Abstract
BACKGROUND AND PURPOSE: To use MR spectroscopy to aid in the diagnosis of demyelinating disease and to help differentiate tumefactive demyelinating lesions from neoplastic processes.
MATERIALS AND METHODS: MR imaging of the brain was obtained in 4 patients who presented clinically with focal neurologic deficits. MR imaging initially revealed parenchymal mass lesions. Single-voxel MR spectroscopy was then performed utilizing a point-resolved spectroscopy sequence protocol with a short echo time (30 msec).
RESULTS: MR imaging revealed a focal ring-enhancing mass in one patient, multiple ring-enhancing lesions in the second patient, a large area of edema and mass effect without associated enhancement in the third patient, and multiple solid and peripherally enhancing lesions in the fourth patient. MR spectroscopic results in all 4 patients demonstrated marked elevation of the glutamate and glutamine peaks (2.1–2.5 ppm). Other nonspecific (and in a sense confounding) findings included elevation of the choline peak (3.2 ppm), elevation of the lactate peak (1.3 ppm), elevation of the lipid peak (0.5–1.5 ppm), and decrease in the N-acetylaspartate peak (2.0 ppm). All 4 patients were eventually given the diagnosis of multiple sclerosis based on CSF analysis, brain biopsy, and/or clinical follow-up.
CONCLUSION: MR spectroscopic metabolite information may be useful in the diagnosis of demyelinating disease by demonstrating elevation of the glutamate/glutamine peaks because elevation of these peaks is typically not seen in aggressive intra-axial neoplastic processes. This is particularly beneficial in the rarer cases of tumefactive demyelinating lesions, which are very difficult to differentiate from neoplasms by imaging findings alone.
Tumefactive demyelinating lesions can mimic intracranial neoplasms in clinical presentation. MR imaging, although sensitive in detecting such lesions, is not specific for their etiology. The neuroradiologic observation of a large deep white matter lesion with mass effect, clinically revealed by signs of increased intracranial pressure, is highly suspicious for central nervous system (CNS) neoplasm. In rare cases, a demyelinating disorder can present with atypical signs, symptoms, and imaging features suggestive of a brain tumor.1 These so-called tumefactive demyelinating lesions can pose a considerable diagnostic challenge to both the clinician and the radiologist. The difficulty in diagnosing tumefactive demyelinating disease, most commonly secondary to multiple sclerosis (MS), often leads to surgical biopsy; however, the histopathologic findings can be misleading as well.2,3
Literature reports, studying the potential utility of proton MR spectroscopy (1H-MR spectroscopy) in differentiating tumefactive demyelinating lesions from neoplasms, have focused on the classical metabolites of N-acetylaspartate (NAA), choline (Cho), creatine (Cr), lactate (Lac), and mobile lipids (Lip).2,4 Recent publications have suggested that MR spectroscopy may not be suitable to differentiate MS from neoplasms because of the overlap in decreased NAA/Cr ratio, increased Cho/Cr ratio, and the variable and nonspecific presence of Lac and Lip in both of these entities.2,5 This study reports on the observation that an abnormal elevation of the glutamate/glutamine (β,γ-Glx) peaks is the more critical MR spectroscopy finding in tumefactive lesions of MS in the brain, a finding that is not usually seen in the confounding aggressive intra-axial neoplasms.
Materials and Methods
Using a 1.5T superconducting magnet, MR images of the brain were obtained in 4 patients. MR images revealed parenchymal mass lesions in all cases. With Institutional Review Board approval to perform MR spectroscopy as clinically indicated in the evaluation of cerebral pathology, single-voxel 1H-MR spectroscopy was then performed in the areas of abnormality utilizing a point-resolved spectroscopy sequence (PRESS) protocol with a short echo time (30 msec). In 2 of the patients, MR spectroscopy was also obtained in the corresponding contralateral normal hemisphere. In the other 2 patients, control spectra were not obtained because additional lesions were also located in the contralateral regions of the brain. All spectra were obtained by using the automatic MR spectroscopy software with spin-echo technique, TR 1500 msec, 1024 point acquisition, 1000-Hz bandwidth, 128 averages, chemical shift selection suppression (CHESS) with a 35-Hz bandwidth, automated shimming with first- and second-order shim channels, zero-order phase correction, zero filling to 2048 points, water reference postprocessing, noise removal low pass 700-msec Hanning filter, and sixth-order polynomial baseline correction. Although multivoxel spectroscopy allows for smaller voxels with less partial volume artifact and simultaneous analysis of multiple regions within a given patient, the standard 2 × 2 × 2 cm3 (8 mL) single-voxel technique was used because single-voxel spectroscopy has more precise spatial localization, more homogeneous shimming, better water suppression, less complex data processing, and a shorter time of acquisition that results in improved reproducibility between patients, in particular with the necessary short echo acquisition required to observe the β,γ-Glx metabolites.6 All 4 patients were eventually given the diagnosis of tumefactive MS based on CSF analysis in 2 of the 4 patients, brain biopsy in 3 of the 4 patients, and clinical follow-up in 3 of the 4 patients.
