Abstract
BACKGROUND AND PURPOSE: To investigate associations between cerebral ischemic events and signal hyperintensity in T1-weighted MR imaging (T1WI) of carotid plaque according to stenosis severity and to estimate persistence of T1WI signal hyperintensity.
METHODS: A total of 222 patients (392 atherosclerotic carotid arteries) underwent plaque imaging using 3D inversion-recovery-based T1WI (magnetization-prepared rapid acquisition with gradient-echo [MPRAGE]). Carotid plaque with intensity on MPRAGE of >200% that of adjacent muscle was categorized as “high signal intensity” and correlated with ipsilateral ischemic events within the previous 6 months. A total of 58 arteries (35 patients) underwent repeat MR imaging a total of 70 times at a median interval of 279 days (range, 10–1037 days).
RESULTS: Ipsilateral ischemic events were more frequent in patients with MPRAGE high signals than in patients with low signals in the 0%–29%, 30%–69%, and 70%–99% stenosis groups: Relative risk (95% confidence interval) was 2.50 (0.96–6.51), 7.55 (1.84–31.04), and 1.98 (1.01–3.90), respectively. In the 70 cases of repeat MR imaging, 29 of 30 cases with high signals on the preceding MR imaging maintained high signals. Of the 58 arteries that underwent repeat MR imaging, 4 of 22 carotid arteries with high signals developed ipsilateral subsequent ischemic events within 1 year, whereas none with low signals developed subsequent events.
CONCLUSIONS: Carotid plaque signal hyperintensity on T1WI is strongly associated with previous ipsilateral ischemic events, persisting over a period of months, and may indicate risk of subsequent events. Larger clinical trials are warranted to clarify associations between signal hyperintensity and risk of subsequent cerebral ischemic events.
Atherosclerotic carotid plaque represents a major cause of cerebral ischemia.1 Superiority of carotid endarterectomy to medical treatment has been confirmed for symptomatic carotid artery with severe stenosis (70%–99%) by the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST), but ≥7 operations were performed to avoid 1 stroke.2,3 At the same time, symptomatic patients with 50%–69% stenosis have been shown to benefit from moderate reduction of stroke risk by surgery, whereas patients with <50% stenosis do not benefit from surgery.2,3 However, cerebral ischemic episodes are not restricted to severe stenosis of the carotid artery,2–4 and a substantial fraction of ischemic strokes in the territory of the carotid artery are unrelated to carotid stenosis.1 Methods of noninvasively identifying “at risk” plaques are thus required.
Many studies have focused on MR imaging to characterize carotid plaques by using various imaging sequences. However, standardized sequence parameters and evaluation criteria to identify “at risk” plaques in MR imaging have not yet been established. Signal hyperintensity of carotid plaque in inversion recovery-based 3D T1-weighted imaging (alternatively known as magnetization-prepared rapid acquisition with gradient echo [MPRAGE])5 was associated with recent ischemic events,6,7 and was related to complicated plaques (type VI as proposed by the American Heart Association).8,9
We have performed carotid plaque imaging using MPRAGE since December 2001 for patients with suspected or confirmed carotid artery stenosis. The present study investigated associations between ischemic events and MPRAGE signal hyperintensity according to severity of stenosis in the carotid artery, and estimated persistence of MPRAGE signal hyperintensity as a potential risk factor for ischemic events.
Methods
Population
Since December 2001, MR imaging of the carotid artery has been performed for patients with suspected or confirmed atherosclerosis of the carotid artery after provision of oral informed consent on admission to the Departments of Neurology or Neurosurgery of our hospital. We reviewed the medical records of 222 consecutive patients who underwent MR imaging between December 2001 and June 2004. This study was performed in accordance with the ethics guidelines of our hospital. Of the 444 carotid arteries, 45 occluded arteries (at origin of the internal carotid artery, n = 39; common carotid artery, n = 1; top of the internal carotid artery or horizontal portion of the middle cerebral artery, n = 5) and 7 surgically treated carotid arteries (endarterectomy, n = 5; stent grafting, n = 2) were excluded from the study. A total of 392 carotid arteries from 222 patients were thus enrolled in this study.
