Abstract
BACKGROUND AND PURPOSE: Endovascular treatment (EVT) of carotid cavernous fistulas (CCFs) is based on various techniques, mainly those using detachable balloons. Coronary covered stent grafts have been sporadically used in the intracranial arteries and only 2 traumatic CCFs have been reported in the literature; moreover, there is poor information about the long-term follow-up. We present 8 cases of CCFs treated by the placement of a covered stent, 5 of which have a 1-year clinical and angiographic follow-up.
METHODS: Eight patients with posttraumatic CCF were treated by positioning a covered stent in the intracranial internal carotid artery (ICA) to occlude the fistula. They received periodic clinical and angiographic follow-up to evaluate the patency and the stability of clinical results.
RESULTS: In all cases, the symptoms related to the CCF regressed after treatment and did not recur in the follow-up. Two patients presented residual filling of the CCF at the end of the procedure. The angiographic follow-up revealed in 6 patients of 7 a good patency of the ICA; in 1 patient, there was an ICA asymptomatic occlusion. One patient required transvenous coil occlusion of the cavernous sinus.
CONCLUSION: When standard treatments fail, covered stent grafts can be used as a valid alternative in the treatment of CCFs, but more data are needed, especially in the long-term follow-up.
Direct carotid cavernous fistulas (CCFs) are spontaneous or traumatic communication between the internal carotid artery and the cavernous sinus, classified by Barrow et al1 as type A CCFs. Among the various causes are head trauma, surgical trauma, fibromuscular dysplasia, arterial dissection, collagen deficiency syndromes, and rupture of internal carotid artery (ICA) cavernous aneurysms.2–5
Their clinical presentation is related to their size and to the type of venous drainage, which can lead to a variety of symptoms, such as visual loss, proptosis, bruit, chemosis, cranial nerve impairment, intracranial hemorrhage (rare), etc. Direct surgical closure, or ICA surgical ligation, has been now supplanted by endovascular methods, including occlusion of the ostium of the fistula with detachable balloons and transarterial or transvenous catheter coil embolization. Sacrifice of the ICA has been required in certain instances.5–8 In our institution, balloon occlusion of the fistula is the technique most commonly used because of a 30-year experience and because of a series of advantages: the balloon is easily flow-guided to the site of the fistula, repeated inflations and deflations of the balloon may help in understanding the underlying anatomy, and it is very cheap. Despite that, the use of detachable balloons at times suffers technical problems, such as early detachment/deflation of the balloon or occasional rupture of the balloon caused by its contact with bone fragments. It can also dilate the ostium of the fistula or the cavernous sinus itself, as in a procedure of balloon angioplasty, possibly causing a delayed recurrence.
In the 8 cases we present, we were unable to obtain a complete and permanent occlusion of the CCF with balloons alone, so we decided to try the placement of an endovascular stent graft before having to occlude the ICA.
Patients and Techniques
Between March 2002 and August 2005, 8 patients (5 male and 3 female, aged 14–70 years) who presented symptoms related to a posttraumatic CCF after severe head trauma were treated by positioning a Jostent coronary stent graft (previously JoMed, Helsingborg, Sweden; now Abbott Vascular, Redwood City, Calif), after failure at occluding the fistula with detachable balloons (Table).
