Abstract
BACKGROUND AND PURPOSE: Visualizing with MR imaging and obtaining quantitative indexes of degeneration of the substantia nigra in Parkinson disease have been long-sought goals. We investigated the potential role of area and T1 contrast measurements in differentiating patients from controls and their age-related changes.
METHODS: Eight patients with Parkinson disease, 8 age-matched controls, and 8 young controls were imaged. We obtained the pixel-wise difference between 2 sets of inversion-recovery images, acquired parallel to the bicommissural plane, with different inversion times. Pixel-intensity ratios between lateral and medial nigral regions, and nigral area and substantia-nigra/midbrain area ratios were computed.
RESULTS: Compared with that of controls, loss of substantia nigra was evident in patients, its borders taking a smoother and more irregular appearance. Patients were characterized by a lateral-to-medial gradient, due to reduced hypointensity of the lateral portion of the substantia nigra and relative sparing of its medial portion. The visible nigral area was significantly smaller in patients compared with matched controls (P = .04). The substantia nigra/midbrain area ratio enabled considerably better separation (P = .0001). The lateral/medial pixel-intensity ratio was significantly higher in patients compared with matched controls (P = .01) and in young controls compared with age-matched controls (P = .01).
CONCLUSION: Inversion-recovery sequences may provide a convenient way to visualize nigral degeneration. Relative area and pixel-intensity measurements may integrate other techniques (such as diffusion-tensor imaging on nigrostriatal pathways) in the neuroradiologic diagnosis and follow-up of Parkinson disease by quantitatively assessing the degeneration of the substantia nigra.
Parkinson disease is common, involving degeneration of dopaminergic neurons in the substantia nigra. Incidence increases with age; therefore, this increase results in an expanding burden on the health care systems of aging Western societies.
From a neuroradiologic point of view, diagnosis and staging of Parkinson disease has traditionally proved challenging because standard T1, T2, and proton-density sequences do not show disease-specific changes.1 At the present time, the most sensitive imaging techniques for the early diagnosis of Parkinson disease are positron-emission tomography (PET) and single-photon emission tomography (SPECT).2 However, even though PET and SPECT have demonstrated superior sensitivity to disease stage (and may hold a promise for use in the preclinical stage), compared with MR imaging, their availability is presently limited and the cost of each imaging session is higher.2
During recent years, a range of techniques has been proposed for MR imaging of Parkinson disease, falling broadly into 4 groups. The first group is based on measurement of the T2 and T2* relaxation times, which are known to change because of increased iron deposition occurring in the basal ganglia in Parkinson disease. Ordidge et al3 and Gorell et al4 demonstrated significant differences due to increased nigral iron content in patients, and subsequently Graham et al5 reported similar findings, obtained by using a specific MR imaging sequence.
The second group is based on measuring the nigral area and area ratios on the basis of T2- and proton-density–weighted images; Oikawa et al6 reported no significant differences in nigral thickness as measured on the resulting images. A similar approach, based on delineating the nigral borders on the basis of surrounding fibers on diffusion-weighted MR imaging (DWI), has been investigated by Adachi et al,7 who reported no significant area differences.
The third group is based on diffusion-tensor imaging measurements, particularly mean diffusivity and fractional anisotropy; promising results have been obtained differentiating controls, patients with Parkinson disease, and patients with progressive supranuclear palsy.8–10 However, these results have been obtained through measurements on structures of the basal ganglia and their connections and do not necessarily reflect the degree of degeneration of the substantia nigra itself.
The fourth group is based on changes in the spin-lattice relaxation time (T1), which, as discussed by Hutchinson et al11 and Hutchinson and Raff, 12,13 seem to accompany nigral degeneration because of degenerative changes in the intracellular compartment and in iron deposition.
Inversion-recovery sequences are a convenient and widely available way to obtain images whose contrast heavily depends on the spin-lattice relaxation time (T1) of tissues. Hutchinson et al11 and Hutchinson and Raff 12,13 demonstrated good separation between controls and patients with Parkinson disease by using a combination of inversion-recovery sequences, but their work received seemingly limited attention. We set out to replicate their findings and to compare different approaches for the analysis of the resulting inversion-recovery images.
