhanced axial fast spin-echo T1-weighted image (340/14/1 [TR/TE/excitations]) reveals marked reduction of the mass size with continued abnormal enhancement along the dura.
Discussion
The occurrence of diffuse large cell non-Hodgkin lymphoma in the setting of CLL was first described by Richter in 19289 and appointed as “Richter syndrome” in 1964.10 Richter transformation is an unusual and serious complication of CLL, with an incidence occurring between 1% and 10% of CLL cases, a 5–6 month median survival duration despite multiagent therapy,3,8,11,12 and a significantly higher incidence in patients younger than 55 years of age.13 Hodgkin disease in the setting of CLL has also been described as a variant of Richter transformation.3 Diffuse large cell non-Hodgkin lymphoma has been demonstrated in 6% of patients with Waldenstrom macroglobulinemia.14 Reports have documented the presence of 2 types of Richter syndrome: the “classical” type as a terminal event of CLL and the “variant” type as the first clinical presentation of previously undetected subclinical CLL.15,16 There has been controversy about whether the transformation represents the emergence of a more malignant clone or the chance occurrence of 2 independent neoplasms.3,4,8
The incidence of Richter transformation in the study by Robertson et al8 was similar in patients who had and who had not received prior fludarabine therapy, implying that therapy does not reverse a component of CLL-associated immunodeficiency leading to the development of Richter transformation.3 Follow-up has been suggested to evaluate whether increased immunosuppression correlates with an increased incidence of Richter transformation.3
Only an awareness of the patient’s pre-existing condition and the possibility of transformation of CLL to non-Hodgkin lymphoma can lead to the inclusion of Richter transformation. The mass in this patient was dural-based, and because previously reported cases of central nervous system Richter transformation4,5,8 do not specify a dural mass, this case may be even more atypical.
Acknowledgments
We thank Negar Imani, who assisted in preparing the images for this case report.
References
- 1.Agard G, Hamidou M, Leautez S, et al. Localisation neuroméningée d’um syndrome de Richter. Rev Méd Interne 1999;20:64–67 [DOI] [PubMed] [Google Scholar]
- 2.Bayliss KM, Kueck BD, Hanson CA, et al. Richter’s syndrome presenting as primary central nervous system lymphoma: transformation of an identical clone. Am J Clin Pathol 1990;93:117–23 [DOI] [PubMed] [Google Scholar]
- 3.Giles FJ, O’Brien SM, Keating MJ. Chronic lymphocytic leukemia in (Richter’s) transformation. Semin Oncol 1998;25:117–25 [PubMed] [Google Scholar]
- 4.Lane PK, Townsend RM, Beckstead JH, et al. Central nervous system involvement in a patient with chronic lymphocytic leukemia and non-Hodgkin’s lymphoma (Richter’s syndrome), with concordant cell surface immunoglobulin isotypic and immunophenotypic markers. Am J Clin Pathol 1988;89:254–59 [DOI] [PubMed] [Google Scholar]
- 5.Mahé B, Moreau P, Bonnemain B, et al. Isolated Richter’s syndrome of the brain: two recent cases. Nouv Rev Fr Hematol 1994;36:383–85 [PubMed] [Google Scholar]
- 6.O’Neill BP, Habermann TM, Banks PM, et al. Primary central nervous system lymphoma as a variant of Richter’s syndrome in two patients with chronic lymphocytic leukemia. Cancer 1989;15:1296–300 [DOI] [PubMed] [Google Scholar]
- 7.Resende LS, Bacchi CE, Resende LA, et al. Isolated Richter’s syndrome in central nervous system: case report [in Portuguese]. Arq Neuropsiquiatr 2005;63(2B):530–31. Epub 2005 Jul 25 [DOI] [PubMed] [Google Scholar]
- 8.Robertson LE, Pugh W, O’Brien S, et al. Richter’s syndrome: a report on 39 patients. J Clin Oncol 1993;11:1985–89 [DOI] [PubMed] [Google Scholar]
- 9.Richter MN. Generalized reticular cell sarcoma of lymph nodes associated with lymphatic leukemia. Am J Pathol 1928;6:285–99 [PMC free article] [PubMed] [Google Scholar]
- 10.Lortholary P, Boiron M, Ripault J, et al. Leucémie lymphoïde chronique secondairement associée à une réticulopathie maligne (syndrome de Richter). Nouv Rev Fr Hematol 1964;4:621–44 [PubMed] [Google Scholar]
- 11.Rai KR, Keating MJ. Staging and prognosis of chronic lymphocytic leukemia. Up-to-date May 10, 2005. Available at http://patients.uptodate.com/topic.asp?file=leukemia/12607
- 12.Rai KR, Dohner H, Keating MJ, et al. Chronic lymphocytic leukemia: case-based session. Hematology Am Soc Hematol Educ Program 2001. :140–56 [DOI] [PubMed]
- 13.Mauro FR, Foa R, Giannarelli D, et al. Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: a single institution study of 204 cases. Blood 1999;94:448–54 [PubMed] [Google Scholar]
- 14.Tardif S, de Kerviler E, Chaibi P, et al. CT and MR patterns of spinal involvement in Richter syndrome. J Comput Assist Tomogr 1995;19:146–49 [DOI] [PubMed] [Google Scholar]
- 15.Jelic S, Jovanovic V, Milanovic N, et al. Richter syndrome with emphasis on large-cell non-Hodgkin lymphoma in previously unrecognized subclinical chronic lymphocytic leukemia. Neoplasma 1997;44:63–68 [PubMed] [Google Scholar]
- 16.Strauchen JA, May MM, Crown J. Large cell transformation of subclinical small lymphocytic leukemia/lymphoma: a variant of Richter’s syndrome. Hematol Oncol 1987;5:167–74 [DOI] [PubMed] [Google Scholar]