Abstract
Marijuana use is increasing as more states are legalizing cannabis for both medicinal and recreational purposes. National survey data estimate that over 2 million Americans with established cardiovascular diseases currently use or have used marijuana in its variety of forms including inhalation and vaping. Cannabinoid receptors are distributed in multiple tissue beds and cells, including platelets, adipose tissue, and myocytes. Observational data suggest associations between marijuana and a broad range of adverse cardiovascular risks. Marijuana is becoming increasingly potent, and smoking marijuana carries many of the same cardiovascular health hazards as smoking tobacco. Synthetic cannabinoids have been linked to more sustained and deleterious pharmacodynamic effects. Marijuana is classified as a Schedule I substance, thus limiting its rigorous study for cardiovascular health effects. We summarize cardiovascular considerations related to marijuana use, pharmacological interactions, and future steps to provide clearer guidance regarding its cardiovascular safety. Screening for marijuana use is encouraged, especially in young patients presenting with cardiovascular disease.
Keywords: arrhythmia, cannabis, coronary artery disease, marijuana, vascular disease
Condensed Abstract
Marijuana is increasingly used for both medicinal and recreational purposes, including among patients with established cardiovascular disease. Yet, its cardiovascular effects are not fully understood, and comprehensive scientific studies and recommendations are lacking. We summarize cardiovascular considerations related to marijuana use, pharmacological interactions, and future steps to provide more clear guidance regarding the cardiovascular safety of marijuana.
Introduction
The use of marijuana and its derivatives is increasing as more states are legalizing these products for both medicinal and recreational use(1, 2). This accompanies an increasing prominence of vaping and new tobacco products such as electronic cigarettes and water pipe (hookah) smoking, both of which have prompted statements by the American Heart Association(3, 4). Furthermore, vaping-related mortalities are on the rise with increasing reports of pulmonary illnesses and respiratory failure(5). With growing use, patients are increasingly inquiring about the cardiovascular safety of marijuana, especially when used alongside other commonly prescribed cardiovascular therapies. Yet, the cardiovascular effects of marijuana are still not fully understood and comprehensive scientific studies and recommendations are lacking to guide the cardiovascular community(6). Limited observations have implicated 9-tetrahydrocannabinoid (THC), the active ingredient in marijuana, in contributing to a broad range of cardiovascular events(7–9), however the level of evidence has not been robust. In addition, cannabinoids may have drug interactions with a variety of cardiovascular medications. In this review, we discuss relevant mechanisms of potential cardiovascular risks related to marijuana, pharmacological interactions with common cardiovascular therapies, and synthesize a practical approach to approaching marijuana use in cardiovascular clinical care settings.
Current Use of Marijuana in the US
Chemical Properties and Common Uses
Marijuana is a greenish-gray mixture of the dried leaves, flowers, stems and seeds of the Cannabis sativa or Cannabis indica plant. The plant also contains more than 500 other chemicals, including more than 100 compounds that are chemically related to THC, called cannabinoids. Specifically, common compounds include cannabinol (CBN), cannabidiol (CBD), and THC, which is the most psychoactive chemical in marijuana(10). Cannabinoids are available in oral, sublingual, and topical formulations.
The effects of marijuana are mediated through the endocannabinoid system(11, 12). Cannabinoid (CB) receptors are distributed in multiple tissue beds and cell types (Figure 1). CB-1 receptors are present in high concentrations in the central and peripheral nervous systems, but also exist on platelets, adipose tissue, myocytes, liver, pancreas, and skeletal muscle(11). Therefore, exogeneous cannabinoids can exert effects on multiple systems (Table 1)(12). In settings of tissue injury, endocannabinoids are generated in excess with enhanced CB-1 receptor signaling. CB-2 receptors are present on immune cells, osteoclasts, and osteoblasts.
Figure 1. Intracellular mechanisms of marijuana effects.
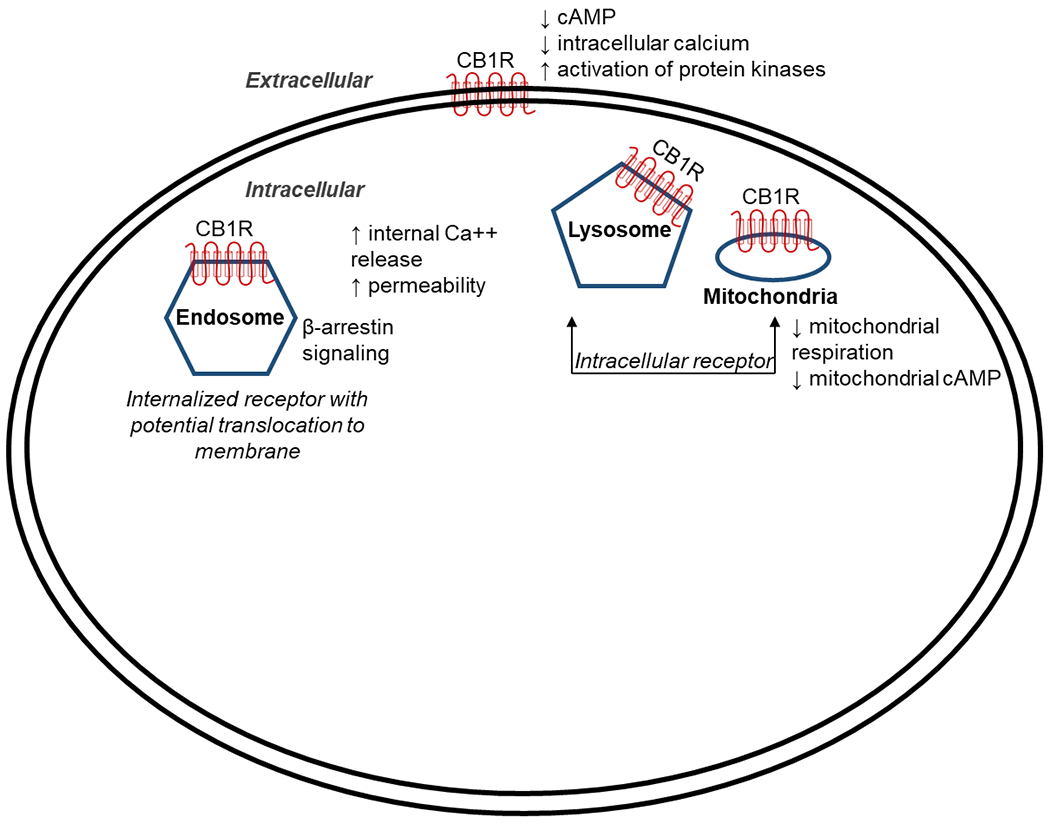
Cannabinoid receptor 1 (CB1R) is typically located on the cell surface and generally inhibits cyclic adenosine monophosphate (cAMP) formation that in turn decreases calcium influx. It can be internalized as a ligand-induced receptor mediating signaling pathways via β-arrestin. In contrast, intracellular CB1Rs do not translocate and can increase intracellular calcium through release of internal lysosomal calcium stores via increased membrane permeability. Additionally, CB1Rs located in mitochondria will decrease mitochondrial respiration and cAMP formation, thus regulating cellular energy metabolism.