Treatment of CCFs with covered stents and 6-month and 1-year follow-up
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| Sex/age | M/31y | M/23y | F/71y | F/45y | F/15y | M/31y | M/29y | M/35y |
| Indication | Ocular symptoms | Ocular symptoms | Ocular symptoms | Ocular symptoms | Ocular symptoms and venous drainage in the subarachnoid cerebral veins | Large dilation of the basilar venous plexus with compression of the midbrain requiring urgent treatment | Ocular symptoms | Recent worsening of ocular symptoms |
| Time between trauma and treatment | 2 months | 1 month | 7 months | 1 month | 1 month | 1 day | 11 days | 20 years |
| Location of the fistula* | 3 | 5 | 4 | 5 | 5 | 3,4,5 | 5 | 5 |
| U.P. | 3 | 2 | 2 | 3 | 1 | 1 | 1 | 1 |
| Stent size | 4/26 mm | 4/19 mm | 4/16 mm | 4.5/19 mm | 4/19 mm | 4/19 and 4/16 mm | 4.5/26 mm | 4.5/26 mm |
| Outcome | Complete occlusion of the CCF without complications | Complete occlusion of the CCF without complications. On day 2 angiography, a residual fistula was treated with a 5-mm angioplasty balloon | Complete occlusion of the CCF without complications | Complete occlusion of the CCF without complications | Complete occlusion of the CCF without complications | Complete occlusion of the CCF without complications related to the procedure | Near-complete occlusion of the CCF without complications | Near-complete occlusion of the CCF without complications. On day 2 angiography, a residual fistula was treated with a 5-mm angioplasty balloon |
| 6-month follow-up | Asymptomatic ICA occlusion | Good ICA patency | Good ICA patency | Good ICA patency | Intimal hyperplasia (30% stenosis) | Not available for follow-up because of death from multiorgan failure | (2 months) Persistence of the CCF that required transvenous coil occlusion of the cavernous sinus | (3 months) Moderate persistence of the CCF with regression of the ocular symptoms |
| 1-year follow-up | Stable result | Stable result | Stable result | Stable result | Stable result | NA | NA | NA |
According to Debrun ICA segmentation.44
Note:—y indicates years; U.P., unsuccessful procedures; ICA, internal carotid artery; AV, arteriovenous; NA, not available.
Three patients (cases 3, 4, and 6) presented bilateral CCFs, but only 1 CCF in each patient was treated with a covered stent, whereas the second regressed spontaneously in 1 case (case 4) or was occluded with detachable balloons (completely in case 3 and subtotally in case 6).
In all patients, CT and 4-vessel angiography were performed (Fig. 1). A CT scan revealed traumatic cerebral contusions and intracranial hemorrhages in all patients. Various attempts to exclude their CCFs using detachable balloons were unsuccessful because of technical problems and the characteristics of the fistula itself. Before performing a permanent occlusion of the ICA, we decided to try to deploy a covered stent.
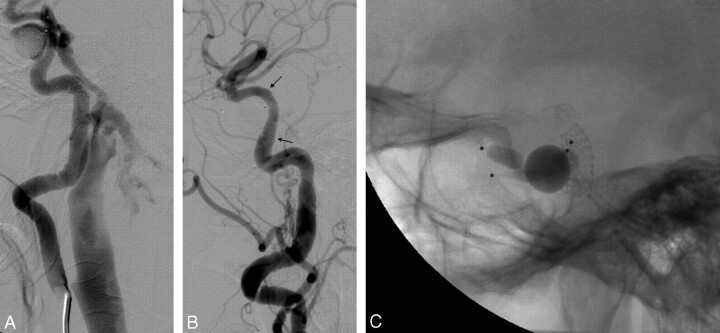
Fig 1.
Case 3. A and B, lateral right ICA angiograms show the CCF before (A) and after (B) treatment; arrows point to stent extremities.
C, lateral view showing the stent and 4 detachable balloons previously used; 2 balloons partially inflated were used in an attempt to occlude the right CCF, whereas the 2 deflated balloons occluded the left CCF 2 months earlier.
Case 6 required an urgent treatment during the second day after trauma because he had bilateral CCFs causing a large dilation of the basilar venous plexus, compressing the brain stem. He was referred to our center from a different hospital solely for the treatment of the CCF, and he was then transferred back to his hospital at the end of the procedure. A first attempt to occlude both fistulas with detachable balloons obtained partial results, without resolution of the venous dilation. Therefore, we decided to occlude the larger CCF with a covered stent; the treatment required 2 covered stents because of multiple tears in the cavernous ICA, but it was effective in deflating the basilar venous plexus.
All stent-graft placement procedures were performed via a transfemoral approach under general anesthesia. All procedures were performed in a dedicated monoplane or biplane neuroangiography suite Integris Allura (Philips Medical Systems, Best, the Netherlands).
