Abstract
BACKGROUND AND PURPOSE: Hyperintensity of the subcortical white matter (SWM) of the precentral gyrus and hypointensity of the precentral gyrus gray matter (PGGM) on fluid-attenuated inversion recovery (FLAIR) are described as potentially useful diagnostic findings in amyotrophic lateral sclerosis (ALS). A detailed study of the prevalence of these findings in various age groups has not been described.
METHODS: One hundred twenty-two patients underwent axial FLAIR brain examinations as part of either hearing loss or tinnitus evaluation. Examinations were randomly selected to reflect an even spread through the decades from ages 15 to 78 years and were reviewed by 2 readers, blinded to patient’s age and sex, for the presence/absence of the above 2 signs. If SWM hyperintensity was present, it was graded as intense as caudate nucleus (grade 1) or insula (grade 2).
RESULTS: We identified 32 cases of grade 1 and 5 cases of grade 2 SWM hyperintensity, and 28 cases of PGGM hypointensity. Both signs showed significant Spearman correlation with increasing age (r = 0.55, P < .001 for grade 1, r = 0.45, P < .001 for grade 2 SWM hyperintensity, r = 0.45, P < .001 for PGGM hypointensity). Analysis of variance showed there was a significant difference between the different age groups (P < .001) for both signs. Grading of the SWM and PGGM signals were highly reproducible with very good interobserver agreement (r = 0.88, P < .001, and r = 0.97, P < .001, respectively).
CONCLUSION: This study suggests a statistically significant relationship between increasing age and the frequency of precentral gyrus SWM hyperintensity and PGGM hypointensity on FLAIR, and reinforces previous reports that these signs can be seen in patients who do not have ALS.
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease characterized by progressive weakness, with evidence of upper and lower motor neuron involvement and specific degeneration in the corticospinal tracts (CST).1 MR imaging is the most useful neuroimaging method for depicting the location and extent of the imaging characteristics in ALS.1 In patients with ALS, it has been suggested that there is an increased incidence of T2 hyperintensity in the CST, including the precentral gyrus, centrum semiovale, posterior third of the posterior limb of the internal capsule, cerebral peduncles, and ventral brain stem.1–7 In addition, T2 hypointensity of the precentral gyrus gray matter in patients with ALS1–7 and T1-weighted hyperintensity of the anterolateral column of the spinal cord8 have been described in patients with ALS . Other MR techniques, including MR spectroscopy showing significantly elevated levels of choline and myo-inositol and decreased levels of glutamate and N-acetylaspartate in the precentral gyrus in patients with ALS,2 hypointensity along the CST and bilateral subcortical regions of the precentral gyri in patients with ALS on T1-weighted spin-echo magnetization transfer contrast sequence,7 and a decrease of fractional anisotropy in CST in diffusion tensor MR imaging,9,10 have also been shown to be helpful in detecting and providing information regarding the metabolism and pathology of ALS.
It has been suggested that fluid-attenuated inversion recovery (FLAIR) imaging can find more abnormalities compared with T2-weighted spin-echo1,3 or T1 spin-echo and proton attenuation imaging.3 In particular, hyperintensity of the subcortical white matter (SWM) of the precentral gyrus on FLAIR images has been described as a potentially useful diagnostic finding in ALS1–3 and may be a specific sign of ALS that is not seen in healthy, asymptomatic patients.3
In patients with ALS, it has also been suggested that there is an increased incidence of the hypointensity of the precentral gyrus gray matter (PGGM), also known as the “motor dark line,” on both T2-weighted spin-echo and FLAIR images.1–3 However, it has been suggested that PGGM hypointensity on T2-weighted spin-echo images is not specific to ALS and could be found in healthy persons2,3,7 and in patients with other neurologic diseases.11,12
In our experience, hyperintensity of the subcortical white matter of the precentral gyrus and hypointensity of the PGGM on FLAIR are not uncommon findings in elderly patients without clinical evidence of ALS. The aim of this study was to assess the prevalence of these 2 signs in patients at various ages who had clinical evidence of ALS. To our knowledge, a detailed study of the prevalence of this finding in various age groups has not been described.