Table 1.
Distribution and potential effects of cannabinoid receptor 1 (CB1R)(12)
| Brain |
| • Inhibition of pathological excitotoxicity associated with seizures/epilepsy via inhibition of glutamate release • Neuroprotective in patients with Alzheimer’s Disease, Huntington’s disease, and Parkinson’s disease • Appetite activation via the hypothalamus |
| Endocrine |
| • Communication with leptin, orexin, ghrelin to improve appetite stimulation |
| Gastrointestinal |
| • Gastrointestinal motility and absorption regulation via the enteric nervous system and intestinal mucosa that may aid in the management of nausea/vomiting and other inflammatory bowel processes • Upregulation of CB1R in hepatic cells may lead to hepatic insulin resistance, fibrosis, and lipogenesis |
| Cardiovascular |
| • CB1R activation in cardiomyocytes, vascular endothelial cells, and smooth muscle cells may lead to oxidative stress, inflammation, fibrosis, vasodilation, and negative inotropy |
Due to lack of evidence surrounding human expression of the cannabinoid receptor 2, limited data are available related to downstream effects of its receptor signaling.
In addition to naturally derived cannabinoids, various related formulations have been synthesized. The US Food and Drug Administration has approved 3 cannabinoids for medical use: 1) Cannabidiol, an oral solution for the treatment of seizures in rare forms of epilepsy; 2) Dronabinol (synthetic THC) to treat refractory chemotherapy-associated nausea/vomiting and human immunodeficiency virus-related anorexia/weight loss; and 3) Nabilone (synthetic chemical structure similar to THC) for refractory chemotherapy-associated nausea/vomiting.
The potency of marijuana has been steadily increasing over time. Synthetic cannabinoids (SCB), including ‘Spice’ and ‘K2’ have existed for over a decade, which may have undergone potentially dangerous pharmacological alteration. These SCBs are not under specific federal regulation(13). SCBs may be up to 100-fold more potent than THC and have been linked to more sustained and deleterious downstream pharmacodynamic effects(14, 15). Similarly, hydrophonic methods of cultivation used in small-scale recreational production may include potentially harmful Plant Growth Regulators and produce more potent marijuana.
Burden of Disease
Marijuana is the most commonly used drug of abuse according to the 2015 National Survey on Drug Use and Health. It is currently classified as a Schedule I drug by the US Drug Enforcement Administration, meaning that it is a drug with “no currently accepted medical use and a high potential for abuse.” However, it is worthwhile recognizing this is a policy distinction, and there is existing evidence for medical use.
Data from the National Survey on Drug Use and Health, an annual survey of the US civilian, non-institutionalized population demonstrates that in 2016 and 2017, over 39 million respondents reported use of marijuana in the last year (Figure 2). Its use is more prevalent among men than women—a gender gap that widened in the years 2007 to 2014. A recent analysis of the Behavioral Risk Factor Surveillance System found that adults with medical conditions were significantly more likely to report current marijuana use(16). Most (77.5%) of marijuana users reported smoking as their method of administration(16).
Figure 2. Reported use (in thousands) of marijuana in the last year, from 2016-2017 National Survey on Drug Use and Health.
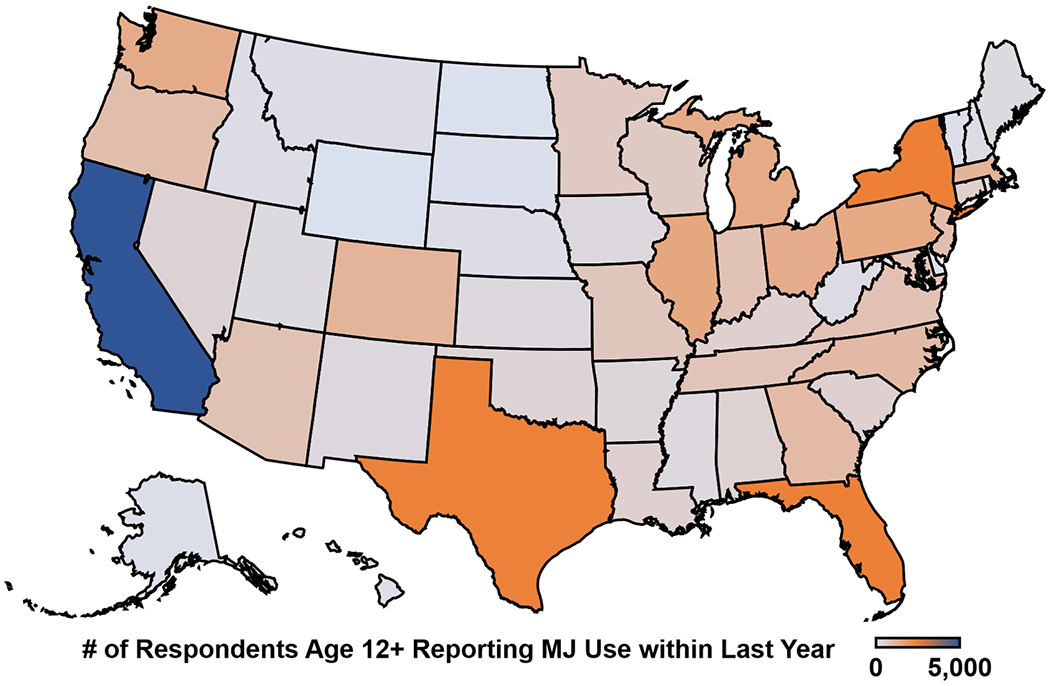
Data were extracted from the National Survey on Drug Use and Health, an annual survey of the US civilian, non-institutionalized population, in 2016-2017. Across the US, over 39 million respondents reported use of marijuana in the last year. Mapping software was powered by Bing (GeoNames, HERE, MSFT).
We conducted a dedicated query of the National Health and Nutrition Examination Survey (NHANES) from 2005-2016 to estimate marijuana use in cardiovascular disease. In NHANES, marijuana use was defined as those responding “yes” to ever using hashish or marijuana. Cardiovascular disease was defined broadly as those responding “yes” to being ever told by a healthcare provider they had congestive heart failure, coronary heart disease, or a heart attack. In 2015-2016, response rates to both sets of questions were 49.4%. By applying sampling weights to available respondent data, we estimated that 2 million (2.3%) of the 89.6 million adults who reported marijuana use have cardiovascular disease in the US in 2015-2016 (Figure 3). However, given substantial non-response, these data may be subject to response bias.
Figure 3. Estimated 1.7 to 2.7 million adults reporting prior or current marijuana use who have cardiovascular disease, 2005-2016 from National Health and Nutrition Examination Survey (NHANES).
