Abstract
SUMMARY: A patient with human immunodeficiency virus–related posterior fossa progressive multifocal leukoencephalopathy had serial diffusion-weighted imaging using b-values of 1000 and 3000 before and during highly active antiretroviral therapy (HAART). High-b-value images provided a superior definition of the leading edge of the lesion and additional information about the integrity of white matter tracts. Following HAART, there was a marked reduction of lesional apparent diffusion coefficient and reconstitution of anisotropy in the affected middle cerebellar peduncle.
The prognosis of untreated AIDS-related progressive multifocal leukoencephalopathy (PML) is poor,1 but highly active antiretroviral therapy (HAART) may improve outcome of PML in human immunodeficiency virus (HIV)-infected patients.2 There are relatively few case reports on diffusion-weighted imaging (DWI) in PML.3–5 None have described its use in monitoring therapeutic response or high-b-value imaging.
Case Report
A 43-year-old man presented with slurred speech, left-handed weakness, and swallowing difficulties. He had been HIV-infected for 13 years and had never received HAART. The CD4 count was 160 cells/μL (normal = 350–1250 cells/μL), and the plasma HIV viral load was 425,300 copies/mL.
JC virus DNA was detected in the CSF, and MR imaging demonstrated a nonenhancing T2 hyper- and T1 hypointense lesion in the left middle cerebellar peduncle (MCP), consistent with PML.
Two diffusion-weighted protocols were performed: 1 using b = 1000 smm−2 (DWI1000); the other, b = 3000 smm−2 (DWI3000), including 3 sets of DWIs in mutually orthogonal diffusion-encoding directions.
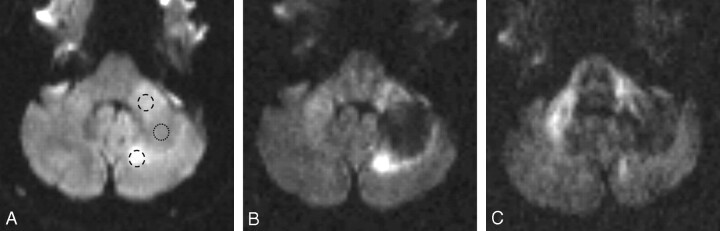
The trace-weighted images of DWI1000 showed a lesion with an isointense center and a relatively ill-defined broad area of peripheral hyperintensity, whereas the trace-weighted images of DWI3000 showed a hypointense center and a much better defined hyperintense rim. Apparent diffusion coefficient (ADC) measurements were 1.10 ± 0.03 × 10−3 mm2 s−1 at the lesion center corresponding to a relative ADC ratio (rADC) of 247%, by using the unaffected side as reference. The rADCs at the anterior and posterior lesion margins were 124% and 104%, respectively. On DWI3000 images showing diffusion in the section direction (supero-inferior) and read direction (left to right), there was loss of anisotropy in the left MCP, compared with the right (Fig 1).
Fig 1.
Pretreatment MR imaging study.
A, Trace image of DWI1000 shows a lesion in the left MCP, which is hyperintense anteriorly and posteriorly and of intermediate signal intensity centrally. The rounded ring-shaped markings indicate where the regions of interest have been placed on the corresponding ADC maps.
B, Trace image of DWI3000 shows a hypointense lesion center and a peripheral high-signal-intensity rim, which is much more clearly defined than on the trace images of DWI1000 (Fig 1A).
C, Anisotropic image DWI3000, sensitive to water movement in the section (supero-inferior) direction, shows high signal intensity in the right MCP, which is a normal finding because the MCP represents a barrier to free water movement in this direction. The PML lesion in the mid and posterior portions of the left MCP appears no longer hyperintense, implying a loss of structural integrity. Note also that water diffusion is not impeded by the superior cerebellar peduncles, which run in a craniocaudal direction.
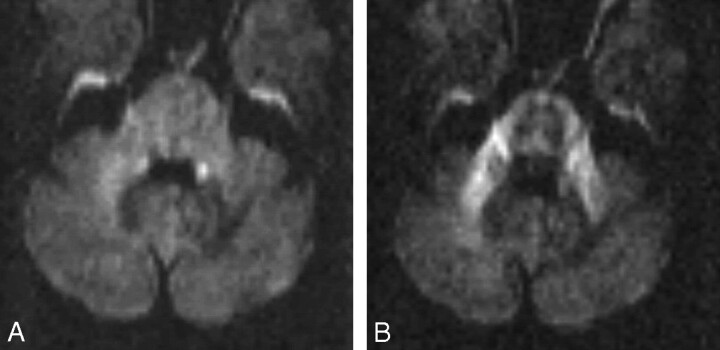
The patient began HAART with zidovudine, tenofovir, and ritonavir/lopinavir and steadily improved during the next 4 weeks. The CD4 count rose to 300 cells/μL, and the HIV viral load dropped to 1400 copies/mL. MR imaging 6 weeks after commencement of HAART showed some decrease in lesion size, which remained T2 hyper- and T1 hypointense. On the trace-weighted images, the lesion appeared much less hypointense and no longer had a hyperintense rim (Fig 2). The ADC at the lesion center had dropped to 0.68 ± 0.04 (rADC = 140%). The DWI3000 in the read (left to right) and section directions showed an apparent restoration of diffusion anisotropy in the left MCP (Fig 2). Twelve months later the patient remains well.