Patient characteristics were recorded retrospectively by reviewing medical records. Ischemic events ipsilateral to the carotid artery within the previous 6 months were recorded, including cerebral infarction, transient ischemic attack, and retinal ischemia (amaurosis fugax and retinal artery occlusion). Emboligenic cardiac diseases (including persistent and paroxysmal atrial fibrillation, mitral valve stenosis, implantation of prosthetic heart valves, dilated cardiomyopathy, endocarditis, and acute myocardial infarction within the previous 6 months) were also recorded. Recorded risk factors of atherosclerosis included hypertension, diabetes mellitus, hyperlipidemia, and cigarette smoking.
MR Imaging
MR imaging was performed using a Magnetom Sonata 1.5T system (Siemens, Erlangen, Germany) with standard neck array and spine array coils (Fig 1). Plaque imaging was performed using MPRAGE in transaxial section with null blood condition (effective inversion time, 660 ms; TR, 1500 ms) and the water excitation technique to suppress fat signals. TR was defined as the interval between successive inversion pulses. Other imaging variables were: TE, 5.0 ms; FOV, 180 × 180 mm; matrix, 256 × 204; section thickness, 1.25 mm; 56 partitions; covering 70 mm around the carotid bifurcation; data acquisition time, 5 minutes. Multislab 3D time-of-flight (TOF) MR angiography (MRA) was also performed to facilitate delineation of lumen shape and plaque morphology (TE, 4.4 ms; TR, 35 ms; same spatial resolution as MPRAGE). Contrast MRA was performed after MPRAGE and 3D TOF MRA using rapid infusion of 0.1 mmol/kg body-weight gadolinium-diethylene-triaminepentaacetic acid (Gd-DTPA) at a rate of 2.0–3.0 mL/s after a test bolus of 1 mL Gd-DTPA for timing evaluation at the same rate. Typical imaging variables comprised: TR, 3.2 ms; TE, 1.3 ms; section thickness, 1.0 mm; 64 partitions; FOV, 360 × 200 mm; matrix, 512 × 208; data acquisition time, 14 seconds; near coronal section.
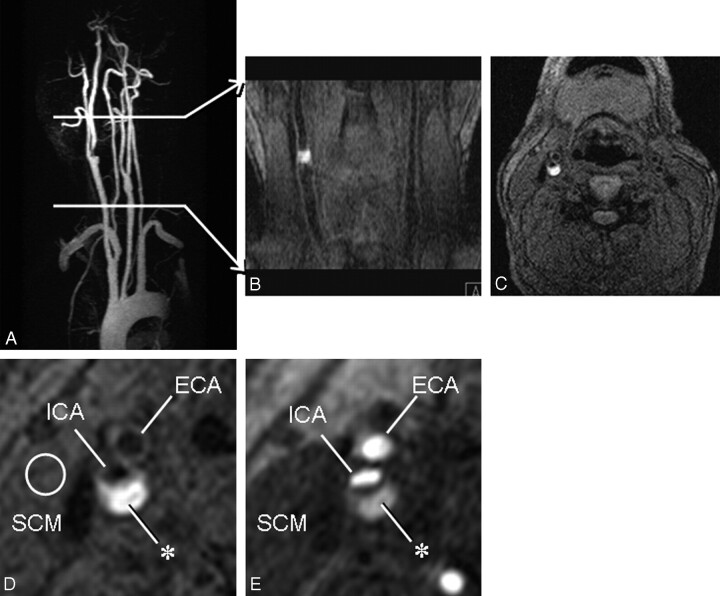
Fig 1.
Volume of plaque imaging.
A, Maximum intensity projection for contrast MRA of the cervical arteries. Volume of plaque imaging with MPRAGE is indicated between the 2 transverse bars.
B, Near-coronal multiplanar reconstruction of MPRAGE.
C, Source image of MPRAGE 3 mm cranial to the right carotid bifurcation shows relatively homogeneous signal intensity in the neck.
D, Zoomed source image of MPRAGE demonstrates dark lumen of the right internal carotid artery (ICA) and the external carotid artery (ECA). Circle indicates region of interest placed in the SCM.
E, Zoomed source image of TOF MRA at the same position demonstrates bright lumen of the carotid arteries. Plaque of the ICA (asterisk) demonstrates signal hyperintensity. SCM, sternocleidomastoid muscle.