Methods
Subjects
All patients who underwent an axial FLAIR examination of the brain as part of either a hearing loss or tinnitus evaluation (ie, “exclude acoustic neuroma or retrocochlear cause”) from April 2000 to November 2004 were selected after hospital ethics committee approval. The request for evaluation was reviewed for any evidence of concomitant neurologic disease, and any patients with such evidence were excluded. Patients were divided into 6 age groups: age less than 30 years, between 31 and 40 years, between 41 and 50 years, between 51 and 60 years, between 61 and 70 years, and between 71 and 80 years. A similar number of patients were randomly selected from each age group to reflect an even spread through the decades from age 15 to age 78. A total of 122 patients with no provided clinical evidence of ALS were included in this study.
MR Imaging
MR examinations were performed on a 1.5T whole-body unit (LX platform, SIGNA; General Electric, Milwaukee, Wis) using a quadrature head coil. Axial fast FLAIR MR imaging was performed as follows: TR = 9000 ms, TE = 140 ms, TI = 2200 ms, FOV = 240 mm, matrix = 256 × 192, NEX = 1, section thickness = 5 mm, intersection gap = 2 mm, scan time = 3 minutes 36 seconds, and 20 sections covering the whole brain.
Data Collection
Visual evaluation of the FLAIR MR images was performed independently by 2 readers, a radiologist with 10 years of MR imaging reporting experience (reader A) and an MR fellow (reader B). Both were blinded to the patients’ age and sex.
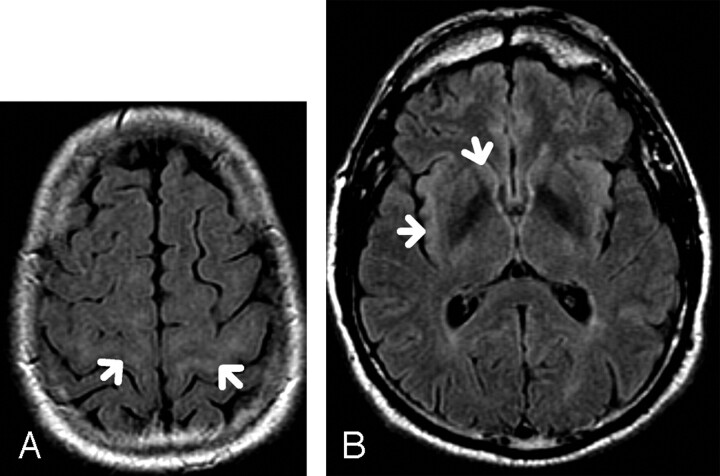
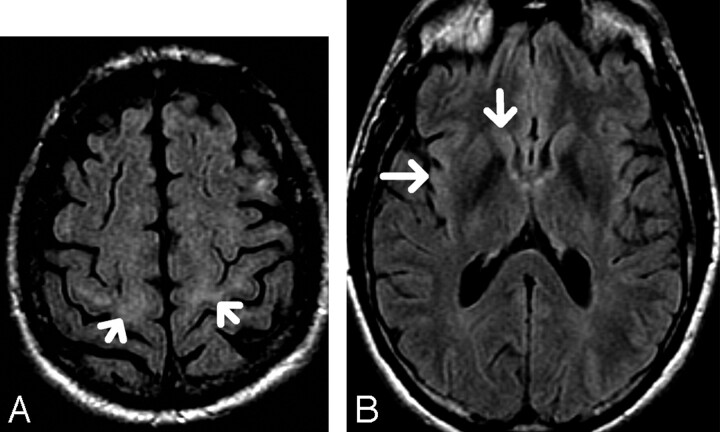
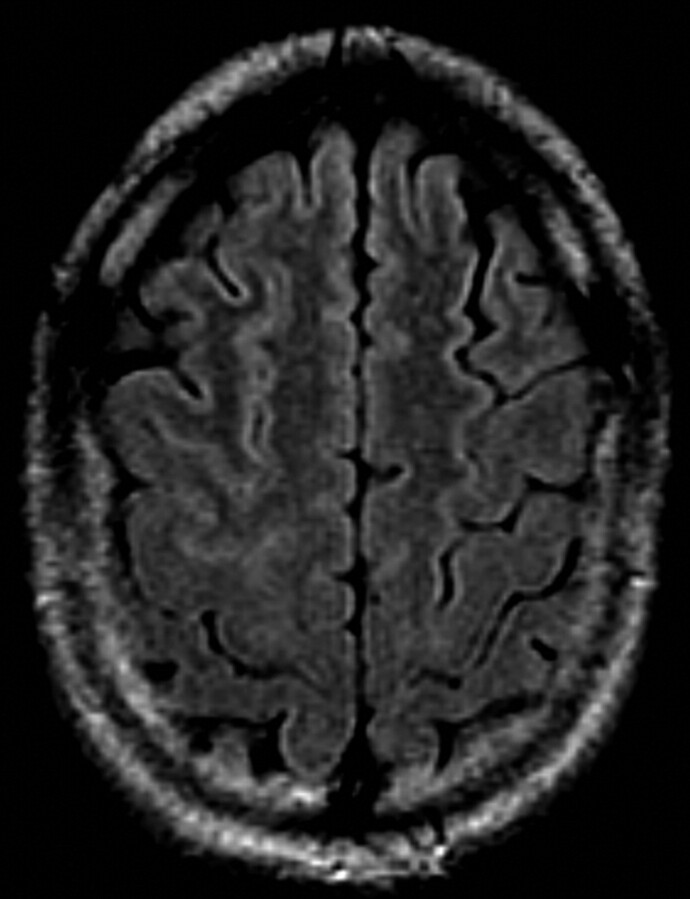
A classification scheme of the precentral gyrus SWM FLAIR hyperintensity similar to that used by Hecht et al3 was used. The presence of hyperintensity in the SWM of the precentral gyrus, and at least 2 sections above the lateral ventricles, was graded as “mildly hyperintense” if isointense to the caput of caudate nucleus (designated “1”) (Fig 1A, -B) or “distinctly hyperintense” if isointense to the insular cortex (designated “2”) (Fig 2 A, -B). The absence of hyperintense signal intensity was designated “0” (Fig 3).
Fig 1.
A, 65-year-old patient. Axial FLAIR. There is subcortical white matter hyperintensity (arrows) that is isointense to the caudate nucleus, classified as “mildly hyperintense” and graded as “1.
B, Axial FLAIR. Signal intensities at the caudate nucleus and insular cortex in the same patient with grade 1 SWM hyperintensity.
Fig 2.