Patient 1 was a 42-year-old Hispanic man who presented with rapid onset of right upper and lower extremity paralysis, right-sided tingling, and difficulty speaking. Physical examination revealed severe right upper and lower extremity weakness as well as diminished sensation along the right side of the body. Brain MR imaging with contrast was performed, which revealed a 2.3-cm ring-enhancing left frontal lobe mass with associated vasogenic edema. MR spectroscopy of this mass was also performed. Based on MR imaging, the mass was initially thought to represent a neoplasm. A week later, the patient underwent biopsy of this lesion, and pathology revealed a macrophage-rich lesion with perivascular lymphocyte cuffing. Luxol fast blue stain demonstrated total myelin loss and innumerable purple granules within the macrophages while Bielschowsky silver stain showed relative axonal preservation, consistent with demyelinating disease. There was no evidence of cell neoplasia. As a result, the patient was given a diagnosis of tumefactive MS.
Patient 2 was a 17-year-old Hispanic man who initially presented with headaches, slurred speech, and left visual field loss. The patient stated that he had transient right-sided weakness and numbness at age 14 that resolved after 1 month. Brain MR imaging with contrast was performed, revealing multiple, small, bilateral ring-enhancing lesions in both cerebral hemispheres as well as in the right middle cerebellar peduncle (brachium pontis). These lesions were thought to represent either metastases or brain abscesses. As a result, the patient underwent brain biopsy. Biopsy results revealed a macrophage-rich lesion with perivascular lymphocyte cuffing, exuberant gliosis, chronic inflammation, and axonal sparing. The pathologists favored a diagnosis of demyelinating disease but could not exclude subacute infarct, progressive multifocal leukoencephalopathy, and neoplasm. Thus, the patient had a subsequent brain MR imaging with contrast several months later that revealed numerous white matter lesions in both corona radiata with involvement of the periventricular white matter as well as the subcortical U fibers. A lesion was also seen in the lower pons. Some of these lesions were enhanced after contrast administration. Most of the ring-enhancing lesions seen on the patient’s original brain MR imaging had resolved in the interim. MR spectroscopy of the right internal capsule enhancing lesion was also performed. The temporal course of the imaging findings, in conjunction with the prior biopsy results, was strongly suggestive of demyelinating disease and the patient was given the diagnosis of tumefactive MS.
Patient 3 was a 50-year-old, previously healthy man who presented with right-sided facial droop and the inability to communicate. On examination, the patient was noted to have a fever of 101°F, a moderate degree of expressive and receptive aphasia, mild right facial droop, and mild-to-moderate right upper and lower extremity weakness. MR imaging with contrast was performed, which revealed a large amount of increased T2-weighted signal intensity in the deep white matter of the left frontal and temporal lobes with mild mass effect and no pathologic enhancement. MR spectroscopy of this space-occupying process was also performed. Based on the MR imaging findings, this lesion was initially thought to represent either a low-grade glioma or tumefactive demyelinating disease. CSF analysis revealed elevated myelin basic protein at 11.95 ng/mL (normal range = 0.07–4.10 ng/mL). An infectious work-up was unrevealing. The remainder of the laboratory findings were unremarkable. The patient was treated with IV steroids for 9 days and showed clinical and radiographic improvement, and thus brain biopsy was not performed. The patient was discharged with a diagnosis of tumefactive MS.