Follow-Up MR Imaging
Of the 222 patients, 28 patients underwent one repeat MR imaging and 7 patients underwent 2 repeat MRIs for follow-up of carotid atherosclerosis up to June 2005, depending on clinical demands. Among the 28 patients with one repeat MR imaging, 4 carotid arteries were excluded because of occlusion and 6 arteries were excluded as a result of surgical treatment before initial MR imaging (n = 1) and between initial and repeat MR imaging (n = 5). Among the 7 patients with 2 repeat MRIs, 2 arteries were excluded because of occlusion. MPRAGE signals from plaques were thus compared a total of 70 times in 58 arteries from 35 patients. For arteries that underwent 2 repeat MRIs, first repeat MR imaging was compared with initial MR imaging, and the second repeat MR imaging was compared with the first repeat MR imaging.
Evaluation of MR Imaging
Carotid stenosis was measured using contrast MRA according to the methods defined by the NASCET10 and categorized into 3 groups: mild or no stenosis (0%–29%), moderate stenosis (30%–69%), and severe stenosis (70%–99%). One observer evaluated signal intensity of plaques on MPRAGE relative to signal intensity in adjacent muscle (typically the sternocleidomastoid muscle) as measured by placing a round region of interest 5–8 mm in diameter on a standard console of the clinical MR system (Fig 1D). If the plaque displayed signal intensity >200% of muscle intensity at any place or section in the plaque, that plaque was categorized as “high signal intensity.” Otherwise, the plaque was categorized as “low signal intensity” (Fig 2).
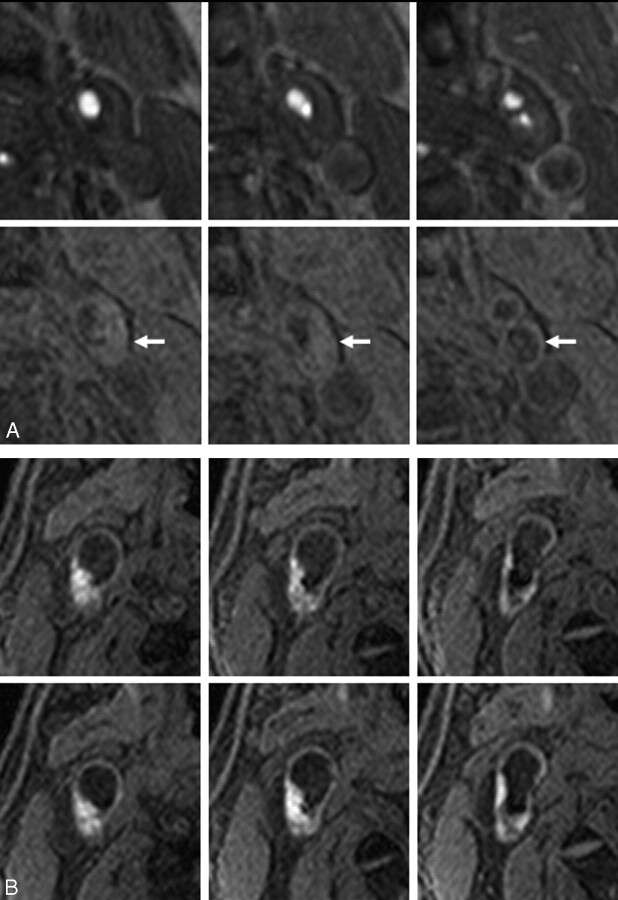
Fig 2.
Examples of classic carotid plaques.
A, An example of low signal intensity plaque. Top and bottom rows show 3 corresponding sections with 2.5-mm intervals of TOF MRA and MPRAGE, respectively. A 76-year-old man has left carotid artery stenosis and no history of ipsilateral ischemic events. The carotid plaque (arrows) displays no signal hyperintensity relative to the adjacent muscle.
B, An example of high signal intensity plaque. Top and bottom rows show 3 corresponding sections with 2.5-mm intervals in initial and follow-up MR imaging. A 58-year-old man experienced cerebral infarction in the territory of the right middle cerebral artery 12 days before initial MR imaging, which reveals a right carotid plaque with heterogeneous MPRAGE signal hyperintensity (top row). At 4 months after initial MR imaging, the patient again developed cerebral infarction in the right middle cerebral artery territory. Follow-up MR imaging at 5 months after initial MR imaging (bottom row) shows mild increase of MPRAGE high signal intensity region.