Subjects and Methods
To assess sensitivity with respect to pathologic and age-related changes, we defined 3 groups: patients (PD), age-matched controls (MC), and young controls (YC). Written informed consent was obtained from all subjects according to institutionally approved procedures, regulations, and forms.
Each group included 3 women and 5 men. A clinical diagnosis of probable Parkinson disease was established in the PD group by a senior neurologist according to established criteria.14 MC and YC groups were screened for absence of clinical and neuroradiologic evidence of neurologic pathology and for the absence of any first relative with the diagnosis of extrapyramidal syndrome. In the PD, MC, and YC groups, mean age was, respectively, 66.5 ± 5.0 years, 63.6 ± 7.3 years, and 28.5 ± 3.3 years. Given the exploratory nature of the present study, patients were scanned as a part of their clinical follow-up while they were on their usual medications. Given that the Unified Parkinson Disease Rating Scale (UPDRS) score as determined under this condition may not be representative of disease severity, patients were characterized by disease duration, which was 2.8 ± 1.2 years (range, 2–5 years). As examined, all patients matched the criteria for disease stage II on the Hoehn and Yahr scale15—that is bilateral disease without postural instability.
Scans were obtained on a Magnetom Avanto 1.5T system (Siemens, Erlangen, Germany). Similar to the work of Hutchinson et al11 and Hutchinson and Raff,12,13 we used 2 inversion-recovery sequences, denoted respectively as white-matter-suppressed (WMS) and gray-matter-suppressed (GMS), whose parameters were adjusted to minimize signal intensity from deep brain white matter and gray matter in the MC group. Hutchinson et al11 and Hutchinson and Raff 12,13 reported using TI = 420 ms, TE = 20 ms, and TR = 2000 ms for the GMS sequence; and TI = 250 ms, TE = 20 ms, and TR = 1450 ms for the WMS sequence.11–13 For reasons that require further verification, using these parameters on controls and patients failed to deliver the expected contrast between the substantia nigra and surrounding structures with our experimental setup. We thereafter empirically determined (by successive adjustments of the TI) TI = 475 ms, TE = 12 ms, TR = 3000 ms; and TI = 240 ms, TE = 12 ms, and TR = 3000 ms as the parameters, respectively, minimizing the signal intensity from nigral gray matter and from deep brain white matter in MC.
The MR signal intensity was modulus-reconstructed; as a consequence, all T1 changes (both lengthening and shortening) with respect to values found in MC resulted in signal-intensity increase.16
Matrix size was set to 192 × 256; FOV, to 192 × 256 mm; and section thickness, to 1.5 mm, obtaining 1 × 1 × 1.5 mm voxels. To minimize positioning variability, we aligned sections with the bicommissural plane. The intersection gap was set to 0.15 mm, and interleaved acquisition was used. Six excitations were performed for both sequences. Turbo spin-echo sequences were used, the echo-train length was set to 15, and no parallel imaging was performed. Subjects were imaged with a receive-only head coil. Twelve sections were acquired, and the total acquisition time was approximately 7 minutes.
Preliminary visual analyses indicated that the subtraction images enabled better delineation of the substantia nigra compared with the GMS images alone and that they appeared to be less affected by noise than the ratio images used in previous studies.11–13 Therefore, all measurements and visual analyses were conducted on the image obtained by pixel-wise subtraction of the WMS image from the GMS image (GMS-WMS), interpolated to 384 × 512 to improve region-of-interest drawing accuracy. Image quality (eg, absence of motion during and between the 2 sequences) was checked after each acquisition. A senior neuroradiologist, blinded to patient names, birth dates, and groups, drew regions of interest on the images. All image processing was performed by using the ImageJ program (National Institutes of Health, Bethesda, Md).