Patient 4 was a 43-year-old woman who presented with a history of multiple neurologic complaints and a report of recent prior MR imaging scan at another facility that showed enhancing lesions throughout the brain parenchyma. The patient had an initial brain MR imaging at our institution that revealed numerous solid and peripherally enhancing white matter lesions, with the largest residing in the right temporal lobe, in which MR spectroscopy was also performed. While the diagnosis of tumefactive demyelinating disease was suggested because a few lesions showed only partial ring enhancement, metastatic neoplastic lesions could not be entirely excluded because other lesions were solidly enhancing or fully ring-enhancing. Given the patient’s declining clinical course, a brain biopsy of the right temporal lesion was performed that revealed nonspecific inflammatory cells with no evidence of neoplastic tissue. A follow-up brain MR imaging obtained 1 month later demonstrated some lesions improving and some worsening as well as interval development of a new large enhancing right frontal lobe lesion. Based on the changing nature of these white matter lesions on MR imaging and CSF analysis, which revealed several oligoclonal bands and an elevated immunoglobulin G index, the patient was given the diagnosis of tumefactive MS.
Results
MR imaging revealed a focal ring-enhancing mass in one patient (Fig 1A), multiple ring-enhancing lesions in the second patient (Fig 2A), a large area of increased T2-weighted signal intensity and mass effect without associated enhancement in the third patient (Fig 3A), and multiple solid and peripherally enhancing lesions in the fourth patient (Fig 4A). Corresponding voxel localization images and proton MR spectra of the lesions are also shown (Figs 1B, -C, 2B, -C, 3B, -C, 4B, -C). Additionally, contralateral cerebral hemisphere control voxel localization images and proton MR spectra are depicted in 2 patients (Figs 1D, -E, 3D, -E). Using peak height measurements, metabolite ratios were calculated for NAA, Cho, and β,γ-Glx with reference to Cr. Presence of Lac and Lip was also determined. Peak height measurements were used as is customary for β,γ-Glx evaluation because there is normal peak overlap of NAA and β,γ-Glx on the short TE spectra preventing accurate integration of peak area under the curves. Normal peak height values for β,γ-Glx/Cr are 0.36 ± 0.04; therefore, ratios greater than 0.5 are considered abnormal.7 Varied nonspecific and in a sense confounding MR spectroscopy findings included elevations of the Cho peak (3.2 ppm), the Lac peak (1.3 ppm), and of mobile Lip (0.5–1.5 ppm). A mild decrease in the NAA peak (2.0 ppm) was also identified. More importantly, however, MR spectroscopic results in all 4 patients demonstrated significant marked elevation of the β,γ-Glx peaks (2.1–2.5 ppm) with respect to the Cr peak (3.0 ppm). Additionally, even in the smaller lesions where the single voxel technique may induce partial volume artifact and limit metabolite abnormality detection due to the large interrogation voxel size compared with the lesion size, the elevation of β,γ-Glx was still clearly evident (Figs 2B, -C, 4B, -C). The 1H-MR spectroscopy metabolite ratios with reference to Cr are listed in the Table.
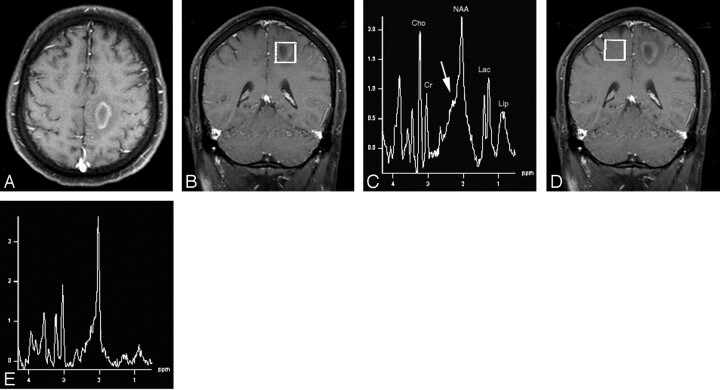
Fig 1.
A, Axial T1-weighted postcontrast MR image (TR/TE = 437/14) depicts a ring-enhancing lesion in the posterior medial left frontal lobe white matter with surrounding vasogenic edema.
B, Voxel localization for proton MR spectroscopy of the mass.
C, Proton MR spectrum of the mass shows marked elevation of choline, lactate, and lipid, metabolites normally indicative of aggressive neoplastic lesions. Of note, however, is elevation of the β,γ-Glx peaks (arrow) that suggests an inflammatory demyelinating process, which, in conjunction with the anatomic findings, is indicative of tumefactive multiple sclerosis. NAA indicates N-acetylaspartate; Cho, choline; Cr, creatine, Lac, lactate, Lip, lipid.