A, 76-year-old patient. Axial FLAIR. There is subcortical white matter hyperintensity (arrows) which is isointense to the insular cortex, classified as “distinctively hyperintense” and graded as “2.”
B, Axial FLAIR. Signal intensities at the caudate nucleus and insular cortex in the same patient with grade 2 SWM hyperintensity.
Fig 3.
61-year-old patient. Axial FLAIR. There is absence of subcortical white matter hyperintensity.
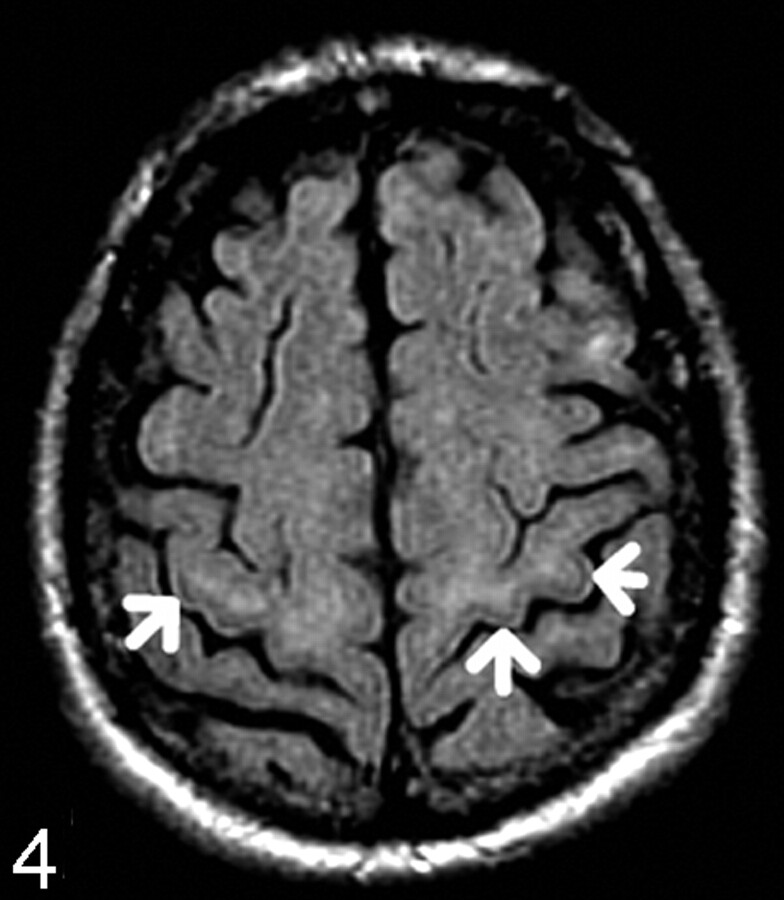
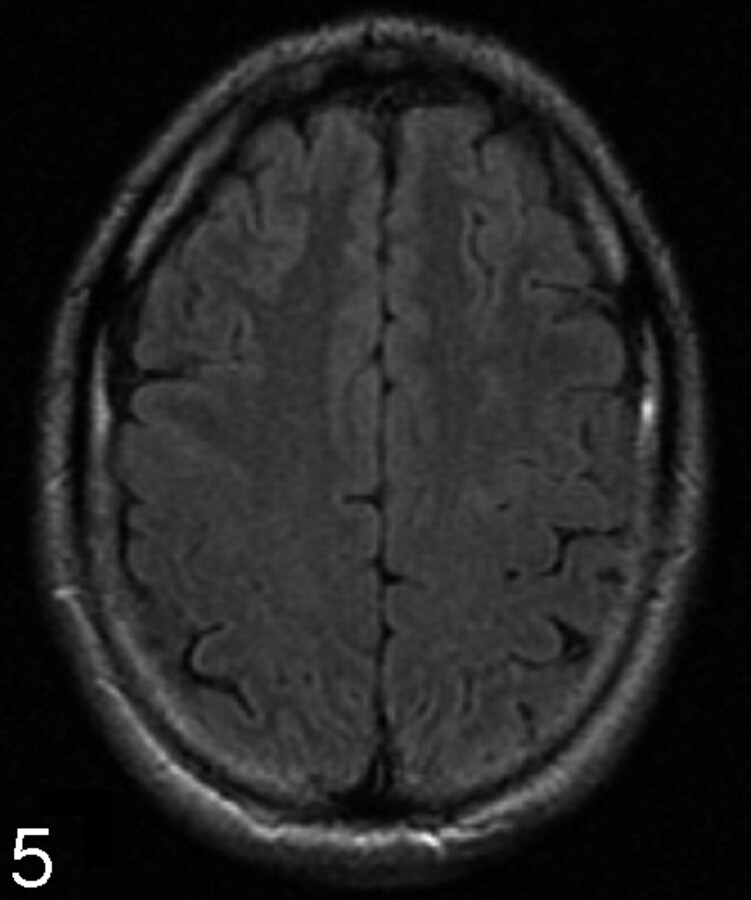
The PGGM was assessed for the presence of hypointensity, designated “1” (Fig 4). The absence of this line was designated “0” (Fig 5). The data were recorded according to the age groups in a Microsoft Excel spreadsheet.
Fig 4.
65-year-old patient. Axial FLAIR. There is a hypointense line in the precentral gyrus gray matter (arrows), designated “1.”
Fig 5.
61-year-old patient. Axial FLAIR. There is absence of a hypointense line in the precentral gyrus gray matter, designated “0.”
Statistical Analysis
Data were analyzed independently for each reader. Correlation of the presence of SWM hyperintensity on FLAIR images with increasing age was assessed by computing Spearman correlation coefficient and analysis of variance (ANOVA). P values below .05 were considered significant. Interobserver variability was assessed by calculating the Pearson correlation coefficient.