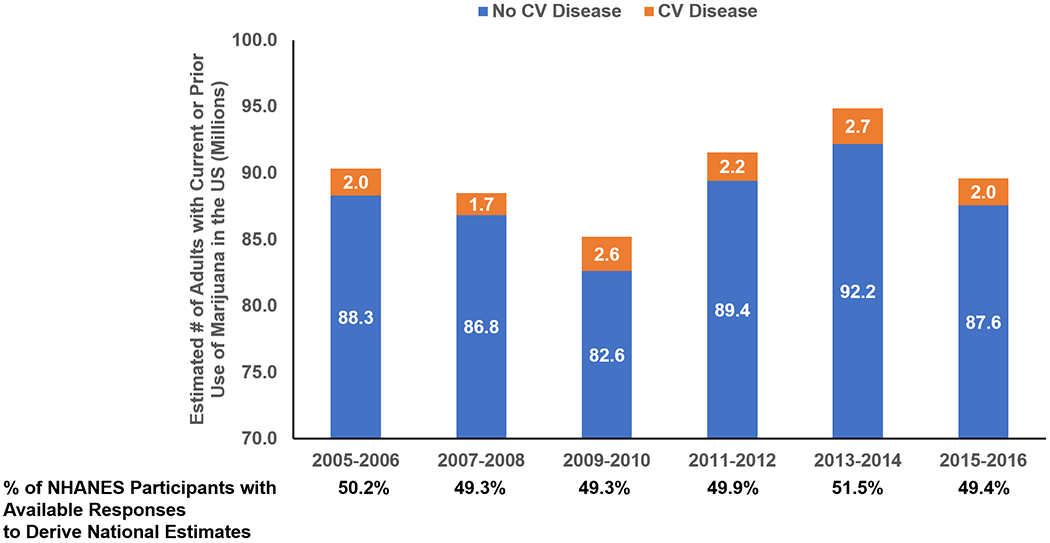
Marijuana use was defined as those responding “yes” to ever using hashish or marijuana. Cardiovascular (CV) disease was defined broadly as those responding “yes” to being ever told by a healthcare provider they had congestive heart failure, coronary heart disease, or a heart attack. Response rates to both questions ranged from 49.3% to 51.5% throughout the study timeframe.
An analysis of the National Inpatient Sample (NIS) from 2010-2014 identified 465,959 hospitalizations (representing 2.3 million weighted hospitalizations in the US population) of people with history of marijuana use, using administrative coding. The most common non-psychiatric primary discharge diagnoses included diabetes mellitus, acute myocardial infarction, and nonspecific chest pain among others(17). Importantly, patients with coronary atherosclerosis and peripheral vascular disorders independently faced the highest risks of in-hospital mortality(17).
Legalization
In recent years, there has been increasing legalization at the state level both with regards to medical use and recreational use. Recreational marijuana is currently legal in 11 states and the District of Columbia; other states are currently contemplating similar legalization policies. Medical marijuana is legal in 33 states as of July 2019.
Mechanisms of Cardiovascular Disease Associated with Marijuana
With increasing patterns of use and potency of marijuana, recent increases in cannabis-related adverse health effects have been reported(18). However, these associations have been largely based on case reports, case series, or observational studies(19). When reported, marijuana has often been self-reported, and few have collected “doses” or blood levels. Many epidemiological studies may be confounded by factors related to healthcare access and other adverse health behaviors (concurrent tobacco use and other drugs of abuse). Acknowledging the limited scope of data, few mechanisms of cardiovascular risk have emerged(7) (Figure 4).
Figure 4. Potential mechanisms of cardiovascular (CV) risk with exposure to marijuana.
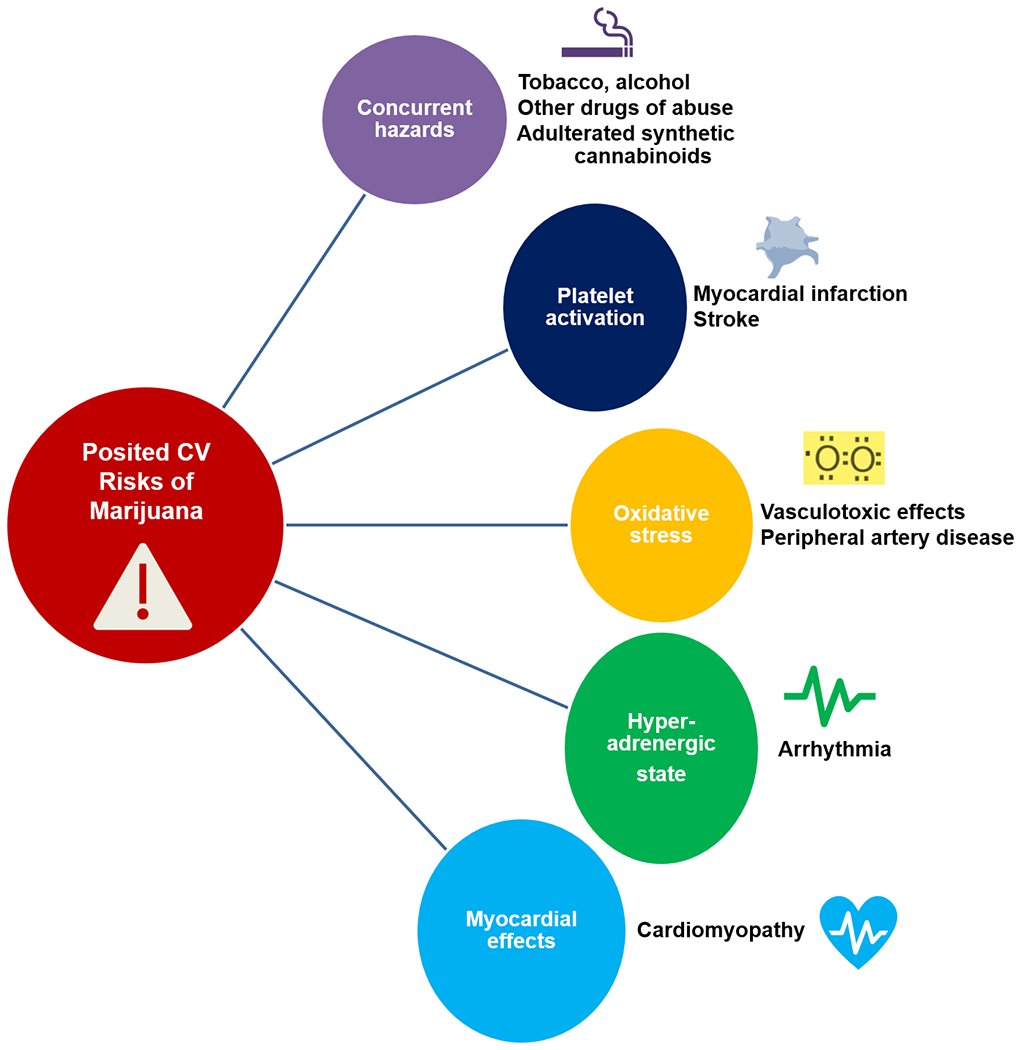
The quality of evidence supporting these posited mechanistic relationships is modest.