For carotid plaque with high signal intensity, volume of the region with signal intensity >200% of the muscle intensity was calculated using Dr. View/PRO version 5.2 software (Asahi Kasei Information Systems, Tokyo, Japan) on a stand-alone workstation.
Statistical Analysis
Two-tailed t tests or Mann-Whitney tests were used for comparison of means, 2-sided Fisher exact tests for comparison of proportions, paired t tests for comparison of paired variables, and χ2 tests for linear trends of ischemic events according to stenosis severity. Associations between MPRAGE signal intensity and ipsilateral ischemic events were analyzed by considering each artery independently. All analyses were performed using Prism version 4.0 software for Windows (GraphPad Software, San Diego, Calif).
To calculate interobserver variability in categorization of carotid plaque as high or low signal intensity, a second observer categorized carotid plaque signals for the first 100 arteries after completing consensus reading of the last 10 carotid plaques as training between first and second observers. For calculation of intraobserver variability, the first observer repeated categorization of plaque signals for the first 100 arteries at >1 month after first observation. All interpretations of MR imaging were performed in a blinded manner. Interobserver and intraobserver agreement was calculated using κ statistics.
Results
Association between Signal Hyperintensity and Previous Ischemic Events
A total of 74 carotid arteries were associated with ipsilateral ischemic events within the previous 6 months (cerebral infarctions, n = 45; transient ischemic attack, n = 20; retinal ischemia, n = 9). Patients displaying carotid arteries with and without ipsilateral ischemic events exhibited no significant differences in age, sex, hypertension, diabetes mellitus, hyperlipidemia, or cigarette smoking status. A total of 24 carotid arteries were present in patients with atrial fibrillation, whereas 2 carotid arteries were from a single patient with a prosthetic aortic valve (Table 1). No patients were diagnosed with mitral stenosis, dilated cardiomyopathy, endocarditis, or acute myocardial infarction.
Table 1:
Baseline characteristics of carotid arteries according to symptoms
| Symptomatic(n = 74) | Asymptomatic(n = 318) | P | |
|---|---|---|---|
| Age (years) | 69.9 ± 8.3* | 70.0 ± 7.6 | .8872 |
| Female sex | 14.9 (11/74) | 17.3 (55/318) | .7309 |
| AF and prosthetic heart valve | 5.4 (4/74) | 6.9 (22/318) | .7982 |
| Hypertension | 79.7 (59/74) | 83.3 (265/318) | .4957 |
| Diabetes mellitus | 39.2 (29/74) | 39.9 (127/318) | 1.0000 |
| Hyperlipidemia | 62.2 (46/74) | 56.0 (178/318) | .3630 |
| Cigarette smoking | 29.7 (22/74) | 23.3 (74/318) | .2930 |
Note:—AF indicates atrial fibrillation. Values for age represent mean ± SD; other values represent percentage of carotid arteries, with raw numbers provided in parentheses.
MPRAGE high signal intensity was assigned to 170 of 392 carotid plaques (Fig 2). The κ values for interobserver and intraobserver agreement were 0.729 and 0.792, respectively (good agreement). After excluding carotid arteries from patients with atrial fibrillation or prosthetic heart valves, a total of 370 carotid arteries were included in evaluation of association with previous ischemic events. Relative risks (95% confidence interval) of carotid arteries with MPRAGE high signals compared with carotid arteries with MPRAGE low signals for 0%–29%, 30%–69%, 70%–99% stenosis groups were 2.50 (0.96–6.51), 7.55 (1.84–31.04), and 1.98 (1.01–3.90), respectively (Table 2). In addition, risk of high signal intensity carotid arteries with 0%–29% and 30%–69% stenoses resembled risk of low signal intensity carotid arteries with 70%–99% stenosis: relative risks (95% confidence intervals [CI]) were 0.87 (0.34–2.24) and 1.34 (0.65–2.78), respectively. Frequency of ischemic events in MPRAGE high signal intensity plaques increased with stenosis severity (P = .0133). Median interval between MR imaging and previous ischemic events for MPRAGE high and low signal intensity groups was 20 days (range, 0–180 days) and 51 days (range, 6–179 days), respectively. Mann-Whitney tests revealed no significant differences between the 2 intervals (P = .0854).