Results
SWM Hyperintensity
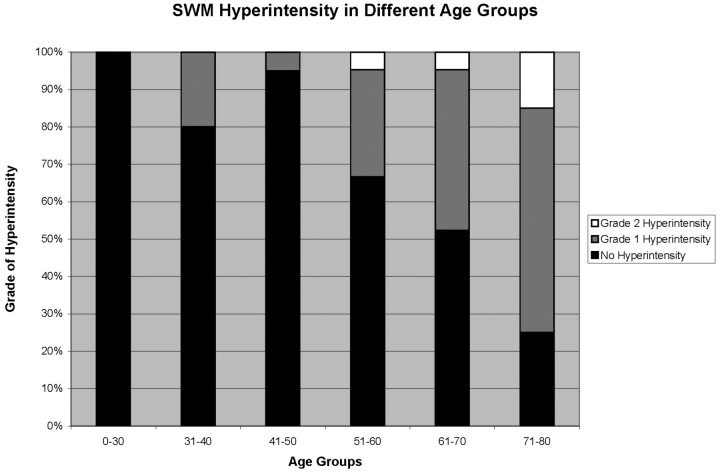
Of the 122 patients, reader A, the senior MR specialist, identified 32 cases of grade 1 SWM hyperintensity and 5 cases of grade 2 SWM hyperintensity. Reader B, the MR fellow, identified 25 cases of grade 1 SWM hyperintensity and 4 cases of grade 2 SWM hyperintensity (Table 1). Grade 2 SWM hyperintensity was found only in patients older than 50 years, and the frequency increased with age, especially in the 71–80 age group (3 of 20 patients for both readers). The incidence of grade 1 SWM hyperintensity also increased with age, although this was also observed in younger patients, including those in the 31–40 age group (Fig 6). No SWM hyperintensity was observed in the <30 age group for either reader. SWM hyperintensity showed significant Spearman correlation with increasing age (r = 0.55, P < .001). When patients were divided into 6 age groups, ANOVA showed there was a significant difference in signal intensity between the groups (P < .001).
Table 1:
SWM hyperintensity in different age groups
| Age (n) | Grade 0 |
Grade 1 |
Grade 2 |
|||
|---|---|---|---|---|---|---|
| Reader A | Reader B | Reader A | Reader B | Reader A | Reader B | |
| 0–30 years (20) | 20 | 20 | 0 | 0 | 0 | 0 |
| 31–40 years (20) | 16 | 19 | 4 | 1 | 0 | 0 |
| 41–50 years (21) | 20 | 20 | 1 | 1 | 0 | 0 |
| 51–60 years (21) | 14 | 15 | 6 | 6 | 1 | 0 |
| 61–70 years (20) | 10 | 13 | 9 | 6 | 1 | 1 |
| 71–80 years (20) | 5 | 6 | 12 | 11 | 3 | 3 |
Note:—SWM indicates subcortical white matter.
Fig 6.
Percentage of subjects identified with SWM hyperintensity in different age groups (average of reader A and B).
Pearson correlation coefficient showed the grading and determination of the SWM hyperintensity were highly reproducible with very good interobserver agreement (r = 0.88, P < .001). No interobserver variation in grading was seen in the 0–30 and 41–50 age groups. There was only 1 case with a difference in opinion (grade 1 versus 2) by the readers in the 51–60 age group, and 7 cases of a difference in opinion (grade 0 versus 1) in the 31–40 and 61–80 age groups. No case of a grading difference of 0 versus 2 was seen.