Smoking-Related Cardiotoxicity.
While the dominant psychogenic substance differs in tobacco (nicotine) and marijuana (THC), when smoked, many cardiotoxic chemicals are similarly produced (Table 2). When the combustion products of both substances are profiled, both contain a similar array of chemicals. Although marijuana is smoked with fewer puffs, larger puff volumes and longer breath holds may yield greater delivery of inhaled elements.
Table 2.
Comparison of use patterns, regulation, and cardiovascular (CV) effects of marijuana and tobacco smoking
| Marijuana Smoking | Tobacco Smoking | |
|---|---|---|
| Estimated Current Use | >39 million* | 34.3 million** |
| Recent Trends in Use | Rising | Declining |
| Psychoactive Substance | Tetrahydrocannabinol | Nicotine |
| Composition | Similar particular matter & chemical toxin profile | Similar particular matter & chemical toxin profile |
| Typical Use Pattern | More frequent puffs | Larger puff and inhaled volume, longer breath-hold |
| FDA Approved Products for Medicinal Use | Cannabidiol (seizures); Dronabinol & Nabilone (nausea, anorexia, weight loss) | None |
| DEA Controlled Substance | Yes (Schedule I) | No |
| Current Level of Epidemiological Evidence of CV Toxicity | + | +++ |
| Safe Dose/Level | ? | None |
People reporting use in the past year according to the 2016-2017 National Survey on Drug Use and Health
Based on the U.S. Department of Health and Human Services, current smokers defined as people who reported smoking at least 100 cigarettes during their lifetime and who, at the time they participated in a survey about this topic, reported smoking every day or some days
Abbreviations: DEA = Drug Enforcement Administration; FDA = Food and Drug Administration
Coronary Artery Disease
Mechanistically, marijuana use may pose potential cardiovascular risk in patients with atherosclerotic cardiovascular disease, especially early after acute coronary syndromes(20, 21). In the acute setting, cannabis smoking can lead to increases in heart rate and blood pressure, secondary to sympathetic nervous system activation(22), augmenting myocardial oxygen demands(8). Aronow and colleagues determined that exercise time until angina onset was reduced after smoking a single marijuana cigarette compared with placebo in a small experiment of 10 patients with coronary artery disease(23). Chronic use promotes tolerance and may be associated with less pronounced physiological effects(24). Other postulated mechanisms include production of oxidant gases resulting in cellular stress, platelet activation, increased oxidized low-density lipoprotein cholesterol formation, and induction of an inflammatory response.
Epidemiological studies have identified a potential temporal link between marijuana use and myocardial infarction. In a meta-analysis of 36 studies, the top 3 triggers of myocardial infarction included use of cocaine, eating a heavy meal, and smoking marijuana(26). Furthermore, in a systematic analysis of 33 studies, 28 found an increased risk of acute coronary syndromes with marijuana use(22). This observed risk association appears temporally related to recency of use. For instance, among 3,882 patients with myocardial infarction in the Determinants of Myocardial Infarction Onset Study(27, 28), 3% smoked marijuana in the prior year; 37 of whom had smoked within 24 hours and 9 within one hour of MI (27). In addition, marijuana use, which is more prevalent in younger adults, is not infrequently detected among patients presenting with early-onset MI. In the Partners YOUNG-MI registry of patients who presented with first MI under the age of 50, marijuana use was reported or tested positive in >6%(29). Marijuana use was associated with twice the hazard of death among these patients even after adjusting for tobacco use(29). Another mechanism of coronary pathology is coronary vasospasm in the absence of coronary artery disease.
Arrhythmias
A broad range of arrhythmias, including atrial fibrillation/flutter, atrioventricular block/asystole, sick sinus syndrome, ventricular tachycardia, and Brugada pattern, have been described with marijuana use(30–32). Increased catecholamines and β-adrenergic stimulation with THC may theoretically increase arrhythmogenicity (33). In an NIS study from 2010-2014, Desai and colleagues found that 66,179 of 2,459,856 (3%) of those with reported marijuana use experienced arrhythmias (mostly atrial fibrillation)(30).
Cerebrovascular Disease
Cerebrovascular events have also been reported in association with marijuana use(35–38), including with SCBs(39). Mechanisms related to potential cerebrovascular risks including direct vasculotoxic effects, alterations in hemodynamics, or incident atrial fibrillation/flutter(35, 40). One population survey found that individuals who had smoked marijuana in the past year experienced 3.3 times higher rate of cerebrovascular events(36). A case series described 14 patients with ischemic stroke who had exposure to cannabis during or before symptoms began, with 5 experiencing recurrent stroke with re-exposure(37). Among 334 patients who experienced acute ischemic stroke under the age of 45 over a 9-year period, 17% were cannabis users. These patients were typically younger and more likely to be men(41).
Peripheral Artery Disease
Thrombosis and ischemia of other vascular beds has also been reported (42). Delta-8 and delta-9-tetrahydrocanabinols can induce peripheral vasoconstriction (43). Cannabis arteritis has been reported in young men who developed distal ischemia leading to necrosis of fingers or toes(43–45), commonly with concurrent use of tobacco(45). Arteriographic evaluation reveals anomalies resembling Buerger’s disease(43). Exposure to secondhand smoke from marijuana for one minute impaired femoral artery flow-mediated dilatation, a measure of endothelial dysfunction, for at least 90 minutes, which was longer than impairment by tobacco secondhand smoke(25).
Cardiomyopathy
Cannabis use has been associated with myocardial dysfunction, independent of coronary artery disease. Rabbits who have received a selective CB2 agonist demonstrate concentration-dependent decreases in cardiac contractility(46). Case reports have suggested associations of cannabis with stress cardiomyopathy(47) and myocarditis/myopericarditis, an entity referred to as “toxic myocarditis”(48, 49).
Metabolic Alterations
Early studies had shown that cannabinoids contribute to weight gain in patients with human immunodeficiency virus leading to the rationale for use of dronabinol as an appetite stimulant(50, 51). Furthermore, a trial of rimonabant, an endocannabinoid receptor antagonist, demonstrated weight loss and improved metabolic abnormalities(52). However, multiple recent epidemiologic studies have suggested that cannabis may be protective against weight gain and related alterations in metabolism(53–55). In one study, cannabis users had lower low density lipoprotein-cholesterol; when cannabis was discontinued, a subset had an increase in weight greater than non-users(53). In 2016, a small randomized double-blind trial showed that in patients with diabetes mellitus, tetrahydrocannabivarin (compared with placebo) significantly decreased fasting plasma glucose levels and improved pancreatic β-cell function(56).