Only midbrain sections superior to the decussation of the superior cerebellar peduncles and on which the borders of the substantia nigra could be satisfactorily identified (as judged by the blinded neuroradiologist) were included in the analysis. The upper limit of the included sections was at the level at which the anterior lateral midbrain outline (ie, the profile of the cerebral peduncle) was no longer clearly identifiable (at the level at which the cerebral peduncles merge with the base of the brain).
The microscopic brain stem atlas of Olszewski and Baxter17 and the stereotaxic atlas of Duvernoy and Vannson18 were used as reference throughout the whole study. Furthermore, coronal and horizontal plates from the diencephalon atlas of Van Buren and Borke19 served as reference to ensure that the subthalamic nucleus was not included in the sections used for analysis.
Considering the difficulty of separating the pars compacta and the pars reticulata, we decided to include in the nigral region of interest the substantia nigra as a whole, appearing as a hypointense area on the subtraction image. The cerebral peduncles, appearing bright on the subtraction image, were taken as the anterior-lateral limit of the substantia nigra.
Although the extension of the substantia nigra is relatively easy to delineate in healthy individuals, it is difficult to define in patients because of the diffuse nature of degeneration. Because the relationship between loss of dopaminergic neurons and T1 relaxation time is not straightforward, the purpose of the present study was not to measure the actual extension of the residual substantia nigra but, more simply, to measure the extension of its portion having a T1 similar to that found in healthy individuals.
To overcome the difficulties associated with the fact that nigral borders can have a smooth appearance in patients, the neuroradiologist visually determined, as reference for region-of-interest drawing, a level approximately half-way between the pixel intensity of the visible substantia nigra and that of surrounding structures. The overall extension of the visible substantia nigra was considered for the region-of-interest drawing, regardless of the presence of focal hyperintense spots within its area.
As represented in Fig 1A, to test the hypothesis that the nigral area is significantly reduced in patients, we drew regions of interest by using the criteria defined previously, including the full extension of the substantia nigra characterized by a signal intensity similar to that of MC.
Fig 1.
Positioning of the region of interest (ROI) for area measurement, drawn on the visible substantia nigra (A), and of the ROI for relative contrast measurement, drawn including the whole expected extension of the substantia nigra (B).
Because considerable variability is found in midbrain size among individuals, a further region of interest was placed on each section, including the whole midbrain, with the purpose of serving as a reference for relative area measurements.
As represented in Fig 1B, to test the hypothesis that lateral-to-medial nigral contrast is significantly different in patients and controls because of the gradient of degeneration, 2 regions of interest were placed on each section, on each side, evenly dividing the whole extension of the substantia nigra as would be expected from known anatomy (rather than the extension of the substantia nigra with unaltered signal intensity), in a lateral and medial region.
For all measurements performed, a one-way analysis of variance (ANOVA) was used to evaluate group differences, followed by a 2-tailed unpaired t test between the PD group and the MC group, and between the MC group and the YC group. Given the small sample size and that all patients were classified as having the same disease stage on the Hoehn and Yahr scale,15 correlation of measurements with disease duration and stage was not investigated in this study.
Results
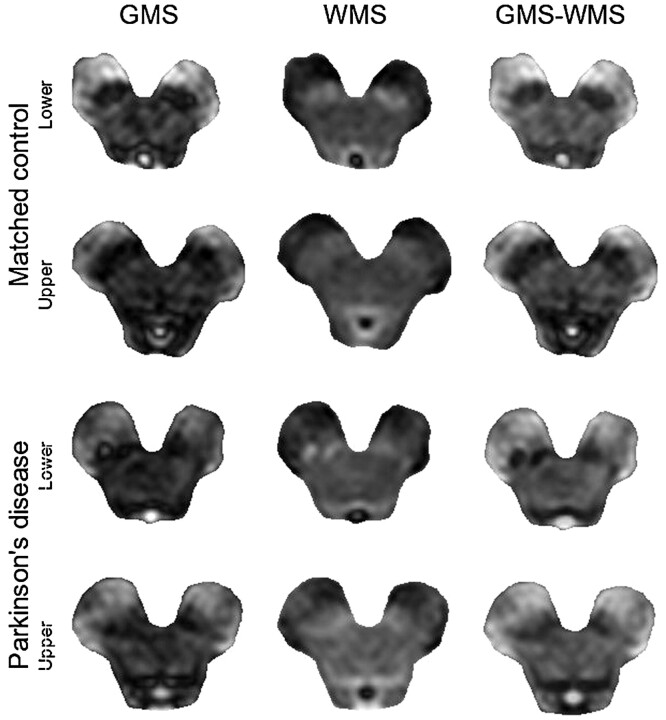
In all control subjects, the substantia nigra appeared as a hypointense area on the subtraction images, whose morphology closely matched known midbrain anatomy. On these images, we were unable to distinguish the substantia nigra pars compacta and the pars reticulata.17 Figure 2 depicts 2 central sections from an MC and from a PD. The outlines of other midbrain nuclei were not clearly visible, either on the raw images or on the subtraction images.