D, Voxel localization for proton MR spectroscopy of contralateral control.
E, Normal Proton MR spectrum of contralateral control.
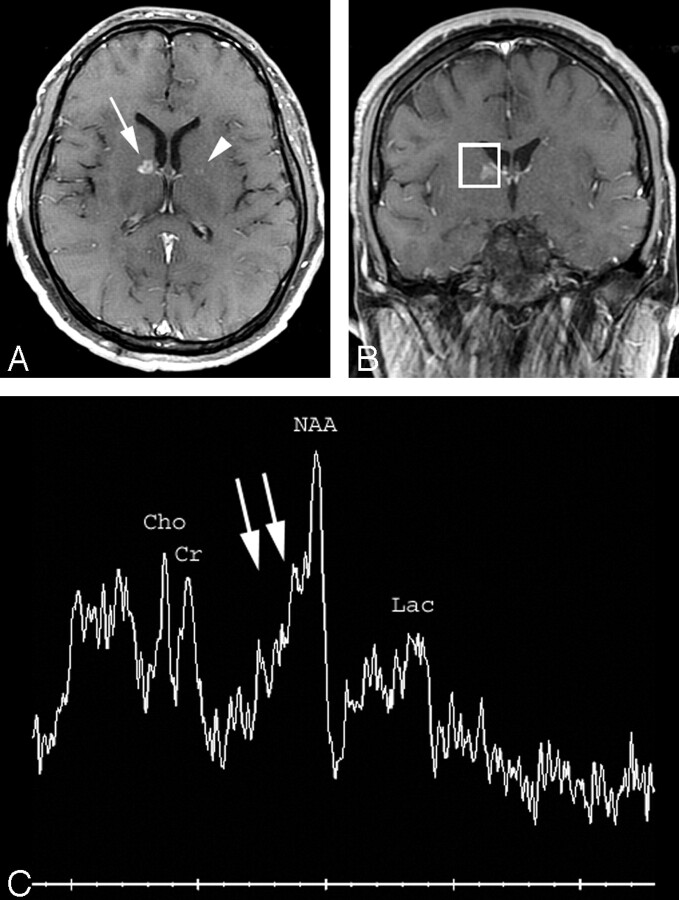
Fig 2.
A, Axial T1-weighted postcontrast MR image (TR/TE = 466/14) shows a small ring-enhancing lesion in the genu of the right internal capsule (arrow) and a barely perceptible lesion in the left globus pallidus (arrowhead). Multiple additional similar small ring-enhancing lesions were identified throughout the brain parenchyma.
B, Voxel localization for proton MR spectroscopy of the right internal capsule lesion.
C, MR spectroscopy of the right internal capsule lesion demonstrates marked elevation of the β,γ-Glx peaks (double arrows) compared with creatine (peak height ratio 1.1 [normal less than 0.5]) compatible with tumefactive multiple sclerosis. There is also mild decrease of N-acetylaspartate and probable mild presence of lactate. NAA indicates N-acetylaspartate; Cho, choline; Cr, creatine; Lac, lactate.
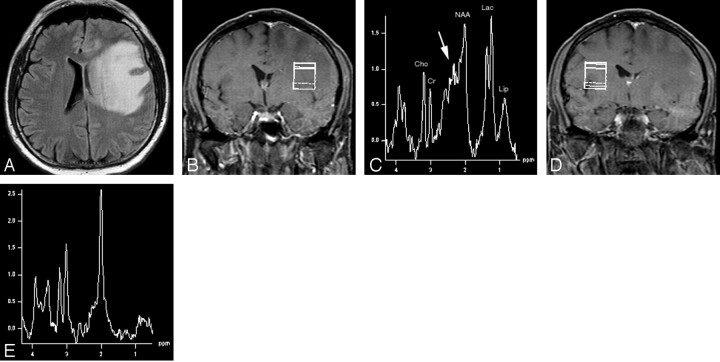
Fig 3.
A, Axial fluid-attenuated inversion recovery MR image (TR/TE/TI = 9000/109/2500) demonstrates a large region of increased T2-weighted signal intensity in the left frontal and temporal lobes, primarily in the white matter and extending into the corpus callosum. The lesion is exerting mass effect with compression of the left lateral ventricle and left to right subfalcine herniation. There was no significant enhancement of the lesion on postcontrast images.