We obtained angiographic series in all CCF patients, injecting the contralateral ICA and a vertebral artery while hand compressing the neck at the injured side, as part of the preliminary study of the lesion. In all cases in this series, there was very good collateral flow, allowing carotid occlusion, if needed.
Systemic heparinization was administrated intravenously at the beginning of the interventional procedure (a bolus of 5000 IU) and was continued for 48 hours with a flow rate of 1000 IU/h.
Guiding catheters (7F or 8F) were placed in the ICA. In all 8 cases, an Excel 14 microcatheter (Boston Scientific, Natick, Mass) was navigated distal to the fistula into a branch of the middle cerebral artery (MCA). The microguidewire was then replaced with a 0.014-inch, 300-cm exchange guidewire HT-BMW (ACS Guidant, Santa Clara, Calif) or Transend ES (Boston Scientific), so that the Jostent coronary stent could reach the rupture site of the ICA. After careful angiographic confirmation of the correct position, the stent was released, inflating slowly up to 8–10 atm. Postdilation was often needed to completely exclude the lesion using a coronary balloon of a larger diameter. At the end of the procedures, the covered stents were correctly apposed to the ICA wall. In all cases, we had periprocedural spasm of the ICA, because of the stiffness of the system, for which no medical treatment was needed.
According to our protocol for covered stent placement in traumatic CCFs, immediately after the procedure, an anticoagulant and a double platelet-inhibiting therapy (ticlopidine or clopidogrel and aspirin) was prescribed. Anticoagulant therapy (heparin 1000 IU/h) was discontinued after 48 hours, whereas ticlopidine (250 mg twice a day) or clopidogrel (75 mg daily) was discontinued after 3 months, and the patients were left on aspirin alone (100–200 mg daily).
All patients were neurologically evaluated by experienced neurologists in the days before their discharge and than periodically during the follow-up.
Results
Final angiograms at the end of the procedure confirmed total exclusion of the fistula and normal patency of the ICA in all patients (Fig 1) except cases 7 and 8; they had very large ICAs, and the covered stents could not occlude the fistulas despite postdilation with coronary balloons, resulting in a minimal residual filling of the cavernous sinus from the ICA.
No thromboembolic complications occurred in any patients. On the day after the procedure, a confirmatory angiogram was performed in all patients; patient 6 had his confirmatory angiogram 6 days later in the other hospital, confirming complete occlusion of the CCF treated with the grafts.
In case 2, on the second-day confirmatory angiography, there was a residual arterial flow into the cavernous sinus. A 5-mm angioplasty balloon was then inflated to 10 atm to further dilate the stent with complete and persistent occlusion of the fistula and related symptoms.
The patient in case 7 underwent a confirmatory angiography 2 months after the covered stent placement because of a persistent flow into the fistula. Stent dilation with 5- and 6-mm angioplasty balloons resulted in only temporary occlusion of the fistula, so we decided to occlude the cavernous sinus by transvenous coil embolization.
Patient 8, on the second-day confirmatory angiography, presented a remaining flow into the fistula that required dilation of the stent with a 5-mm angioplasty balloon with an improved result but incomplete occlusion of the fistula (Fig 2). Two months later, a confirmatory angiography showed a reduction of flow in the fistula, with normalization of eye chemosis and proptosis.
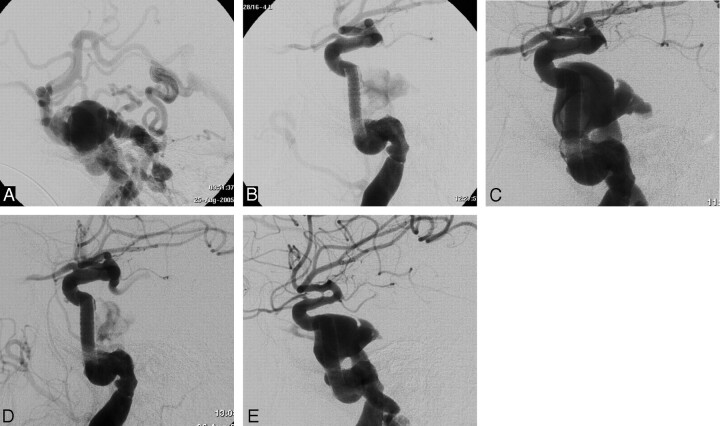
Fig 2.