Potential Pharmacologic Interactions with Cardiovascular Medications
Cannabinoids can interfere with the action of multiple classes of cardiovascular therapies by inhibiting the cytochrome P450 (CYP450) family(10, 57, 58). Additional pharmacokinetic interactions may occur at the level of membrane transporters. Glycoprotein P (P-gp) expression is affected by the duration of exposure to cannabinoids(59). With chronic exposure, the expression of P-gp is downregulated, but with short exposure it is upregulated. Cannabinoids inhibit breast cancer-resistant protein and increase accumulation of its substrates(60). Additionally, a withdrawal phenomenon has been reported after abrupt discontinuation due to the high affinity of THC to cannabinoid binding receptors (13).
Cannabidiol
Cannabidiol is a substrate of CYP3A4 and CYP2C19 and is a more potent inhibitor of CYP3A and CYP2D6 as compared with other cannabinoids (Table 3)(10). Additionally, it also influences uridine 5′-diphospho (UDP)-glucuronosyltransferases.
Table 3.
Pharmacokinetic characteristics of cannabinoids
| Cannabinoid Compound | Substrate Pathway | Affected Metabolism Pathways | |
|---|---|---|---|
| Inhibitor | Inducer | ||
| Cannabidiol | CYP3A4 CYP2C19 |
CYP3A4 CYP2D6 CYP2C8/9/19 CYP1A1/2 CYP1B1 CYP2B6 |
|
| Tetrahydrocannabinol | CYP2C9 CYP3A4 |
CYP3A CYP2D6 CYP2C9 CYP2B6 |
CYP1A1/2 |
| Cannabinol | CYP2C9 CYP3A4 |
CYP3A CYP2D6 CYP2C9 CYP2B6 |
|
| Synthetic Cannabinoids | CYP2C9 CYP1A2 CYP2D6 |
CYP1A CYP2C8/9/19 CYP3A |
|
Cannabinol and Tetrahydrocannabinol
THC and CBN are substrates of CYP2C9 and CYP3A4 and both similarly inhibit a variety CYP450 enzymes (10). CBN additionally inhibits UDP-glucuronosyltransferases enzymes and THC has been shown to induce the CYP1A enzyme.
Synthetic cannabinoids
There is very little evidence surrounding the pharmacokinetic and pharmacodynamic effects of SCBs. In vitro studies have shown that SCBs are potential substrates of CYP2C9, CYP1A2, CYP2D6, and other CYP450 enzymes (depending on the specific formulation of SCB) (Table 3)(10, 13).
Affected Medication Classes
Cannabinoids affect key classes of cardiovascular medications including antiarrhythmics, calcium channel blockers, statins, β-blockers, and warfarin (Table 4)(10, 13, 61–63). The anticipated changes in drug levels are described, but limited clinical data are available guiding need for dose or therapeutic changes.
Table 4.
Medications affected by cannabinoids
| Mechanism | Cannabinoid involved | Key Therapy Affected | Anticipated Change in Drug Level |
|---|---|---|---|
| CYP3A4 Inhibition | CBD, THC, CBN, SCB | Anti-Arrhythmic [Amiodarone, Quinidine, Lidocaine] | ↑ |
| Calcium Channel Blockers [Dihydropyridine + Non- Dihydropyridine] | ↑ | ||
| Isosorbide dinitrate/mononitrate | ↑ | ||
| HMG-CoA Reductase inhibitors [Atorvastatin, Lovastatin, Simvastatin] | ↑ | ||
| CYP2C9 Inhibition | CBD, THC, CBN, SCB | Warfarin | ↑ |
| HMG-CoA Reductase inhibitors [Rosuvastatin, Fluvastatin] | ↑ | ||
| Non-steroidal anti-inflammatory drugs [Celecoxib, Ibuprofen, Naproxen] | ↑ | ||
| CYP2D6 Inhibition | CBD, THC, CBN | Beta Blockers [carvedilol, metoprolol] | ↑ |
| Anti-arrhythmic [flecainide, mexiletine, propafenone] | ↑ | ||
| CYP1A Inhibition/Induction | CBD, CBN, SCB | Theophylline, Caffeine | Inhibition: ↑ Induction: ↓ |
CBD: cannabidiol; CBN: Cannabinol; SCB: Synthetic Cannabinoids; THC: Tetrahydrocannabinol
Cardiovascular Clinical Care Applications
When to Screen and Test
In light of accumulating data suggesting prevalent use of marijuana, including among patients with established cardiovascular disease, it is important to integrate screening, counseling, and testing when appropriate into clinical care (Central Illustration). Cardiovascular specialists should be aware of local regulations and state-specific legalization status of marijuana products. We would advocate for routine screening for marijuana use. There are a variety of tools available for assessing for marijuana use, although most were validated in adolescents and young adults and no 1 tool has been universally accepted(64, 65). Whenever possible, questions should encompass frequency, quantity, and methods of administration (i.e. joints, hand pipes, vaporizers, edibles, oils)(64). Based on epidemiologic predilection, screening may be particularly high yield in states with reported high marijuana use density and among young patients presenting with cardiovascular disease. It may be reasonable to also perform urine toxicology in the setting of myocardial infarction and new-onset heart failure. Marijuana testing is required prior to an evaluation for heart transplantation. Use of non-prescription SCBs should be avoided given higher potential for pharmacologic manipulation and increased potency. Patients should be reminded that marijuana (when smoked) yields an inhaled chemical profile comparable to tobacco smoking. Patients should be screened for and counseled regarding the hazards of concurrent use of other illicit drugs, especially those with known adverse cardiovascular effects (e.g. cocaine, methamphetamines).
Central Illustration. Practical approach to screening for marijuana use among patients with cardiovascular disease.
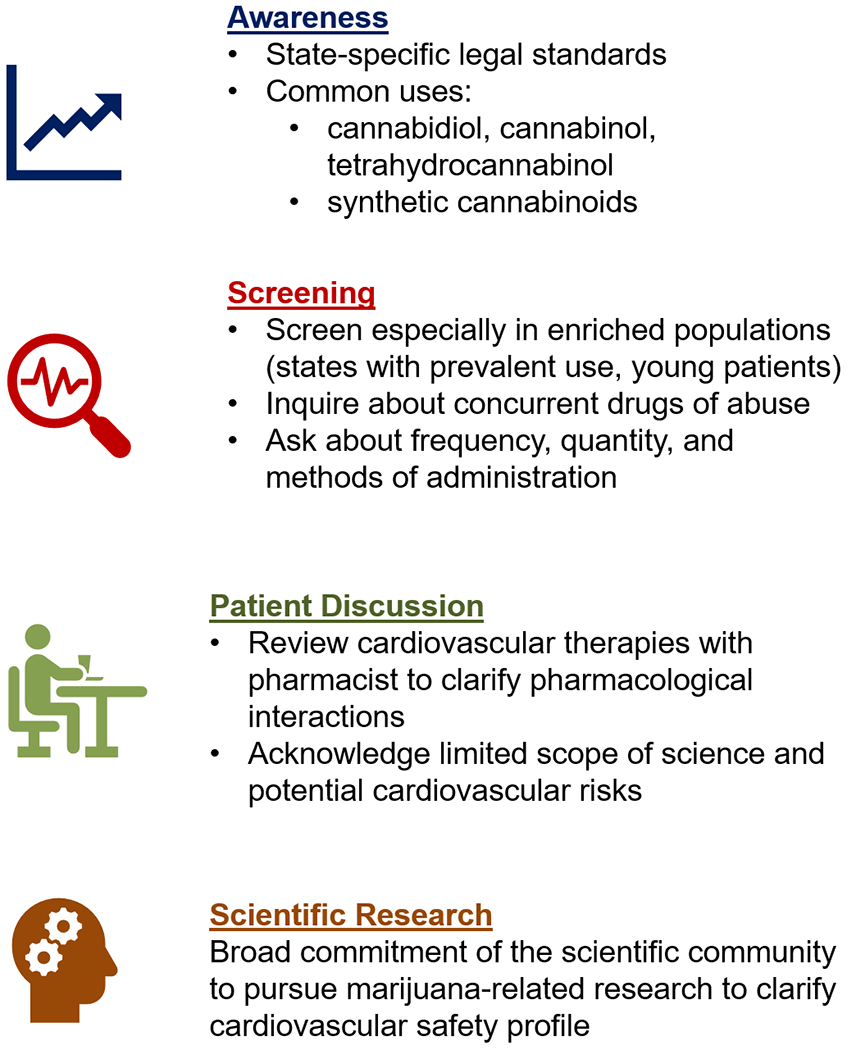
In light of the accumulating data regarding marijuana use and cardiovascular effects, it is increasingly important for clinicians to screen patients for use, educate about its potential effects, and contribute to ongoing research in the field.