B, Voxel localization for proton MR spectroscopy of the mass.
C, 1H-MR spectroscopy of the abnormality shows mild choline elevation, presence of lipid, and marked elevation of lactate. There is very significant elevation of the β,γ-Glx peaks (arrow). This large solitary nonenhancing mass lesion is another example of the varied imaging appearance of tumefactive multiple sclerosis. NAA indicates N-acetylaspartate; Cho, choline; Cr, creatine; Lac, lactate; Lip, lipid.
D, Voxel localization for proton MR spectroscopy of contralateral control.
E, Normal proton MR spectrum of contralateral control.
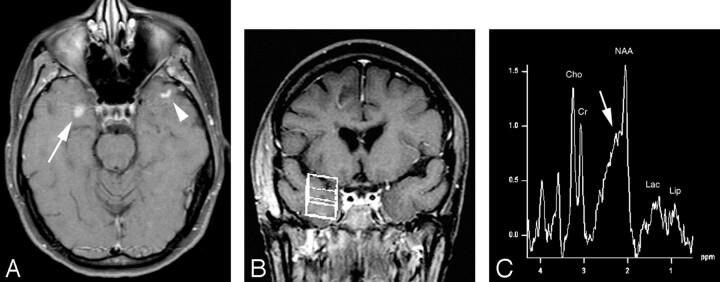
Fig 4.
A, Axial T1-weighted postcontrast MR image (TR/TE = 481/14) shows a solidly enhancing lesion in the right temporal lobe (arrow). There is also a curvilinear focus of enhancement in the left temporal subcortical white matter (arrowhead) suggestive, but not diagnostic, of the diagnosis of demyelinating disease.
B, Voxel localization for proton MR spectroscopy of the right temporal enhancing mass.
C, MR spectroscopy of the right temporal lobe lesion shows mild elevation of choline and mild decrease of N-acetylaspartate metabolites with probable presence of lipid and lactate possibly leading to the incorrect assumption of neoplastic disease. Again, however, there is elevation of the β,γ-Glx peaks (arrow) consistent with the patient’s correct diagnosis of tumefactive multiple sclerosis.
Proton MR spectroscopy metabolite ratios of lesions (and controls)
| Patient No. | NAA/Cr | Cho/Cr | Glx/Cr | Lac | Lip |
|---|---|---|---|---|---|
| 1 | 2.2 (1.9) | 2.0 (0.6) | 1.2 (0.5) | + | + |
| 2 | 1.6 | 1.1 | 1.1 | + | − |
| 3 | 2.1 (1.6) | 1.2 (0.8) | 1.5 (0.4) | ++ | + |
| 4 | 1.6 | 1.4 | 1.0 | + | + |
Note:—NAA indicates N-acetylaspartate; Cr, creatine; Cho, choline; Glx, glutamine and glutamate; Lac, lactate; Lip, lipid; +, mildly elevated; ++, markedly elevated; −, not evident.