Case 8. A, Lateral angiogram of the ICA showing total steal of the flow toward the cavernous sinus; vertebral and contralateral ICA angiographies (not reported) showed steal also from posterior and contralateral circulation.
B, Lateral ICA angiogram at the end of the stent-graft placement procedure showing nearly complete occlusion of the CCF.
C and D, ICA angiogram in lateral projection of day 2 after treatment showing partial reopening of the fistula (C) and the result after angioplasty with a coronary balloon.
E, Lateral ICA angiogram 3 months later showing persistence of the fistula with regularization of intracranial hemispheric circulation (note different diameter of distal ICA and posterior communicating artery between D and E).
After the procedure, symptoms improved in all patients; CCFs were completely occluded and eventually resolved, with no new symptoms related to possible periprocedural complications. Patient 6 died 2 weeks after the procedure of post-traumatic multiorgan failure. Patient 1 developed an asymptomatic occlusion of the left ICA with optimal collateral blood flow from the contralateral ICA at the 1-month angiographic follow-up.
At the 6-month and 1-year follow-up examinations, there were no changes in the angiographic and neurologic features in the other cases, except an asymptomatic 30% in-stent stenosis caused by intimal hyperplasia in case 5 (Fig 3), which seemed to have improved at the 1-year follow-up. During the follow-up period, no clinical symptoms were related to thromboembolic events caused by the presence of the stent graft.
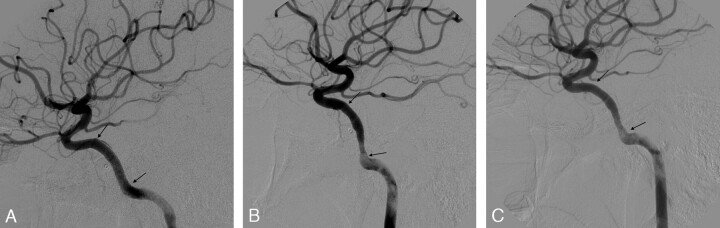
Fig 3.
Case 5. Lateral angiograms show the postprocedural occlusion of the CCF (A) and the intimal hyperplasia causing a 30% reduction of the ICA lumen at the 6-month follow-up (B), with a possible improvement at the 1-year follow-up (C). Arrows point to the stent extremities. The marker of the previously released balloon is visible anterior to the ascending segment of the carotid siphon.
Discussion
Despite the enormous progress in endovascular techniques in the past few decades, the first treatment for CCFs in many centers around the world remains the occlusion of the fistula with detachable balloons.7–14 Transarterial or transvenous coil occlusion of CCFs is also commonly used because many interventional neuroradiologists are more familiar with the use of coils than of detachable balloons and also because balloons are not available everywhere; however, coil treatment is not always safe and effective and has high costs.7, 15, 16
In the past decade, the endoluminal placement of endovascular stent grafts became established as an effective alternative to most surgical repair techniques in the aorta, peripheral and visceral arteries.17–21 Cardiologic covered stents are now commonly used in coronary artery stenosis and bypass ruptures.22, 23 No covered stent is dedicated to intracranial arteries, but there are some sporadic reports that describe the use of coronary covered stents in the neurovascular field.24–35
Stents covered with polytetrafluoroethylene (PTFE) have been used with success in the treatment of pathologic conditions of the coronary arteries because of their low rate of stent-related coronary stenosis and acute stent thrombosis preventing debris protrusion and neointimal proliferation through the stent.36, 37 The main problem in the placement of this type of stent within the ICA is the stiffness of its profile; it is composed of the superposition of 2 stainless steel stents and an ultrathin PTFE layer between them. This rigid configuration does not permit the easy navigation of the stent in the tortuous ICA and can be the cause of periprocedural spasm or dissection. Arterial spasm is a common complication during endovascular procedures, especially when using stiff materials. Other factors in determining vasospasm may be young age and increased arterial reactivity after head injuries. In most cases, the arterial spasm is not clinically relevant, and it resolves spontaneously within a few minutes, but it can also be treated with intra-arterial infusion of nimodipine.