Table 2:
Risk of ipsilateral ischemia according to MPRAGE signal intensity and stenosis after excluding patients with atrial fibrillation and prosthetic heart valves
| Stenosis | 0%–29% (n = 152) |
30%–69% (n = 114) |
70%–99% (n = 100) |
Total (n = 366) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | A | F (%) | S | A | F (%) | S | A | F (%) | S | A | F (%) | |
| MPRAGE | ||||||||||||
| High signal intensity | 6 | 26 | 18.8 | 18 | 44 | 29.0 | 27 | 36 | 42.9 | 51 | 106 | 32.5 |
| Low signal intensity | 9 | 111 | 7.5 | 2 | 50 | 3.8 | 8 | 29 | 21.6 | 19 | 190 | 9.1 |
| P | 0.0889 | 0.0004 | 0.0498 | <0.0001 | ||||||||
| Relative risk (95% CI) | 2.500 (0.9603–6.508) | 7.548 (1.836–31.041) | 1.982 (1.008–3.900) | 3.573 (2.201–5.801) | ||||||||
Note:—MPRAGE indicates magnetization-prepared rapid acquisition with gradient echo; S, symptomatic within previous 6 months; A, asymptomatic within previous 6 months; F, frequency of carotid arteries in patients with ipsilateral symptom; CI, confidence interval. P values were calculated using the Fisher exact test between MPRAGE high and low groups for symptoms (S) in each stenosis group. Relative risk of ischemic events was calculated for MPRAGE high carotid arteries compared with low carotid arteries in each stenosis group.
In MRPAGE high signal intensity plaques, volume of the high signal intensity region was larger in symptomatic plaques than in asymptomatic plaques. Mean (± SD) volume for 0%–29%, 30%–69%, and 70%–99% stenosis groups in symptomatic plaques was 249 ± 301, 186 ± 327, and 166 ± 331 mm3, respectively—larger than in asymptomatic plaques at 48 ± 72, 123 ± 169, and 115 ± 200 mm3, respectively. Mann-Whitney tests revealed a significant difference for 0%–29% stenosis (P = .0247) but not for 30%–69% (P = .5102) or 70%–99% stenosis (P = .3177).
Follow-Up MR Imaging
A total of 70 comparisons in 58 arteries were performed between successive MRIs at a median interval of 279 days (range, 10–1037 days). Initial status of high or low signal intensity was maintained on repeat MR imaging for most comparisons; only 4 of 40 low signal intensity carotid arteries changed to high signal intensity, and only 1 of 30 high signal intensity carotid arteries changed to low signal intensity (Table 3). Volume of the region with signal intensity >200% of muscle intensity tended to be similar between successive MRIs (P = .690) (Fig 3). Mean stenosis did not change significantly in the 70 comparisons, at 44.2 ± 30.0% for the preceding MR imaging and 45.0 ± 30.2% for follow-up MR imaging (P = .487).
Table 3:
Number of carotid arteries displaying MPRAGE high and low signal intensity according to interval between repeat and the preceding MRI, and signal intensity change on repeat MRI
| Interval | Number on Preceding MRI |
Number Associated with Signal Intensity Change on Repeat MRI |
||
|---|---|---|---|---|
| High | Low | High to Low | Low to High | |
| <90 days | 5 | 9 | 1 | 0 |
| 90–179 days | 8 | 7 | 0 | 1 |
| 180–364 days | 9 | 8 | 0 | 0 |
| ≥ 365 days | 8 | 16 | 0 | 3 |
| Total | 30 | 40 | 1 | 4 |
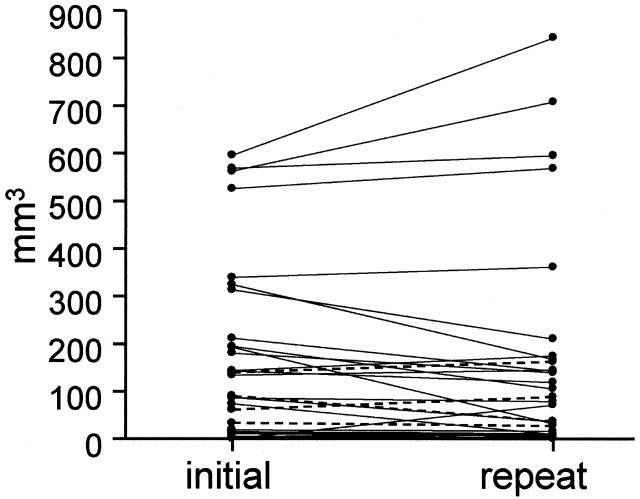
Fig 3.