Discussion
MS is a chronic inflammatory disease of the CNS characterized by focal areas of demyelination.8,9 It is the most common form of primary demyelinating disease of the CNS, and is classically defined by a relapsing-remitting or progressive clinical course. The usual pathologic findings demonstrate multiple well-circumscribed lesions that lack mass effect or edema, occurring in characteristic locations such as the periventricular white matter.10 A subset of patients, however, presents with lesions that produce confounding clinical symptoms and imaging findings suggestive of a neoplastic mass demonstrating ill-defined borders, mass effect, perilesional edema, central necrosis, contrast enhancement, and variable involvement of gray matter structures.11–16
MR spectroscopy has the potential for discriminating the presence and relative amount of various chemical metabolites in the brain and is therefore a useful technique to further assess various intracranial diseases, particularly to specifically determine which major category of disease is manifest by the observed lesion (eg, neoplastic, inflammatory, ischemic). A neoplastic profile generally includes the following: an attenuated NAA peak, consistent with neuronal loss; an elevated Cho resonance, indicative of increased turnover (synthesis and/or degradation) of cell membrane and myelin components; an attenuated Cr peak, reflecting depressed cellular energetics and/or cell death from lesional necrosis; and, in some cases, detectable Lip and Lac peaks, indicating, respectively, areas of cellular necrosis with the release of free lipids and anaerobic metabolism resulting in the production of lactate as a by-product.17,18
The acute lesion of MS is associated with the breakdown of the blood-brain barrier and is pathologically characterized by a dense perivascular inflammatory infiltrate that contributes to demyelination and axonal damage.19 Elevation of the Cho level is consistently found in acute MS lesions.4,20–23 Explanations for this finding have included reactive astrogliosis, demyelination, and inflammation, leading to cell membrane breakdown. Another common finding in the acute MS lesion is reduced NAA levels.4,20–22,24 Neuronal destruction, axonal damage, and neuronal mitochondrial dysfunction have been implicated. In pathologically confirmed acute MS lesions, evidence shows early axonal degeneration22 and decreased axonal attenuation4 that is associated with decreased NAA values. A more variable finding in the acute lesion of MS is the presence of Lac.4,20–23,25 Pathologically, the highest Lac is found in plaques with high inflammatory activity,4 suggesting that elevated Lac values may be related to inflammation, probably produced by activated macrophages.26 All of these pathologic processes can lead to an MR spectroscopy pattern indistinguishable from neoplasm. Based on this assertion, recent publications have suggested that MR spectroscopy may not be suitable to differentiate MS from neoplasm.2,5
In our analysis of the MR spectra in all 4 of the presented patients, there was the interesting observation of marked elevation of the β,γ-Glx peaks at 2.1–2.5 ppm. In reviewing the literature, Krouwer et al,27 who used MR spectroscopy with a short TE technique, evaluated a diverse group of 6 patients who had non-neoplastic diseases and concluded that MR spectroscopy could not differentiate between neoplastic and non-neoplastic disease processes based on NAA, Cho, Cr, Lip, and Lac metabolite levels. Although we agree with this assertion based on those metabolites alone, the authors did not specifically identify changes in the β,γ-Glx peaks, and actually the 2 patients with biopsy and proved fulminant demyelinating disease showed abnormal elevation of the β,γ-Glx peaks in the spectra presented in the article. Regarding MR spectroscopy in patients with typical MS, occasional peak elevation in the 2.1–2.6 ppm region was described by Grossman et al and was termed “marker peaks.”28 As noted, however, subsequent publications state a continued inability to distinguish MS from neoplasm. Besides simply not recognizing the β,γ-Glx metabolites, this may also be due to the fact that elevation of the β,γ-Glx peaks is not always present in regions of typical MS without active demyelination.28 We present the much rarer cases of tumefactive demyelinating disease where there is significant active demyelination resulting in marked elevation of the β,γ-Glx peaks, and we specifically note that these are the more challenging cases that do not have the typical appearance of demyelination but rather mimic neoplastic disease. As such, these cases are most problematic for diagnosis with MR imaging alone but may be the suitable instance where MR spectroscopy can be most helpful based on this observation of elevated β,γ-Glx.
In our experience, the spectroscopic pattern of marked elevation of the β,γ-Glx metabolites has been associated with inflammatory processes of the CNS and is not seen with aggressive intra-axial neoplastic disorders. Moreover, this lack of β,γ-Glx elevation in intra-axial neoplasms has been shown in a large review of neoplasm MR spectroscopy patterns by Majos et al.29 In this large series, elevation of β,γ-Glx was only noted in the extra-axial meningioma. Although there is no specific known mechanism for the elevation of these metabolites in inflammatory conditions, we propose the following hypothesis. Glutamate and glutamine are known to function as neurotransmitters, though this function concerns only a very small fraction of the total concentration of these metabolites in the brain. These molecules are primarily found in astrocytes, and therefore the observed peaks in the normal MR spectra mainly act as an astrocytic marker.30 In the normal MR spectrum, the quantity of glutamate exceeds that of glutamine (10 mmol/L versus 5 mmol/L, respectively); whenever there is elevation of these metabolites, the increase is primarily due to an increase in glutamine. This is due to the fact that glutamate, when present in excess levels, is actually a neurotoxic substance. Therefore, when an increase in glutamate occurs, glutamine synthetase is activated, which converts glutamate to glutamine, a substance that is nontoxic to the brain.31 In inflammatory conditions, cell breakdown of both neural and glial elements occurs, as does an associated adjacent astrocytic response, leading to the local accumulation of many metabolites and likely accounting for the high concentration of β,γ-Glx. This rise is most likely due to the participation of β,γ-Glx in the redox cycle that governs lactate accumulation, a protective function of the astrocyte.