Fig 2.
Appearance of GMS (left column), WMS (middle column), and difference images (GMS-WMS, right column) from an MC (2 upper rows) and a PD (2 lower rows). Reduced hypointensity of the substantia nigra is evident on the difference images from the patient.
The anterior lateral margin of the substantia nigra was more sharply delineated than its posterior medial margin because the contrast with the cerebral peduncle was generally higher than the contrast with the midbrain tegmentum. Compared with those of the controls, the borders of the substantia nigra appeared smoother and more irregular in patients, especially in its lateral part. Furthermore, focal hyperintensities surrounded by areas of relatively unaltered nigral signal intensity could be seen in some patients.
The PD group was characterized by a lateral-to-medial nigral intensity gradient due to hyperintensity of the lateral portion of the substantia nigra and relative sparing of its medial portion. PD provided an average of 2.8 usable sections, whereas both MC and YC provided an average of 4.2 usable sections.
In the PD group, on each section parallel to the bicommissural plane, the area of residual substantia nigra was 36.2 ± 13.7 mm2 on the left side and 35.9 ± 14.8 mm2 on the right side. After combining left and right sides, we determined that the total area of the substantia nigra was 72.2 ± 27.4 mm2 in the PD group, 88.8 ± 28.7 mm2 in the MC group, and 91.8 ± 29.4 mm2 in the YC group. The ANOVA indicated the presence of significant differences among groups (P = .04), and the t test P value was .04 for the comparison between the PD and MC groups and .68 for the comparison between the MC and YC groups.
The average area of the midbrain was 5.9 ± 1.1 cm2 in the PD group, 5.8 ± 1.3 cm2 in the MC group, and 5.7 ± 1.1 cm2 in the YC group. No statistically significant differences were found. The ratio of the area of the substantia nigra to the area of the midbrain was 0.12 ± 0.028 in the PD group, 0.15 ± 0.025 in the MC group, and 0.16 ± 0.034 in the YC group. The ANOVA indicated the presence of significant differences among groups (P < .00001), and the t test P values were .0001 for the comparison between the PD and MC groups, and .20 for the comparison between the MC and YC groups.
Averaging left and right sides, we determined that the ratio of mean pixel intensity in the lateral region to the mean pixel intensity in the medial region was 1.42 ± 0.46 (μ ± σ) in the PD group, 1.16 ± 0.19 in the MC group, and 1.28 ± 0.20 in the YC group. The ANOVA indicated the presence of significant differences among groups (P = .006), and the t test P value was .01 for the comparison between the PD and MC groups and .01 for the comparison between the MC and YC groups.
Figure 3 depicts, as a scatterplot on a Cartesian quadrant, the position of each section, with the ratio between pixel intensity in lateral and medial regions of interest as abscissa and the ratio between nigral and midbrain area as ordinate; complete separation between the PD group and controls was not achieved.
Fig 3.
Distribution of young controls, age-matched controls, and patients on a Cartesian quadrant, having as abscissa, the lateral/medial contrast ratio and as ordinate, the nigral/midbrain area ratio. Separation is not achieved; however, measurements from patients tend to cluster in the area corresponding to reduced nigral area and increased lateral-medial contrast. SN indicates substantia nigra.