Clinical Implications
Among patients with cardiovascular disease and known marijuana use, multidisciplinary assessment with a pharmacist is encouraged to determine whether anticipatory dose changes are required for therapies with known interactions. Heightened awareness is needed among cardiovascular specialists of the broad range of potential health consequences of marijuana and its derivatives. Cardiovascular specialists should have open discussions with patients acknowledging the limited scientific data, but potential cardiovascular hazards of marijuana use, especially when used via smoking/inhalation routes. Given the increasing popularity of “vaping” in the US, marijuana is also being delivered in vaporized forms, especially among young adults. Clinicians should counsel patients about the variable concentrations of psychoactive THC delivered via different methods of use; importantly, vaporized cannabis may yield high concentrations with greater pharmacodynamic effects than smoked cannabis(66). Shared decision-making is encouraged if marijuana is used for symptom management or palliative purposes, incorporating estimates of life expectancy and cardiovascular risks.
Heart Transplantation
Heart transplant candidacy may be affected by marijuana use(67). Current International Society for Heart and Lung Transplant guidelines allow each center to develop its own criteria for candidacy regarding marijuana use. Potential concerns include medication adherence due to the psychotropic effects of THC, infectious complications in the setting of immunosuppression, as well as interactions with tacrolimus due to inhibition of CYP3A4(67).
Gaps in Knowledge and Next Steps
Currently, there are no guidelines surrounding marijuana and cardiovascular disease. In 2017, the National Academies of Sciences, Engineering and Medicine released a report on the health effects of marijuana. With regard to cardiometabolic risk, they concluded that the evidence was unclear regarding the association between cannabis use and myocardial infarction, stroke, and diabetes mellitus(68).
Lack of Regulation
With growing use and potential multi-system health effects, it is critical to regulate marijuana(69). On May 31, 2019, at an FDA public hearing, continued efforts to develop new drugs from cannabis were encouraged, while evaluating questions related to safety through development of an internal working group(70).
Need for Research
Significant barriers exist to cannabis research(71, 72) which include, but are not limited to, the heterogeneity of drug (i.e. various forms and routes of administration) and variability in state laws and their implementation(73). Real-world observational studies have inconsistently reported use, dose, and formulation. For instance, cannabis is the source of over 60 compounds with varying pharmacologic activity(73). The scientific community and federal government should remain committed to marijuana-related research so that safe and effective products can be developed. States where marijuana legalization is impending may allow for randomized, stepped roll out as an opportunity to study potential population-level effects.
Due to its Schedule I status, it is illegal to conduct rigorous controlled trials of marijuana products in the US. A search of ClinicalTrials.gov for the terms “marijuana,” “cannabidiol,” and “THC” yielded studies mostly outside the US in a broad range of conditions including neurodegenerative diseases, inflammatory bowel disease, cancer, pain syndromes, addiction, and pediatric epilepsy. Few trials were evaluating cardiovascular risk markers and none of which were actively enrolling as of July 2019 or large enough to assess cardiovascular outcomes.
Conclusions
Marijuana use continues to increase nationally in light of changing policies around legalization. We estimate that over 2 million patients with cardiovascular disease report current or prior use of marijuana. Observational studies have suggested a potential association between marijuana and a range of cardiovascular risks, although the level of evidence has not been robust. Few randomized clinical trials have been conducted or are planned to examine effects of marijuana on cardiovascular risk, due in part to its Schedule I federal designation as a controlled substance. Acknowledging the modest strength of current evidence, screening and testing for use of marijuana in select cardiovascular settings is encouraged. Furthermore, patients at risk of cardiovascular events should be counseled to avoid or at least minimize marijuana use. It is imperative to conduct rigorous scientific research to inform recommendations for patient care and to provide a framework for the cardiovascular community.
Brief Highlights.
We estimate that over 2 million US adults who reported ever using marijuana have cardiovascular disease
Observational studies have suggested an association between marijuana and a range of cardiovascular risks
Marijuana is becoming increasingly potent, and smoking marijuana carries many of the same cardiovascular health hazards as smoking tobacco.
Few randomized clinical trials have been conducted or are planned to explore the effects of marijuana on cardiovascular risk
Screening and testing for use of marijuana are encouraged in clinical settings, especially in care of young patients presenting with cardiovascular disease
Acknowledgments
Funding: None
Disclosures:
Dr. Blankstein receives research support from Amgen Inc, and Astellas Inc.
Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), J Am Coll Cardiol (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda.
Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, and Relypsa, and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH.
The remaining authors have no relationships relevant to the contents of this paper to disclose.
Footnotes
Tweet: Learn about mechanisms of CV risk, drug interactions, and screening tools related to marijuana use in high-risk patients in new @JACCJournals review.