Comparison of high signal intensity volume between successive MR imaging. Comparison was done 34 times between 2 successive MR imagings with high signal intensity in both or either of the 2 MR images. Median interval was 279 days (range, 10–1037 days). High signal intensity volume (mean ± SD) was 149 ± 182 mm3 at the initial MR imaging and 144 ± 217 mm3 at the repeat MR imaging. Paired t test displayed no significant change (P = .690). Broken lines indicate 4 carotid arteries associated with subsequent events within 1 year after initial MR imaging.
For investigation of subsequent events, 6 arteries from patients with atrial fibrillation and 2 arteries with ipsilateral middle cerebral artery occlusion at the horizontal portion were excluded from the 58 arteries. Among the remaining 50 carotid arteries, 4 of 22 carotid arteries with high signals displayed subsequent events, compared with 0 of 28 arteries with low signals (P = .0473) (Table 4). Repeat MR imaging of the 4 carotid arteries with subsequent events was performed at 9, 16, 16, and 27 days after subsequent events. Volume of the 4 arteries did not reveal any specific features compared with the other arteries (Fig 3).
Table 4:
Subsequent ipsilateral events according to stenosis severity and MPRAGE signals: results of 1 year follow-up after initial MRI
| Stenosis | Number at Initial MRI | Subsequent Events |
||
|---|---|---|---|---|
| Ischemic Events | Surgical Treatment | Censored | ||
| Number of low signal intensity carotid arteries | ||||
| 0%–29% Stenosis | 9 | 0 | 0 | 1 |
| 30%–69% Stenosis | 13 | 0 | 0 | 0 |
| 70%–99% Stenosis | 6 | 0 | 3 | 0 |
| Total | 28 | 0 | 3 | 1 |
| Number of high signal intensity carotid arteries | ||||
| 0%–29% Stenosis | 6 | 0 | 0 | 0 |
| 30%–69% Stenosis | 7 | 1 | 1 | 0 |
| 70%–99% Stenosis | 9 | 3 | 5 | 0 |
| Total | 22 | 4 | 6 | 0 |
Note:—MPRAGE indicates magnetization-prepared rapid acquisition with gradient echo. Ischemic events include ischemic stroke and transient ischemic attack. Surgical treatment includes carotid endarterectomy and endovascular stenting.
Discussion
More ischemic events occurred in patients with high signals on MPRAGE than in those who showed low signals in each of the subgroups of patients with mild, moderate, and severe stenosis. Volume of high signal intensity was significantly larger in symptomatic plaque than in asymptomatic plaque for patients with mild stenosis. We also demonstrated in a subgroup that underwent multiple MR imaging that hyperintensity was maintained over a period of months, and MPRAGE high signals may offer an indicator of risk for subsequent events.
MPRAGE is a T1WI and displays intraplaque components that have short T1 as high signal intensity. Mechanisms of the short T1, however, are complex. Many previous studies have shown that lipid-rich necrotic cores display signal hyperintensity on T1WI.11–14 Other studies have shown that intraplaque hemorrhage or thrombus exhibit high signal intensity on T1WI.9,14–16 Although methemoglobin is considered to be a cause of high signal intensity, the duration of methemoglobin in carotid plaques remains unclear. Lipid signals are very weak in advanced plaques.11 Signal hyperintensity in this study was not attributable to lipids, as fat-suppression technique was used. Protein-rich viscous tissue can form another cause of signal hyperintensity.17–19 The actual cause of T1WI signal hyperintensity should thus be investigated further.
In the follow-up study, signal hyperintensity was repeatedly observed (Table 3), and high signal intensity volume did not change significantly. Repeat MR imaging of 4 carotid arteries with subsequent events that was performed 9–27 days after these events exhibited no specific change in volume compared with the other arteries (Fig 3). These results may be attributable to continuous or recurrent intraplaque hemorrhage. However, we cannot conclude that signal hyperintensity is due to recent hemorrhage in this study. Erythrocyte membranes and iron have been shown to be present within the necrotic cores of human atherosclerotic coronary plaques even in the absence of recent hemorrhage, and intraplaque hemorrhage is related to progression and instability of such lesions.20 If this is the case in carotid plaque, as in coronary plaque, the necrotic core of a carotid plaque can be at least partially formed by intraplaque hemorrhage, and thus no clear border would exist between intraplaque hemorrhage and necrotic core.