Discussion
Although other authors claim to have measured specifically the pars compacta of the substantia nigra, on the basis of signal intensity and in comparison with known anatomy, we were not convinced that we could separate the pars compacta and pars reticulata; therefore, we preferred, more simply, to consider the substantia nigra as a whole.
At the present time, the exact relationship between neurodegeneration and changes in the spin-lattice relaxation time remains to be elucidated, changes in the intracellular compartment and in iron deposition both being putative contributors.11–13,20 Until such a relationship is clarified, one should not claim to have measured the actual area of the residual substantia nigra but instead to have measured the nigral area having relatively unaltered T1 with respect to control values.
As with other similar studies, the irregular and smooth appearance of the substantia nigra in the PD group leaves a certain margin for subjectivity in its delineation for area measurements; nevertheless, the observed group difference seemed remarkable when the small sample size and initial disease stage were taken into account. Because pixel values are averaged, lateral-to-medial contrast measurements are much less vulnerable to region-of-interest drawing uncertainty.
The nigral area measured in absolute units on the subtraction images was significantly different between patients and controls. However, the significance of such a comparison was found to be limited by inter-individual variability in midbrain size; when the ratio between nigral area and midbrain area was taken into consideration, separation considerably improved. On the contrary, age-related loss of the substantia nigra appeared not significant in the age range represented by the 2 groups. The fact that patients generally provided fewer usable sections than controls confirmed, as expected, that thinning of the substantia nigra also occurs along the rostrocaudal direction.
In accordance with previous reports and neuropathologic evidence, our finding of a significant difference between patients and controls in lateral-medial pixel-intensity ratio confirms that the lateral part of the substantia nigra is particularly affected by disease. The intermediate values found in YC are of dubious interpretation and require further verification. Age-related changes in iron deposition may determine the observed difference because sequences were optimized to minimize overall nigral signal intensity in MC.
Even though, as represented in Fig 3, complete separation of patients from controls could not be achieved, measurements from patients tended to cluster in the area of the graph corresponding to reduced nigral area and increased lateral-medial nigral contrast.
Compared with advanced MR imaging techniques such as diffusion-tensor imaging and fiber tracking, inversion-recovery imaging may be easier to implement in a clinical routine because acquisition time is limited and postprocessing of data is very simple.
The main limitations of the present study are the small sample size and that all patients were comparable in terms of disease stage. Before the technique can be considered for diagnosis and staging, further study is required, investigating the correlation with the UPDRS score (evaluated on- and off-therapy) in a larger population, with the purpose of obtaining reference values. The proposed technique might give a contribution to the differential diagnosis between Parkinson disease and atypical Parkinsonisms, characterized by different patterns of involvement of the substantia nigra.11,21
The findings of the present study are essentially in line with previous reports by Hutchinson et al11 and Hutchinson and Raff 12,13; interestingly, notwithstanding a certain margin for subjectivity in the delineation of the substantia nigra with unaltered signal intensity, we found that the ratio of its area to that of the midbrain provided better separation between patients and controls compared with lateral-medial contrast ratios alone.11–13
Some authors have reported that the area of the substantia nigra as measured on T2, proton density, and DWI images is not significantly different between controls and patients, characterized by mean disease duration of or below 5 years.6,7 On the basis of this finding, one may conclude that in vivo imaging should not aim at measuring the volume of the substantia nigra as a whole but rather at obtaining parameters that are sensitive to neurodegenerative changes occurring independently from volume reduction. This study confirms that nigral degeneration causes T1 changes and that significant differences between patients and controls can be found on inversion-recovery images, even without absolute quantification of the T1. The discussed measures are complementary to T2 and T2* relaxation time quantification.4,5
Conclusions
Inversion-recovery imaging may provide a convenient way to visualize degeneration of the substantia nigra. The results obtained motivate further studies evaluating the correlation of inversion-recovery imaging findings with patient disease severity on larger patient populations and the potential contribution to the differential diagnosis between Parkinson disease and atypical Parkinsonisms.
Footnotes
The present work has been entirely self-financed, without any financial or material support from third parties.