Other molecules certainly are present and may even add to the increased peaks observed in the MR spectrum at 2.1–2.5 ppm, though no other spectral components are known to exist at this location in sufficient quantity to be representative of this elevated peak. As such, we can only assume at present that these peaks are representative of β,γ-Glx. Nevertheless, the important observation is that elevation of these peaks suggested an inflammatory etiology even though the remaining imaging pattern was not specific and could not reliably differentiate inflammatory processes from primary or secondary neoplastic disease. Thus, this feature could help differentiate demyelinating tumefactive lesions from neoplastic masses, avoiding unnecessary biopsy and potentially harmful surgery, as well as provide a more specific diagnosis during the initial MR examination, allowing the earlier institution of appropriate therapy. Further research is warranted, in particular at higher field strengths where these overlapping spectral peaks can be resolved more accurately,32 possibly resulting in a more definitive diagnostic spectral pattern for inflammatory conditions and the specific relative concentration and spectral location of the metabolites produced by this disorder.
Conclusion
MR spectroscopic metabolite information can be helpful in the noninvasive diagnosis of acute demyelinating disease with the identification of elevation of the β,γ-Glx peaks. This is particularly beneficial in the rarer cases of tumefactive demyelinating lesions, which are extremely difficult to differentiate from neoplasms by imaging findings alone. In particular, we underscore the importance of performing MR spectroscopy by using short TE parameters, in addition to either single or multi-voxel long TE acquisition, in the routine spectroscopic evaluation of mass lesions to detect these metabolites with short T2 values.
References
- 1.Capello E, Roccatagliata L, Pagano F, et al. Tumor-like multiple sclerosis (MS) lesions: neuropathological clues. Neurol Sci 2001;22(suppl 2):113–16 [DOI] [PubMed] [Google Scholar]
- 2.Saindane AM, Cha S, Law M, et al. Proton MR spectroscopy of tumefactive demyelinating lesions. AJNR Am J Neuroradiol 2002;23:1378–86 [PMC free article] [PubMed] [Google Scholar]
- 3.Tan HM, Chan LL, Chuah KL, et al. Monophasic, solitary tumefactive demyelinating lesion: neuroimaging features and neuropathological diagnosis. Br J Radiol 2004;77:153–56 [DOI] [PubMed] [Google Scholar]
- 4.Bitsch A, Bruhn H, Vougioukas V, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol 1999;20:1619–27 [PMC free article] [PubMed] [Google Scholar]
- 5.Law M, Meltzer DE, Cha S. Spectroscopic magnetic resonance imaging of a tumefactive demyelinating lesion. Neuroradiology 2002;44:986–89 [DOI] [PubMed] [Google Scholar]
- 6.Hsu YY, Chang C, Chang CN, et al. Proton MR spectroscopy in patients with complex partial seizures: single-voxel spectroscopy versus chemical-shift imaging. AJNR Am J Neuroradiol 1999;20:643–51 [PMC free article] [PubMed] [Google Scholar]
- 7.Danielsen ER, Ross B. Basic physics of MRS. In: Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. New York: Marcel Dekker;1999;5–22
- 8.Lassman H. Comparative Neuropathology of Chronic Experimental Allergic Encephalomyelitis and Multiple Sclerosis. Berlin, Heidelberg: Springer-Verlag;1983 [PubMed]
- 9.Prineas JW. The neuropathology of multiple sclerosis. In: Koestsier JC, ed. Demyelinating Diseases. Amsterdam: Elsevier Science Publishers;1985;8:213–57 [Google Scholar]
- 10.Nesbit GM, Forbes GS, Scheithauer BW, et al. Multiple sclerosis: histopathologic and MR and/or CT correlation in 37 cases at biopsy and three cases at autopsy. Radiology 1991;180:467–74 [DOI] [PubMed] [Google Scholar]
- 11.Mastrostefano R, Occhipinti E, Bigotti G, et al. Multiple sclerosis plaque simulating cerebral tumor: case report and review of the literature. Neurosurgery 1987;21:244–46 [DOI] [PubMed] [Google Scholar]