Size and location of high signals may be important for vulnerability. In the coronary artery, “at risk” plaques can be morphologically characterized by a large lipid-rich core and thin fibrous cap.8,21 In the case of the carotid artery, however, numerous authors have stressed the importance of intraplaque hemorrhage.22,23 Conversely, other authors have reported no significant differences in frequency of hemorrhage between symptomatic and asymptomatic patients.24–26 The real causes of carotid plaque vulnerability thus remain controversial. Volume of the high signal intensity region was significantly larger in symptomatic carotid plaque than in asymptomatic plaque for the 0%–29% stenosis category, but not for the 30%–69% or 70%–99% categories. Carotid plaques with subsequent events did not display extremely large volumes for high signal intensity regions (Fig 3). Assessment of fibrous cap thickness and integrity is also important when evaluating plaque vulnerability and has been achieved using T2-weighted imaging and TOF MRA.11,27,28 Some authors have reported higher percentages of symptomatic patients for ruptured caps (70%) compared with thick caps (9%) using multicontrast MR imaging.29 Plaque ulceration may be related to stroke risk.30,31 Better prediction of vulnerability may be achieved by combining MPRAGE with these techniques.
This study was performed using a commercially available clinical machine and standard neck- and spine-array coils without additional hardware. Image acquisition time was short (5 minutes for MPRAGE). MPRAGE with fat suppression and null blood condition suppresses background signals and highlights signal hyperintense tissues with short T1, so image interpretation is relatively simple.7,9,32 Although motion artifacts were present to various degrees, predominantly attributable to respiration and swallowing, these were insufficient to result in the exclusion of any patients from the present study. 3D data acquisition is essential for visualizing the entirety of irregularly shaped plaques.
This study examined suspected and confirmed atherosclerotic carotid stenosis that may be related to cerebral ischemia depending upon clinical demands. Some biases in the study population may thus be present. Reasons for MR imaging of the carotid artery varied, including screening of cervical artery stenosis, suspicion of complicated plaque on ultrasonography, inconclusive ultrasonography results due to calcification and high position of stenosis, refusal of conventional angiography by patients, and preoperative evaluation of carotid artery stenosis. Potential embolic sources, such as complicated plaque in the aortic arch and persistent foramen ovale, were not surveyed.
Conclusion
We conclude that carotid plaque hyperintensity on MPRAGE, a heavy 3D T1WI technique, is associated with previous cerebral ischemic events. MPRAGE hyperintense signals persist over a period of months, and may represent a potential indicator of risk for subsequent cerebral ischemia. Longitudinal studies with large subject populations are required to clarify whether MPRAGE hyperintense signals indicate risk of subsequent cerebral ischemic events.
Acknowledgments
We thank Teruo Noguchi from the National Cardiovascular Center for his helpful comments and discussions.
Footnotes
This study was supported by funding from the Japanese Ministry of Health, Labour, and Welfare.