The rigidity of Jostent coronary stent grafts is a well-known limitation of this device among interventional neuroradiologists, but currently no perfect solution for the treatment of CCFs exists. No manufacturer produces covered stents designed or approved for neurovascular use. Cardiovasc Inc (Menlo Park, Calif) has introduced a new coronary stent graft composed of a single steel stent with its extremities folded up to hold the PTFE layer. In our preliminary, unpublished experience with the Cardiovasc Stent Graft, we had the impression of a lower rigidity of the system and an easier progression in the intracranial ICA.
Another important issue with the use of PTFE-covered stents is the short- and long-term patency of the artery. Some reports conclude that PTFE-covered stents reduce the risk of vessel occlusion.22, 23 Although the long-term patency rate of stent grafts in the cerebral arteries remains unknown, some evidence encourages expectation of positive long-term outcome in terms of parent artery patency.30, 33 Moreover, Krings et al38 reported lower in-stent intimal hyperplasia in covered stents than in bare stents in experimentally induced aneurysms. The PTFE layer might reduce the rate of neointimal hyperplasia and stent-related stenosis by inhibiting the migration of inflammatory cells and by attenuating the diffusion of cytokines.35, 36, 39, 40 In contrast, polyethylene terephthalate- and silicone-covered stents have been shown to have poor short-term patency rates because of acute inflammation and exuberant ingrowths of fibrous connective tissue.41–43
When placing covered stent grafts for the treatment of intracranial aneurysms, our anticoagulant and antiplatelet protocol consists of starting double antiplatelet therapy (aspirin and ticlopidine or clopidogrel) 4 days before the procedure and heparin at the beginning of the treatment. Heparin is discontinued 48 hours after the end of the procedure and double antiplatelet is maintained for 2–3 months, followed by aspirin only for 1 year. Similar protocols are used with good results by other authors.30, 33 However in posttraumatic CCFs, we prefer not to start antiplatelet medication before positioning the stent graft because we frequently do a last attempt at occluding the fistula with a detachable balloon during the same session and because a venous drainage through subarachnoid veins is often present with an increased risk of hemorrhage.
We had only 1 case of asymptomatic artery occlusion, in our opinion a result of discontinuation of the antiplatelet therapy. Only 1 case of ICA occlusion after intracranial covered stent placement has been described in the literature so far. Redekop et al35 reported a case of asymptomatic ICA occlusion 1 week after covered stent placement for a posttraumatic petrocavernous ICA pseudoaneurysm. This patient had a distal MCA pseudoaneurysm with high risk of hemorrhage; therefore, after the procedure, heparin therapy was discontinued and single-agent antiplatelet (aspirin 325 mg daily) was initiated.
In case 5, at the 6-month follow-up, we observed an in-stent lumen reduction of 30%, probably due to intimal hyperplasia, that looked improved at the 1-year follow-up and was not responsible for any clinical symptoms. Therefore, we think that an antiplatelet therapy with aspirin and clopidogrel (or ticlopidine) permits good long-term patency results. In-stent stenosis, in intracranial arteries treated with covered stents, has previously been reported in the literature for 2 patients by Islak et al31 and Saatci et al,33 both without any hemodynamic or clinical effect.
In patients 7 and 8, the ICA at the ostium of the CCF exceeded the maximal stent diameter, and there was residual flow between the vessel wall and the stent graft, so we obtained only a slowing of the fistula, not its permanent occlusion. To our knowledge, this is the first report of a similar problem with a covered stent. Moreover, patient 7 required transvenous coil embolization of the cavernous sinus. The largest diameter reached by coronary stent grafts now available is 5 mm; the ICA diameter is usually less than 5 mm, but high-flow, long-standing lesions can widen the ICA. In these situations, endovascular occlusion of CCFs is frequently difficult because large tears of the ICA prevent correct and stable positioning of detachable balloons, and large cavernous sinuses cannot be easily occluded with coils. These CCFs are often treated in various sessions with various devices,7 and stent grafts can be useful in slowing down CCF flow. However larger and self-expandable neurovascular covered stent grafts could be the real solution for high-flow, long-standing CCFs.