Fig 2.
MR imaging study 4 weeks after commencing HAART.
A, Trace images of DWI3000 no longer show any high-signal-intensity rim at the periphery of the lesion, and the left MCP appears only slightly less bright than the right one.
B, On the anisotropic images of DWI3000, sensitive for water movement in the section (supero-inferior) direction, the left MCP appears now nearly as bright as the right MCP, implying that structural integrity has been regained.
Discussion
Not all patients respond in a predictable manner to HAART. Conventional MR imaging has been relatively disappointing in predicting treatment response, yielding contradictory results. Post et al6 found mass effect associated with a higher risk of death, whereas Thurnher et al7 found that mass effect predicted a favorable treatment response, together with temporary enhancement.
The experience of DWI in patients with PML is limited and has only been performed at a b-value of 1000 smm−2 or below. Ohta et al3 demonstrated that changes on DWI preceded those on T2-weighted images. Henderson et al4 first described the appearance of a high-signal-intensity “leading edge,” and Bergui et al5 found the ADC to be much higher in the lesion center than in the periphery, which concurs with our findings. Histopathologic correlation in 1 case4 showed enlarged extracellular spaces with sparse oligodendrocytes and macrophages in the lesion center (where the ADC was most elevated) and loss of myelin, numerous macrophages, and oligodendrocytes with intranuclear inclusions at the lesion periphery. Our ADC measurements indicate that the peripheral high-signal-intensity rim on the trace-weighted images was due to T2 shine-through effects rather than reduction of ADC. The peripheral high-signal-intensity rim on the trace-weighted images was much more clearly defined on DWI3000 than on DWI1000. A higher b-value increases the amount of diffusion-weighting. The center of the lesion, where the ADC is increased, therefore appears darker on DWI3000, allowing a better demarcation of the rim where water diffusion is less significantly altered. At higher b-values, anisotropic white matter becomes more conspicuous, and we used direction-sensitive images at b = 3000 to demonstrate major white matter tracts because dedicated multidirectional diffusion tensor imaging was not available to us.
We successfully used serial ADC measurements to document the response of PML to HAART. As a quantitative technique, it may be a more effective predictor of treatment response than conventional MR imaging; DWI with b = 3000 provided superior definition of the initial high-signal-intensity rim and of white matter anisotropy and their changes following HAART. These changes suggested microstructural reorganization, which was associated with a good clinical outcome and prolonged survival in our patient.
References
- 1.Berger JR, Major EO. Progressive multifocal leukoencephalopathy. Semin Neurol 1999;19:193–200 [DOI] [PubMed] [Google Scholar]
- 2.Tassie JM, Gasnault J, Bentata M, et al. Survival improvement of AIDS-related progressive multifocal leukoencephalopathy in the era of protease inhibitors: Clinical Epidemiology Group. French Hospital Database on HIV. AIDS 1999;13:1881–87 [DOI] [PubMed] [Google Scholar]
- 3.Ohta K, Obara K, Sakauchi M, et al. Lesion extension detected by diffusion-weighted magnetic resonance imaging in progressive multifocal leukoencephalopathy. J Neurol 2001;248:809–11 [DOI] [PubMed] [Google Scholar]
- 4.Henderson RD, Smith MG, Mowat P, et al. Progressive multifocal leukoencephalopathy. Neurology 2002;58:1825. [DOI] [PubMed] [Google Scholar]
- 5.Bergui M, Bradac GB, Oguz KK, et al. Progressive multifocal leukoencephalopathy: diffusion-weighted imaging and pathological correlations. Neuroradiology 2004;46:22–25. Epub 2003 Oct 31 [DOI] [PubMed] [Google Scholar]
- 6.Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol 1999;20:1896–906 [PMC free article] [PubMed] [Google Scholar]
- 7.Thurnher MM, Post MJ, Rieger A, et al. Initial and follow-up MR imaging findings in AIDS-related progressive multifocal leukoencephalopathy treated with highly active antiretroviral therapy. AJNR Am J Neuroradiol 2001;22:977–84 [PMC free article] [PubMed] [Google Scholar]