Twitter Handles: @ersied727 @mvaduganathan
References
- 1.Wilkinson ST, Yarnell S, Radhakrishnan R, Ball SA, D’Souza DC. Marijuana Legalization: Impact on Physicians and Public Health. Annu. Rev. Med. 2016;67:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerdá M, Wall M, Keyes KM, Galea S, Hasin D. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend. 2012;120:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatnagar A, Whitsel LP, Ribisl KM, et al. Electronic cigarettes: a policy statement from the American Heart Association. Circulation 2014;130:1418–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatnagar A, Maziak W, Eissenberg T, et al. Water Pipe (Hookah) Smoking and Cardiovascular Disease Risk: A Scientific Statement From the American Heart Association. Circulation 2019;139:e917–e936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Layden JE, Ghinai I, Pray I, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Preliminary Report. N. Engl. J. Med. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman TM, Fazio S, Shapiro MD. Brief Commentary: Marijuana and Cardiovascular Disease—What Should We Tell Patients? Ann. Intern. Med. 2019;170:119. [DOI] [PubMed] [Google Scholar]
- 7.Singh A, Saluja S, Kumar A, et al. Cardiovascular Complications of Marijuana and Related Substances: A Review. Cardiol. Ther 2018;7:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franz CA, Frishman WH. Marijuana Use and Cardiovascular Disease. Cardiol. Rev. 2016;24:158–162. [DOI] [PubMed] [Google Scholar]
- 9.Thomas G, Kloner RA, Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: what cardiologists need to know. Am. J. Cardiol. 2014;113:187–190. [DOI] [PubMed] [Google Scholar]
- 10.Alsherbiny MA, Li CG. Medicinal Cannabis-Potential Drug Interactions. Med. Basel Switz. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackie K Cannabinoid receptors: where they are and what they do. J. Neuroendocrinol. 2008;20 Suppl 1:10–14. [DOI] [PubMed] [Google Scholar]
- 12.Zou S, Kumar U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai S, Fantegrossi WE. Pharmacological and Toxicological Effects of Synthetic Cannabinoids and Their Metabolites. Curr. Top. Behav. Neurosci. 2017;32:249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacher P, Steffens S, Haskó G, Schindler TH, Kunos G. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat. Rev. Cardiol 2017. [DOI] [PubMed] [Google Scholar]
- 15.Hill GED, Izquierdo DA, Boettcher BT, Pagel PS. Chronic Marijuana and Synthetic Cannabinoid-Induced Toxic Myocarditis and End-Stage Cardiomyopathy: Management With Mechanical Circulatory Support as a Bridge-to-Transplantation. J. Cardiothorac. Vasc. Anesth. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Dai H, Richter KP. A National Survey of Marijuana Use Among US Adults With Medical Conditions, 2016–2017. JAMA Netw. Open 2019;2:e1911936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai R, Shamim S, Patel K, et al. Primary Causes of Hospitalizations and Procedures, Predictors of In-hospital Mortality, and Trends in Cardiovascular and Cerebrovascular Events Among Recreational Marijuana Users: A Five-year Nationwide Inpatient Assessment in the United States. Cureus 2018;10:e3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carliner H, Brown QL, Sarvet AL, Hasin DS. Cannabis use, attitudes, and legal status in the U.S.: A review. Prev. Med 2017;104:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jouanjus E, Raymond V, Lapeyre-Mestre M, Wolff V. What is the Current Knowledge About the Cardiovascular Risk for Users of Cannabis-Based Products? A Systematic Review. Curr. Atheroscler. Rep 2017;19:26. [DOI] [PubMed] [Google Scholar]
- 20.Singla S, Sachdeva R, Mehta JL. Cannabinoids and atherosclerotic coronary heart disease. Clin. Cardiol. 2012;35:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draz EI, Oreby MM, Elsheikh EA, Khedr LA, Atlam SA. Marijuana use in acute coronary syndromes. Am. J. Drug Alcohol Abuse 2017;43:576–582. [DOI] [PubMed] [Google Scholar]
- 22.Richards JR, Bing ML, Moulin AK, et al. Cannabis use and acute coronary syndrome. Clin. Toxicol. Phila. Pa 2019:1–11. [DOI] [PubMed] [Google Scholar]
- 23.Aronow WS, Cassidy J. Effect of marihuana and placebo-marihuana smoking on angina pectoris. N. Engl. J. Med. 1974;291:65–67. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein A, Brickner O, Lerman H, et al. Brain imaging study of the acute effects of Delta9-tetrahydrocannabinol (THC) on attention and motor coordination in regular users of marijuana. Psychopharmacology (Berl.) 2008;196:119–131. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Derakhshandeh R, Liu J, et al. One Minute of Marijuana Secondhand Smoke Exposure Substantially Impairs Vascular Endothelial Function. J. Am. Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawrot TS, Perez L, Künzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet Lond. Engl. 2011;377:732–740. [DOI] [PubMed] [Google Scholar]
- 27.Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805–2809. [DOI] [PubMed] [Google Scholar]
- 28.Frost L, Mostofsky E, Rosenbloom JI, Mukamal KJ, Mittleman MA. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am. Heart J. 2013;165:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFilippis EM, Singh A, Divakaran S, et al. Cocaine and Marijuana Use Among Young Adults With Myocardial Infarction. J. Am. Coll. Cardiol. 2018;71:2540–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai R, Patel U, Deshmukh A, Sachdeva R, Kumar G. Burden of arrhythmia in recreational marijuana users. Int. J. Cardiol. 2018;264:91–92. [DOI] [PubMed] [Google Scholar]
- 31.Korantzopoulos P Marijuana smoking is associated with atrial fibrillation. Am. J. Cardiol. 2014;113:1085–1086. [DOI] [PubMed] [Google Scholar]
- 32.Mithawala P, Shah P, Koomson E. Complete Heart Block From Chronic Marijuana Use. Am. J. Med. Sci. 2019;357:255–257. [DOI] [PubMed] [Google Scholar]
- 33.Aryana A, Williams MA. Marijuana as a trigger of cardiovascular events: speculation or scientific certainty? Int. J. Cardiol 2007;118:141–144. [DOI] [PubMed] [Google Scholar]
- 34.Bachs L, Mørland H. Acute cardiovascular fatalities following cannabis use. Forensic Sci. Int. 2001;124:200–203. [DOI] [PubMed] [Google Scholar]
- 35.Volpon LC, Sousa CLM de M, Moreira SKK, Teixeira SR, Carlotti AP de CP. Multiple Cerebral Infarcts in a Young Patient Associated With Marijuana Use. J. Addict. Med. 2017;11:405–407. [DOI] [PubMed] [Google Scholar]
- 36.Hemachandra D, McKetin R, Cherbuin N, Anstey KJ. Heavy cannabis users at elevated risk of stroke: evidence from a general population survey. Aust. N. Z. J. Public Health 2016;40:226–230. [DOI] [PubMed] [Google Scholar]