PGGM Hypointensity
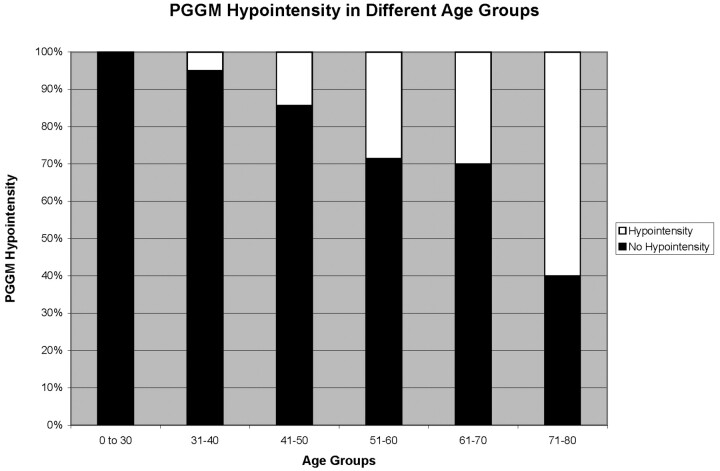
Reader A identified 28 cases of PGGM hypointensity and reader B identified 25 cases of PGGM hypointensity (Table 2). There was progressively increased frequency of the PGGM hypointensity with advancing age (1 patient in the 31–40 group through to 12 patients in the 71–80 group; incidence increasing from 5% to 60%) (Fig 7). This sign was absent in patients younger than 30 years of age for both readers. PGGM hypointensity showed significant Spearman correlation with increasing age (r = 0.45, P < .001). When patients were divided into 6 age groups, ANOVA showed there was a significant difference in signal intensity among the groups (P < .001). Pearson correlation coefficient showed that grading and determination of the PGGM signal intensity were highly reproducible, with excellent interobserver agreement (r = 0.97 P < .001).
Table 2:
Number of subjects identified with PGGM hypointensity in different age groups for readers A and B
| Age (n) | Absent Signal |
Hypointensity |
||
|---|---|---|---|---|
| Reader A | Reader B | Reader A | Reader B | |
| 0–30 years (20) | 20 | 20 | 0 | 0 |
| 31–40 years (20) | 19 | 19 | 1 | 1 |
| 41–50 years (21) | 18 | 18 | 3 | 3 |
| 51–60 years (21) | 15 | 15 | 6 | 6 |
| 61–70 years (20) | 14 | 15 | 6 | 5 |
| 71–80 years (20) | 8 | 9 | 12 | 11 |
Note:—PGGM indicates precentral gyrus gray matter.
Fig 7.
Percentage of subjects identified with PGGM hypointensity in different age groups (average of reader A and B).
Discussion
The incidence of ALS is approximately 2 per 100,000 persons in the United States.13 Patients with ALS usually present in the 6th decade of life, and it invariably leads to death, usually present after 50 years of age.14 There is no definitive diagnostic test for ALS.2 Lower motor neuron involvement is best assessed by electrophysiologic methods. Assessment of upper motor neuron degeneration is often limited in both clinical and electrophysiologic examinations.5 A number of imaging signs on different MR imaging sequences1–8 and MR techniques2,7,9,10 have been described to be useful for the diagnosis of ALS. FLAIR imaging using heavily T2-weighted parameters increases the sensitivity of MR imaging to detect cortical and subcortical tissue changes,3 and it is reported that for patients with ALS, FLAIR imaging can find more abnormalities compared with T2-weighted spin-echo1,3 or T1 spin-echo and proton attenuation imaging.3
Because the neuropathologic substrate of ALS is degeneration of the CST, MR imaging studies have evaluated the CST in these patients. Hyperintensity of the SWM of the precentral gyrus on FLAIR images has been reported to be more frequent in patients with ALS1–3,5 with variable frequency of this finding also reported in normal subjects,1–7 particularly when the precentral gyrus SWM hyperintensity was graded as mild.1,3,4 Zhang et al1 described a specificity of 94% of precentral gyrus SWM hyperintensity on FLAIR images for ALS. Hecht et al3 found distinct grade 2 hyperintense signal intensity of the precentral gyrus SWM only in patients with ALS and suggested it may be a specific sign of ALS that is not seen in patients without ALS.
In our study, we found a statistically significant trend of increasing incidence of grade 2 SWM hyperintensity underlying the precentral gyrus in elderly patients without clinical evidence of ALS. This is in line with a previous study7 reporting that precentral gyrus SWM FLAIR hyperintensity might be seen in elderly volunteers. This sign was present in up to 15% of subjects in the 71–80 age group and was absent in the <50 age groups. Most cases of the grade 2 SWM hyperintensity were found in the 71–80 age group, which would account for the discrepancy from previous results by Hecht et al3 (which included healthy control volunteers ranging in age from 34 to 71 years) and Zhang et al1 (in which the age range of the control group was from 40 to 60 years). Therefore, grade 2 precentral gyrus SWM FLAIR hyperintensity should be interpreted with greater caution in a patient older than 50 years. However, because most patients with ALS present after 50 years of age,14 for most patients, even grade 2 SWM hyperintensity will probably be a sensitive but nonspecific MR imaging finding for ALS.