Although there are several reports of covered stents used in the intracranial vasculature, only 8 CCFs were reported to be treated with covered stents7, 29, 30, 32, 35; of those, only 2 were posttraumatic.30 Felber et al30 reported the use of a Jostent covered stent in intracranial arteries in 7 patients; of those, 2 had posttraumatic CCFs. For these 2 patients (patients 3 and 4 in their series), they reported the complete occlusion of the fistula without complications and with durable results, respectively, at 14 and 11 months.
We report a series of 8 posttraumatic CCFs treated with covered stents. Our results are in line with those reported in the literature and focus on the anticoagulant and antiplatelet therapy to avoid the in-stent thrombosis. Unfortunately, posttraumatic patients could have a higher risk of hemorrhage, and anticoagulant and antiplatelet therapies might often be contraindicated. On the other hand, CCF symptoms frequently appear not immediately after the trauma but after a few days, when the hemorrhagic risk is decreased. Problems can be encountered in aggressive CCFs that require urgent treatment.
Because of the very small number of reports of posttraumatic CCFs treated with covered stents, larger series are necessary to have more reliable results, while we wait for a stent graft developed for neurovascular use. Our clinical results show good outcome, with no clinical complication; in 2 patients, covered stents did not occlude completely the fistula but slowed down the flow with clinical improvement. Furthermore, with the use of the covered stent, we did not have to overdistend or sacrifice the cavernous sinus, possibly limiting damage to the cranial nerves.
Conclusions
Covered stent grafts can sometimes be used as a valid alternative in the treatment of traumatic CCFs, when more standard treatments fail and the anatomy of the ICA is favorable. We believe that our results and follow-up are encouraging for their further use. This has to be done with caution both because of possible periprocedural complications and because of little understanding of long-term follow-up.
References
- 1.Barrow DL, Spector RH, Braun IF, et al. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg 1985;62:248–56 [DOI] [PubMed] [Google Scholar]
- 2.Hoops JP, Rudolph G, Schriever S, et al. [Dural carotid-cavernous sinus fistulas: clinical aspects, diagnosis and therapeutic intervention]. Klin Monatsbl Augenheilkd 1997;210:392–97 [DOI] [PubMed] [Google Scholar]
- 3.Lemaitre J, Jamart S, Lefranc F, et al. [Post-traumatic carotid-cavernous sinus fistula]. Rev Med Brux 1999;20:91–94 [PubMed] [Google Scholar]
- 4.Chuman H, Trobe JD, Petty EM, et al. Spontaneous direct carotid-cavernous fistula in Ehlers-Danlos syndrome type IV: two case reports and a review of the literature. J Neuroophthalmol 2002;22:75–81 [DOI] [PubMed] [Google Scholar]
- 5.Fages-Caravaca EM, Tembl-Ferrairo JI, Lago-Martin A, et al. [Direct carotid cavernous fistulas: endovascular treatment using a detachable balloon]. Rev Neurol 2001;33:533–36 [PubMed] [Google Scholar]
- 6.Boccardi E, Ditchfield A, Valvassori L. Arteriovenous fistulas of intracranial dural sinuses. In: Byrne J, ed. Interventional Neuroradiology. Oxford: Oxford University Press,2002. :155–77
- 7.Luo CB, Teng MM, Yen DH, et al. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma 2004;56:1214–20 [DOI] [PubMed] [Google Scholar]
- 8.Lewis AI, Tomsick TA, Tew JM Jr, et al. Long-term results in direct carotid-cavernous fistulas after treatment with detachable balloons. J Neurosurg 1996;84:400–04 [DOI] [PubMed] [Google Scholar]