References
- 1.Elkeslassy A, Miaux Y, Martin-Duverneuil N, et al. MRI of degenerative extrapyramidal syndromes: Parkinson disease, progressive supranuclear palsy and multiple system atrophy. J Neuroradiol. 1996;23:157–63 [PubMed] [Google Scholar]
- 2.Ravina B, Eidelberg D, Ahlskog JE, et al. The role of radiotracer imaging in Parkinson disease. Neurology 2005;64:208–15 [DOI] [PubMed] [Google Scholar]
- 3.Ordidge RJ, Gorell JM, Deniau JC, et al. Assessment of relative brain iron concentrations using T2-weighted and T2*-weighted MRI at 3 Tesla. Magn Reson Med 1994;32:335–41 [DOI] [PubMed] [Google Scholar]
- 4.Gorell JM, Ordidge RJ, Brown GG, et al. Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology 1995;45:1138–43 [DOI] [PubMed] [Google Scholar]
- 5.Graham JM, Paley MN, Grunewald RA, et al. Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain 2000;123:2423–31 [DOI] [PubMed] [Google Scholar]
- 6.Oikawa H, Sasaki M, Tamakawa Y, et al. The substantia nigra in Parkinson disease: proton density-weighted spin-echo and fast short inversion time inversion-recovery MR findings. AJNR Am J Neuroradiol 2002;23:1747–56 [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi M, Hosoya T, Haku T, et al. Evaluation of the substantia nigra in patients with Parkinsonian syndrome accomplished using multishot diffusion-weighted MR imaging. AJNR Am J Neuroradiol 1999;20:1500–06 [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshikawa K, Nakata Y, Yamada K, et al. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J Neurol Neurosurg Psychiatry 2004;75:481–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seppi K, Schocke MF, Esterhammer R, et al. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the parkinson variant of multiple system atrophy. Neurology 2003;60:922–27 [DOI] [PubMed] [Google Scholar]
- 10.Schocke MF, Seppi K, Esterhammer R, et al. Trace of diffusion tensor differentiates the Parkinson variant of multiple system atrophy and Parkinson’s disease. Neuroimage 2004;21:1443–51 [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson M, Raff U, Lebedev S. MRI correlates of pathology in parkinsonism: segmented inversion recovery ratio imaging (SIRRIM). Neuroimage 2003;20:1899–902 [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson M, Raff U. Structural changes of the substantia nigra in Parkinson’s disease as revealed by MR imaging. AJNR Am J Neuroradiol 2000;21:697–701 [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson M, Raff U. Parkinson’s disease: a novel MRI method for determining structural changes in the substantia nigra. J Neurol Neurosurg Psychiatry 1999;69:815–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;6:33–39 [DOI] [PubMed] [Google Scholar]
- 15.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–42 [DOI] [PubMed] [Google Scholar]
- 16.Hendrick ER. Image contrast and noise. In: Stark DD, Bradley WG, eds. Magnetic Resonance Imaging. Philadelphia: Mosby;1996;53–7
- 17.Olszewski J, Baxter D. Cytoarchitecture of the Human Brain Stem. S. Karker: New York;1954;50 , 52–56, 58, 60, 131, 132
- 18.Duvernoy HM, Vannson JL. Human Brain Stem and Cerebellum: Surface, Structure, Vascularization, and Three-Dimensional Sectional Anatomy with MRI. Berlin, Germany: Springer-Verlag;1995;34–50, 70–75
- 19.Van Buren JM, Borke RC. Variations of the human diencephalon. Berlin, Germany: Springer-Verlag;1972;34–7
- 20.Vymazal J, Righini A, Brooks RA, et al. T1 and T2 in the brain of healthy subjects, patients with Parkinson disease, and patients with multiple system atrophy: relation to iron content. Radiology 1999;211:489–95 [DOI] [PubMed] [Google Scholar]
- 21.Graham DI, Lantos PL. Greenfield’s Neuropathology. Vol.2 . Hodder Arnold: London, UK;2002;329–50 [Google Scholar]