- 37.Singh NN, Pan Y, Muengtaweeponsa S, Geller TJ, Cruz-Flores S. Cannabis-related stroke: case series and review of literature. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2012;21:555–560. [DOI] [PubMed] [Google Scholar]
- 38.Desbois AC, Cacoub P. Cannabis-associated arterial disease. Ann. Vasc. Surg. 2013;27:996–1005. [DOI] [PubMed] [Google Scholar]
- 39.Bernson-Leung ME, Leung LY, Kumar S. Synthetic cannabis and acute ischemic stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014;23:1239–1241. [DOI] [PubMed] [Google Scholar]
- 40.Zachariah SB. Stroke after heavy marijuana smoking. Stroke 1991;22:406–409. [DOI] [PubMed] [Google Scholar]
- 41.Wolff V, Zinchenko I, Quenardelle V, Rouyer O, Geny B. Characteristics and Prognosis of Ischemic Stroke in Young Cannabis Users Compared With Non-Cannabis Users. J. Am. Coll. Cardiol. 2015;66:2052–2053. [DOI] [PubMed] [Google Scholar]
- 42.Raheemullah A, Laurence TN. Repeated Thrombosis After Synthetic Cannabinoid Use. J. Emerg. Med. 2016;51:540–543. [DOI] [PubMed] [Google Scholar]
- 43.Disdier P, Granel B, Serratrice J, et al. Cannabis arteritis revisited--ten new case reports. Angiology 2001;52:1–5. [DOI] [PubMed] [Google Scholar]
- 44.Ducasse E, Chevalier J, Dasnoy D, Speziale F, Fiorani P, Puppinck P. Popliteal artery entrapment associated with cannabis arteritis. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2004;27:327–332. [DOI] [PubMed] [Google Scholar]
- 45.Santos RP, Resende CIP, Vieira AP, Brito C. Cannabis arteritis: ever more important to consider. BMJ Case Rep. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su Z, Preusser L, Diaz G, et al. Negative inotropic effect of a CB2 agonist A-955840 in isolated rabbit ventricular myocytes is independent of CB1 and CB2 receptors. Curr. Drug Saf. 2011;6:277–284. [DOI] [PubMed] [Google Scholar]
- 47.Grigoriadis CE, Cork DP, Dembitsky W, Jaski BE. Recurrent Cardiogenic Shock Associated with Cannabis Use: Report of a Case and Review of the Literature. J. Emerg. Med. 2019;56:319–322. [DOI] [PubMed] [Google Scholar]
- 48.Kariyanna PT, Jayarangaiah A, Singh N, et al. Marijuana Induced Myocarditis: A New Entity of Toxic Myocarditis. Am. J. Med. Case Rep 2018;6:169–172.30533519 [Google Scholar]
- 49.Leontiadis E, Morshuis M, Arusoglu L, Cobaugh D, Koerfer R, El-Banayosy A. Thoratec left ventricular assist device removal after toxic myocarditis. Ann. Thorac. Surg. 2008;86:1982–1985. [DOI] [PubMed] [Google Scholar]
- 50.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015;313:2456–2473. [DOI] [PubMed] [Google Scholar]
- 51.DeJesus E, Rodwick BM, Bowers D, Cohen CJ, Pearce D. Use of Dronabinol Improves Appetite and Reverses Weight Loss in HIV/AIDS-Infected Patients. J. Int. Assoc. Physicians AIDS Care Chic. Ill 2002 2007;6:95–100. [DOI] [PubMed] [Google Scholar]
- 52.Topol EJ, Bousser M- G, Fox KAA, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet Lond. Engl. 2010;376:517–523. [DOI] [PubMed] [Google Scholar]
- 53.Vázquez-Bourgon J, Setién-Suero E, Pilar-Cuéllar F, et al. Effect of cannabis on weight and metabolism in first-episode non-affective psychosis: Results from a three-year longitudinal study. J. Psychopharmacol. Oxf. Engl. 2019;33:284–294. [DOI] [PubMed] [Google Scholar]
- 54.Clark TM, Jones JM, Hall AG, Tabner SA, Kmiec RL. Theoretical Explanation for Reduced Body Mass Index and Obesity Rates in Cannabis Users. Cannabis Cannabinoid Res. 2018;3:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alshaarawy O, Anthony JC. Are cannabis users less likely to gain weight? Results from a national 3-year prospective study. Int. J. Epidemiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jadoon KA, Ratcliffe SH, Barrett DA, et al. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016;39:1777–1786. [DOI] [PubMed] [Google Scholar]
- 57.Foster BC, Abramovici H, Harris CS. Cannabis and Cannabinoids: Kinetics and Interactions. Am. J. Med. 2019. [DOI] [PubMed] [Google Scholar]
- 58.Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab. Rev. 2014;46:86–95. [DOI] [PubMed] [Google Scholar]
- 59.Zhu H- J, Wang J- S, Markowitz JS, et al. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J. Pharmacol. Exp. Ther. 2006;317:850–857. [DOI] [PubMed] [Google Scholar]
- 60.Holland ML, Panetta JA, Hoskins JM, et al. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem. Pharmacol. 2006;71:1146–1154. [DOI] [PubMed] [Google Scholar]
- 61.Owen RP, Sangkuhl K, Klein TE, Altman RB. Cytochrome P450 2D6. Pharmacogenet. Genomics 2009;19:559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamreudeewong W, Wong HK, Brausch LM, Pulley KR. Probable interaction between warfarin and marijuana smoking. Ann. Pharmacother. 2009;43:1347–1353. [DOI] [PubMed] [Google Scholar]
- 63.Cox EJ, Maharao N, Patilea-Vrana G, et al. A marijuana-drug interaction primer: Precipitants, pharmacology, and pharmacokinetics. Pharmacol. Ther. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuttler C, Spradlin A. Measuring cannabis consumption: Psychometric properties of the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU). PloS One 2017;12:e0178194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Legleye S, Guignard R, Richard J- B, Ludwig K, Pabst A, Beck F. Properties of the Cannabis Abuse Screening Test (CAST) in the general population. Int. J. Methods Psychiatr. Res 2015;24:170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spindle TR, Cone EJ, Schlienz NJ, et al. Acute Effects of Smoked and Vaporized Cannabis in Healthy Adults Who Infrequently Use Cannabis: A Crossover Trial. JAMA Netw. Open 2018;1:e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeFilippis EM, Givertz MM. Marijuana use and candidacy for heart transplantation. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2019;38:589–592. [DOI] [PubMed] [Google Scholar]
- 68.Anon. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies of Sciences, Engineering, and Medicine; 2017. Available at: http://nationalacademies.org/hmd/Reports/2017/health-effects-of-cannabis-and-cannabinoids.aspx. [PubMed] [Google Scholar]
- 69.Ayers JW, Caputi T, Leas EC. The Need for Federal Regulation of Marijuana Marketing. JAMA 2019. [DOI] [PubMed] [Google Scholar]
- 70.Sharpless N Scientific Data and Information About Products Containing Cannabis or Cannabis-Derived Compounds. 2019. Available at: https://www.fda.gov/news-events/speeches-fda-officials/remarks-dr-sharpless-fda-public-hearing-scientific-data-and-information-about-products-containing. [Google Scholar]
- 71.Stith SS, Vigil JM. Federal barriers to Cannabis research. Science 2016;352:1182. [DOI] [PubMed] [Google Scholar]
- 72.Shen H Federal red tape ties up marijuana research. Nature 2014;507:407–408. [DOI] [PubMed] [Google Scholar]
- 73.Choo EK, Emery SL. Clearing the haze: the complexities and challenges of research on state marijuana laws. Ann. N. Y. Acad. Sci. 2017;1394:55–73. [DOI] [PubMed] [Google Scholar]