- 9.Prolo DJ, Hanbery JW. Intraluminal occlusion of a carotid-cavernous sinus fistula with a balloon catheter. Technical note. J Neurosurg 1971;35:237–42 [DOI] [PubMed] [Google Scholar]
- 10.Serbinenko FA. [Reconstruction of the cavernous section of the carotid artery in carotid-cavernous anastomosis]. Vopr Neirokhir 1972;36:3–8 [PubMed] [Google Scholar]
- 11.Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg 1990;72:857–63 [DOI] [PubMed] [Google Scholar]
- 12.Fattahi TT, Brandt MT, Jenkins WS, et al. Traumatic carotid-cavernous fistula: pathophysiology and treatment. J Craniofac Surg 2003;14:240–46 [DOI] [PubMed] [Google Scholar]
- 13.Lewis AI, Tomsick TA, Tew JM Jr. Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery 1995;36:239–44 [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi N, Miyachi S, Negoro M, et al. Endovascular treatment strategy for direct carotid-cavernous fistulas resulting from rupture of intracavernous carotid aneurysms. AJNR Am J Neuroradiol 2003;24:1789–96 [PMC free article] [PubMed] [Google Scholar]
- 15.Klisch J, Huppertz HJ, Spetzger U, et al. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: results for 31 patients and review of the literature. Neurosurgery 2003;53:836–56 [DOI] [PubMed] [Google Scholar]
- 16.Remonda L, Frigerio SB, Buhler R, et al. Transvenous coil treatment of a type A carotid cavernous fistula in association with transarterial trispan coil protection. AJNR Am J Neuroradiol 2004;25:611–13 [PMC free article] [PubMed] [Google Scholar]
- 17.Chuter TA, Donayre C, Wendt G. Bifurcated stent-grafts for endovascular repair of abdominal aortic aneurysm. Preliminary case reports. Surg Endosc 1994;8:800–02 [DOI] [PubMed] [Google Scholar]
- 18.Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994;331:1729–34 [DOI] [PubMed] [Google Scholar]
- 19.Kocher M, Utikal P, Koutna J, et al. Endovascular treatment of abdominal aortic aneurysms–6 years of experience with Ella stent-graft system. Eur J Radiol 2004;51:181–88 [DOI] [PubMed] [Google Scholar]
- 20.Ogino H, Banno T, Sato Y, et al. Superior mesenteric artery stent-graft placement in a patient with pseudoaneurysm developing from a pancreatic pseudocyst. Cardiovasc Intervent Radiol 2004;27:68–70 [DOI] [PubMed] [Google Scholar]
- 21.Bartorelli AL, Trabattoni D, Reali M. Percutaneous obliteration of an iatrogenic pseudoaneurysm of the right subclavian artery with a PTFE-covered stent-graft. Int J Cardiovasc Intervent 2001;4:195–96 [DOI] [PubMed] [Google Scholar]
- 22.Briguori C, Sarais C, Sivieri G, et al. Polytetrafluoroethylene-covered stent and coronary artery aneurysms. Catheter Cardiovasc Interv 2002;55:326–30 [DOI] [PubMed] [Google Scholar]
- 23.Elsner M, Auch-Schwelk W, Britten M, et al. Coronary stent grafts covered by a polytetrafluoroethylene membrane. Am J Cardiol 1999;84:335–38, [DOI] [PubMed] [Google Scholar]
- 24.Alexander MJ, Smith TP, Tucci DL. Treatment of an iatrogenic petrous carotid artery pseudoaneurysm with a Symbiot covered stent: technical case report. Neurosurgery 2002;50:658–6211841739 [Google Scholar]
- 25.Auyeung KM, Lui WM, Chow LC, et al. Massive epistaxis related to petrous carotid artery pseudoaneurysm after radiation therapy: emergency treatment with covered stent in two cases. AJNR Am J Neuroradiol 2003;24:1449–52 [PMC free article] [PubMed] [Google Scholar]
- 26.Blasco J, Macho JM, Burrel M, et al. Endovascular treatment of a giant intracranial aneurysm with a stent-graft. J Vasc Interv Radiol 2004;15:1145–49 [DOI] [PubMed] [Google Scholar]
- 27.Burbelko MA, Dzyak LA, Zorin NA, et al. Stent-graft placement for wide-neck aneurysm of the vertebrobasilar junction. AJNR Am J Neuroradiol 2004;25:608–10 [PMC free article] [PubMed] [Google Scholar]