- 12.Hunter SB, Ballinger WE Jr, Rubin JJ. Multiple sclerosis mimicking primary brain tumor. Arch Pathol Lab Med 1987;111:464–68 [PubMed] [Google Scholar]
- 13.Giang DW, Poduri KR, Eskin TA, et al. Multiple sclerosis masquerading as a mass lesion. Neuroradiology 1992;34:150–54 [DOI] [PubMed] [Google Scholar]
- 14.Silva HC, Callegaro D, Marchiori PE, et al. Magnetic resonance imaging in five patients with a tumefactive demyelinating lesion in the central nervous system. Arq Neuropsiquiatr 1999;57:921–26 [DOI] [PubMed] [Google Scholar]
- 15.Kalyan-Raman UP, Garwacki DJ, Elwood PW. Demyelinating disease of corpus callosum presenting as glioma on magnetic resonance scan: a case documented with pathological findings. Neurosurgery 1987;21:247–50 [DOI] [PubMed] [Google Scholar]
- 16.Dagher AP, Smirniotopoulos J. Tumefactive demyelinating lesions. Neuroradiology 1996;38:560–65 [DOI] [PubMed] [Google Scholar]
- 17.Ott D, Hennig J, Ernst T. Human brain tumors: assessment with in vivo proton MR spectroscopy. Radiology 1993;186:745–52 [DOI] [PubMed] [Google Scholar]
- 18.Fulham MJ, Bizzi A, Dietz MJ, et al. Mapping of brain tumor metabolites with proton MR spectroscopic imaging: clinical relevance. Radiology 1992;185:675–86 [DOI] [PubMed] [Google Scholar]
- 19.Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338:278–85 [DOI] [PubMed] [Google Scholar]
- 20.Arnold DL, Matthews PM, Francis GS, et al. Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol 1992;31:435–41 [DOI] [PubMed] [Google Scholar]
- 21.Davie CA, Hawkins CP, Barker GJ, et al. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain 1994;117:49–58 [DOI] [PubMed] [Google Scholar]
- 22.Silver NC, Barker RA, MacManus DG, et al. Proton magnetic resonance spectroscopy in a pathologically confirmed acute demyelinating lesion. J Neurol 1997;244:204–07 [DOI] [PubMed] [Google Scholar]
- 23.Chen CJ. Serial proton magnetic resonance spectroscopy in lesions of Balo concentric sclerosis. J Comput Assist Tomogr 2001;25:713–18 [DOI] [PubMed] [Google Scholar]
- 24.Bruhn H, Frahm J, Merboldt KD, et al. Multiple sclerosis in children: cerebral metabolic alterations monitored by localized proton magnetic resonance spectroscopy in vivo. Ann Neurol 1992;32:140–50 [DOI] [PubMed] [Google Scholar]
- 25.Simone IL, Tortorella C, Federico F, et al. Axonal damage in multiple sclerosis plaques: a combined magnetic resonance imaging and 1H-magnetic resonance spectroscopy study. J Neurol Sci 2001;182:143–50 [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Villegas D, Lenkinski RE, Wehrli SL, et al. Lactate production by human monocytes/macrophages determined by proton MR spectroscopy. Magn Res Med 1995;34:32–38 [DOI] [PubMed] [Google Scholar]
- 27.Krouwer HGJ, Kim TA, Rand SD, et al. Single voxel proton MR spectroscopy of nonneoplastic brain lesions suggestive of a neoplasm. AJNR Am J Neuroradiol 1998;19:1695–703 [PMC free article] [PubMed] [Google Scholar]
- 28.Grossman RI, Lenkinski RE, Ramer KN, et al. MR proton spectroscopy in multiple sclerosis. AJNR Am J Neuroradiol 1992;13:1535–43 [PMC free article] [PubMed] [Google Scholar]
- 29.Majos C, Julia-Sape M, Alonso J, et al. Brain tumor classification by proton MR spectroscopy: comparison of diagnostic accuracy at short and long TE. AJNR Am J Neuroradiol 2004;25:1696–704 [PMC free article] [PubMed] [Google Scholar]
- 30.Danielsen ER, Ross B. The clinical significance of metabolites. In: Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases. New York: Marcel Dekker;1999;23–43
- 31.Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1997;54:959–65 [DOI] [PubMed] [Google Scholar]
- 32.Schubert F, Gallinat J, Seifert F, et al. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage 2004;21:1762–71 [DOI] [PubMed] [Google Scholar]