References
- 1.Barnett HJ, Gunton RW, Eliasziw M, et al. Causes and severity of ischemic stroke in patients with internal carotid artery stenosis. JAMA 2000;283:1429–36 [DOI] [PubMed] [Google Scholar]
- 2.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–25 [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Gutnikov SA,Warlow CP. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 2003;34:514–23 [DOI] [PubMed] [Google Scholar]
- 4.Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke 2000;31:774–81 [DOI] [PubMed] [Google Scholar]
- 5.Mugler JP 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 1990;15:152–57 [DOI] [PubMed] [Google Scholar]
- 6.Moody AR, Allder S, Lennox G, et al. Direct magnetic resonance imaging of carotid artery thrombus in acute stroke. Lancet 1999;353:122–23 [DOI] [PubMed] [Google Scholar]
- 7.Murphy RE, Moody AR, Morgan PS, et al. Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia. Circulation 2003;107:3053–58 [DOI] [PubMed] [Google Scholar]
- 8.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995;15:1512–31 [DOI] [PubMed] [Google Scholar]
- 9.Moody AR, Murphy RE, Morgan PS, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003;107:3047–52 [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991;22:711–20 [DOI] [PubMed] [Google Scholar]
- 11.Toussaint JF, Southern JF, Fuster V, et al. T2-weighted contrast for NMR characterization of human atherosclerosis. Arterioscler Thromb Vasc Biol 1995;15:1533–42 [PubMed] [Google Scholar]
- 12.Rogers WJ, Prichard JW, Hu YL, et al. Characterization of signal properties in atherosclerotic plaque components by intravascular MRI. Arterioscler Thromb Vasc Biol 2000;20:1824–30 [DOI] [PubMed] [Google Scholar]
- 13.Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging 2002;15:62–67 [DOI] [PubMed] [Google Scholar]
- 14.Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001;104:2051–56 [DOI] [PubMed] [Google Scholar]
- 15.Cappendijk VC, Cleutjens KB, Heeneman S, et al. In vivo detection of hemorrhage in human atherosclerotic plaques with magnetic resonance imaging. J Magn Reson Imaging 2004;20:105–10 [DOI] [PubMed] [Google Scholar]
- 16.Cappendijk VC, Cleutjens KB, Kessels AG, et al. Assessment of human atherosclerotic carotid plaque components with multisequence MR imaging: initial experience. Radiology 2005;234:487–92 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi Y, Tachibana O, Muramatsu N, et al. Rathke cleft cyst: MR and biomedical analysis of cyst content. J Comput Assist Tomogr 1999;23:34–38 [DOI] [PubMed] [Google Scholar]
- 18.Ahmadi J, Destian S, Apuzzo ML, et al. Cystic fluid in craniopharyngiomas: MR imaging and quantitative analysis. Radiology 1992;182:783–85 [DOI] [PubMed] [Google Scholar]
- 19.Kucharczyk W, Macdonald PM, Stanisz GJ, et al. Relaxivity and magnetization transfer of white matter lipids at MR imaging: importance of cerebrosides and pH. Radiology 1994;192:521–29 [DOI] [PubMed] [Google Scholar]
- 20.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–25 [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med 2002;8:1257–62 [DOI] [PubMed] [Google Scholar]
- 22.Lusby RJ, Ferrell LD, Ehrenfeld WK, et al. Carotid plaque hemorrhage. Its role in production of cerebral ischemia. Arch Surg 1982;117:1479–88 [DOI] [PubMed] [Google Scholar]
- 23.Imparato AM, Riles TS, Mintzer R, et al. The importance of hemorrhage in the relationship between gross morphologic characteristics and cerebral symptoms in 376 carotid artery plaques. Ann Surg 1983;197:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassiouny HS, Davis H, Massawa N, et al. Critical carotid stenoses: morphologic and chemical similarity between symptomatic and asymptomatic plaques. J Vasc Surg 1989;9:202–12 [PubMed] [Google Scholar]
- 25.Hatsukami TS, Ferguson MS, Beach KW, et al. Carotid plaque morphology and clinical events. Stroke 1997;28:95–100 [DOI] [PubMed] [Google Scholar]
- 26.Svindland A, Torvik A. Atherosclerotic carotid disease in asymptomatic individuals: An histological study of 53 cases. Acta Neurol Scand 1988;78:506–17 [DOI] [PubMed] [Google Scholar]
- 27.Toussaint JF, LaMuraglia GM, Southern JF, et al. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 1996;94:932–38 [DOI] [PubMed] [Google Scholar]
- 28.Hatsukami TS, Ross R, Polissar NL, et al. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959–64 [DOI] [PubMed] [Google Scholar]
- 29.Yuan C, Zhang SX, Polissar NL, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 2002;105:181–85 [DOI] [PubMed] [Google Scholar]
- 30.Eliasziw M, Streifler JY, Fox AJ, et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994;25:304–08 [DOI] [PubMed] [Google Scholar]
- 31.Rothwell PM, Gibson R, Warlow CP. Interrelation between plaque surface morphology and degree of stenosis on carotid angiograms and the risk of ischemic stroke in patients with symptomatic carotid stenosis. On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 2000;31:615–21 [DOI] [PubMed] [Google Scholar]
- 32.Moody AR, Pollock JG, O’Connor AR, et al. Lower-limb deep venous thrombosis: direct MR imaging of the thrombus. Radiology 1998;209:349–55 [DOI] [PubMed] [Google Scholar]