Our data also demonstrated an increased incidence of grade 1 SWM hyperintensity on FLAIR images with increasing age. This is in keeping with results from previous studies,1,3 which described grade 1 SWM hyperintensity in healthy subjects. The presence of grade 1 SWM hyperintensity seems to lack specificity and, when present, should therefore be interpreted with caution.
The pathologic changes of the normal aging brain and neurodegenerative disorders in general (ALS in particular) are similar in that both are characterized by neuronal death. The difference is the selectivity of a particular site or sites and more rapid death of affected neurons with neurodegenerative disorders compared with normal aging. It has been hypothesized that the hyperintense signal intensity of the CST on FLAIR images in patients with ALS may be related to the loss of myelinated fibers, edema, lipid-loaded macrophages, or neuronal death resulting in the increase in signal intensity on T2-weighted images.1–3,5 We postulate that the increased incidence of both grade 1 and 2 SWM signal intensity in normal aging patients compared with the younger normal patients is due to age-related loss of myelinated fibers and/or neuronal death.
Hypointensity of the precentral gyrus gray matter (PGGM) (“dark motor line”) has been described with an increased incidence in patients with ALS on both T2-weighted spin-echo and FLAIR images.1–3 It is reported the frequency of PGGM hypointensity on FLAIR is related to the severity of clinical upper motor neuron sign and is seen in 100% of patients with severe disease.2 There is an increasing incidence of this sign in patients with ALS with increased duration of disease.5 Zhang et al1 reported the PGGM hypointensity on FLAIR images to have a good sensitivity of 74% for diagnosing ALS and was the most frequent positive finding among patients with ALS in their study; it was seen in 77.8% of the patients with ALS and in only 33.3% of the healthy control subjects. However, a study by da Rocha et al7 showed no relation between the PGGM hypointensity and ALS on FLAIR images.
On the T2-weighted spin-echo images, PGGM hypointensity has been suggested to have a highly variable sensitivity and low to moderate specificity for the diagnosis of ALS4; it has also been demonstrated to be found among asymptomatic persons older than 50 years on T2-weighted imaging.7,12,15 Our data suggest that this is also true for FLAIR images. We have demonstrated a definite increased incidence of PGGM hypointensity on FLAIR with increasing age. This is consistent with the results of previous studies,2,7 suggesting that the PGGM FLAIR hypointensity is a nonspecific finding. Most cases of PGGM hypointensity were seen in the 71–80 age group in our study, which may explain the discrepancy between our findings and those by Zhang et al,1 because the age range of their control group was only 40–61 years. It was postulated this phenomenon might be caused by a T2 shortening effect and represented a marker of cortical damage caused by deposition of iron-laden astrocytes and macrophages.1,5 We speculate that the increasing incidence of PGGM hypointensity on both T2-weighted and FLAIR images with increasing age reflects age-related neuronal degeneration and accumulation of iron-laden macrophages.
Our study is not without limitation. Only visual evaluation and no quantitative analysis for signal intensities along the precentral gyrus SWM and PGGM were performed. Visual evaluation could be influenced by the windowing of the MR images, which could lead to a degree of inter-reader variability of the visual impression of the MR images. In addition, the current classification scheme for the precentral gyrus SWM hyperintensity on FLAIR is useful as a guide but requires further justification of its significance. We have used the internal controls of the caudate head and insular cortex to be consistent with previous reports, such as Hecht et al.3 However, despite the lack of quantitative analysis in our study, we have shown no significant inter-reader variability in the interpretation of the grade 1 and grade 2 precentral gyrus SWM hyperintensity and PGGM hypointensity on FLAIR. The second limitation is that we relied on the provided clinical details on the MR imaging request forms to exclude any potential patients with ALS from this study. Therefore, the accuracy of our findings depends on the accuracy of the provided clinical data. Although the low prevalence of ALS suggests that this is unlikely to significantly alter the results, other concomitant conditions not mentioned on the request forms may alter the signal intensity of the precentral gyrus SWM on FLAIR. Further studies with a more vigorously established control group with healthy volunteers, and quantitative analysis of the signal intensities of well-defined regions of interests at the precentral gyrus SWM and reference points, are warranted to definitively establish the expected signal intensity changes of the precentral gyrus SWM on FLAIR with normal aging.