- 28.Chiaradio JC, Guzman L, Padilla L, et al. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery: technical case report. Neurosurgery 2002;50:213–16 [DOI] [PubMed] [Google Scholar]
- 29.de Souza JM, Domingues FS, Espinosa G, et al. Cavernous carotid artery pseudo-aneurysm treated by stenting in acromegalic patient. Arq Neuropsiquiatr 2003;61:459–62 [DOI] [PubMed] [Google Scholar]
- 30.Felber S, Henkes H, Weber W, et al. Treatment of extracranial and intracranial aneurysms and arteriovenous fistulae using stent grafts. Neurosurgery 2004;55:631–38 [DOI] [PubMed] [Google Scholar]
- 31.Islak C, Kocer N, Albayram S, et al. Bare stent-graft technique: a new method of endoluminal vascular reconstruction for the treatment of giant and fusiform aneurysms. AJNR Am J Neuroradiol 2002;23:1589–95 [PMC free article] [PubMed] [Google Scholar]
- 32.Kocer N, Kizilkilic O, Albayram S, et al. Treatment of iatrogenic internal carotid artery laceration and carotid cavernous fistula with endovascular stent-graft placement. AJNR Am J Neuroradiol 2002;23:442–46 [PMC free article] [PubMed] [Google Scholar]
- 33.Saatci I, Cekirge HS, Ozturk MH, et al. Treatment of internal carotid artery aneurysms with a covered stent: experience in 24 patients with mid-term follow-up results. AJNR Am J Neuroradiol 2004;25:1742–49 [PMC free article] [PubMed] [Google Scholar]
- 34.Vanninen RL, Manninen HI, Rinne J. Intrasellar iatrogenic carotid pseudoaneurysm: endovascular treatment with a polytetrafluoroethylene-covered stent. Cardiovasc Intervent Radiol 2003;26:298–301 [DOI] [PubMed] [Google Scholar]
- 35.Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg 2001;95:412–19 [DOI] [PubMed] [Google Scholar]
- 36.Lukito G, Vandergoten P, Jaspers L, et al. Six months clinical, angiographic, and IVUS follow-up after PTFE graft stent implantation in native coronary arteries. Acta Cardiol 2000;55:255–60 [DOI] [PubMed] [Google Scholar]
- 37.Yuan JG, Ohki T, Marin ML, et al. The effect of nonporous PTFE-covered stents on intimal hyperplasia following balloon arterial injury in minipigs. J Endovasc Surg 1998;5:349–58 [DOI] [PubMed] [Google Scholar]
- 38.Krings T, Hans FJ, Moller-Hartmann W, et al. Treatment of experimentally induced aneurysms with stents 2. Neurosurgery 2005;56:1347–59 [DOI] [PubMed] [Google Scholar]
- 39.Sovik E, Klow NE, Brekke M, et al. Elective placement of covered stents in native coronary arteries. Acta Radiol 2003;44:294–301 [DOI] [PubMed] [Google Scholar]
- 40.Gercken U, Lansky AJ, Buellesfeld L, et al. Results of the Jostent coronary stent graft implantation in various clinical settings: procedural and follow-up results. Catheter Cardiovasc Interv 2002;56:353–60 [DOI] [PubMed] [Google Scholar]
- 41.Ahmadi R, Schillinger M, Maca T, et al. Femoropopliteal arteries: immediate and long-term results with a Dacron-covered stent-graft. Radiology 2002;223:345–50 [DOI] [PubMed] [Google Scholar]
- 42.Geremia G, Bakon M, Brennecke L, et al. Experimental arteriovenous fistulas: treatment with silicone-covered metallic stents. AJNR Am J Neuroradiol 1997;18:271–77 [PMC free article] [PubMed] [Google Scholar]
- 43.Link J, Feyerabend B, Grabener M, et al. Dacron-covered stent-grafts for the percutaneous treatment of carotid aneurysms: effectiveness and biocompatibility—experimental study in swine. Radiology 1996;200:397–401 [DOI] [PubMed] [Google Scholar]
- 44.Debrun G, Lacour P, Vinuela F, et al. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg 1981;55:678–92 [DOI] [PubMed] [Google Scholar]