Conclusion
This study suggests a statistically significant relationship between increasing age and the frequency of hyperintensity of the SWM of the precentral gyrus and hypointensity of the PGGM on FLAIR images and reinforces previous studies that have suggested that these signs can be seen in patients without ALS.
References
- 1.Zhang L, Ulug AM, Zimmerman RD, et al. The diagnostic utility of FLAIR imaging in clinically verified amyotrophic lateral sclerosis. J Magn Reson Imaging 2003 May;17:521–7 [DOI] [PubMed] [Google Scholar]
- 2.Bowen BC, Pattany PM, Bradley WG, et al. MR imaging and localized proton spectroscopy of the precentral gyrus in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 2000;21:647–58 [PMC free article] [PubMed] [Google Scholar]
- 3.Hecht MJ, Fellner F, Fellner C, et al. MRI-FLAIR images of the head show corticospinal tract alterations in ALS patients more frequently than T2-, T1- and proton-density weighted images. J Neurol Sci 2001;186:37–44 [DOI] [PubMed] [Google Scholar]
- 4.Chan S, Kaufmann P, Shungu DC, et al. Amyotrophic lateral sclerosis and primary lateral sclerosis: evidence-based diagnostic evaluation of the upper motor neuron. Neuroimaging Clin N Am 2003 May;13:307–26 [DOI] [PubMed] [Google Scholar]
- 5.Hecht MJ, Fellner F, Fellner C, et al. Hyperintense and hypointense MRI signals of the precentral gyrus and corticospinal tract in ALS: a follow-up examination including FLAIR images. J Neurol Sci 2002;199:59–65 [DOI] [PubMed] [Google Scholar]
- 6.Hajnal J, DeCoene B, Lewis P, et al. High signal regions in normal white matter shown by heavily T2-weighted CSF nulled IR sequences. J Comput Assist Tomogr 1992;16:506–13 [DOI] [PubMed] [Google Scholar]
- 7.da Rocha AJ, Oliveira ASB, Fonseca RB, et al. Detection of corticospinal tract compromise in amyotrophic lateral sclerosis with brain MR imaging: relevance of the T1- weighted spin-echo magnetization transfer contrast sequence. AJNR Am J Neuroradiol 2004;25:1509–15 [PMC free article] [PubMed] [Google Scholar]
- 8.Waragai M, Shinotoh H, Hayashi M, et al. High signal intensity on T1 weighted MRI of the anterolateral column of the spinal cord in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 1997;62:88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham JM, Papadakis N, Evans J, et al. Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 2004;63:2111–9 [DOI] [PubMed] [Google Scholar]
- 10.Sach M, Winkler G, Glauche V, et al. Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 2004;127:340–50 [DOI] [PubMed] [Google Scholar]
- 11.Imon Y, Yamaguchi S, Yamamura Y, et al. Low intensity areas observed on T2-weighted magnetic resonance imaging of the cerebral cortex in various neurological diseases. J Neurol Sci 1995;134 Suppl:27–32 [DOI] [PubMed] [Google Scholar]
- 12.Hirai T, Korogi Y, Sakamoto Y, et al. T2 shortening in the motor cortex: effect of aging and cerebrovascular diseases. Radiology 1996;199:799–803 [DOI] [PubMed] [Google Scholar]
- 13.Worms PM. The epidemiology of motor neuron diseases: a review of recent studies. J Neurol Sci 2001;191:3–9 [DOI] [PubMed] [Google Scholar]
- 14.Havercamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population: validation of a scoring system and a model for survival prediction. Brain 1995;118:707–19 [DOI] [PubMed] [Google Scholar]
- 15.Imon Y, Yamaguchi S, Katayama S, et al. Decrease in cerebral cortex intensity on T2-weighted with ageing images of normal subjects. Neuroradiology 1998;40:76–80 [DOI] [PubMed] [Google Scholar]