Abstract
BACKGROUND AND PURPOSE: Transarterial detachable balloon embolization of direct carotid cavernous fistulas (DCCFs) has become an optimal treatment. In a few cases, the parent artery has to be sacrificed to achieve morphologic cure. We present our experience with transarterial balloon-assisted n-butyl-2-cyanoacrylate (n-BCA) embolization of DCCFs in which there was failure to achieve angiographic cure and preservation of parent arteries.
METHODS: Of 141 patients with traumatic DCCFs who had been treated by transarterial embolization with occlusion of the fistula and parent artery preservation, 18 received transarterial balloon-assisted n-BCA embolization—6 for residual fistula after the balloons detached, 7 for recurrent fistula because of premature balloon deflation or migration, and 5 for repeated puncture of the detachable balloon by the bony fragment at the cavernous sinus. A total of 27 procedures were performed with an average 1.5 attempts per patient, and the volume of the n-BCA mixture varied from 0.5 to 2.3 mL with a mean of 0.83 mL.
RESULTS: All DCCFs were successfully occluded by the n-BCA mixture with preservation of parent arteries. One patient with a giant cavernous sinus varix had a fatal subarachnoid hemorrhage. One had a recurrence and was treated by internal carotid artery (ICA) occlusion. Five had asymptomatic pseudoaneurysms at the parent artery. There was no adhesion of the n-BCA mixture to the protective balloon or the microcatheter or n-BCA reflux into the parent arteries.
CONCLUSION: Transarterial balloon-assisted n-BCA embolization is a feasible, efficient, and safe treatment for DCCFs when angiographic cure and ICA preservation are not achieved by transarterial detachable balloon embolization.
Embolization of a direct carotid cavernous fistula (DCCF) by transarterial balloon detachment is a well-established therapeutic option.1–6 The goal of treatment is to occlude the fistula with preservation of the parent artery. Recently, refinements in detachable balloon techniques have led to an increase in the rate of internal carotid artery (ICA) patency to 75%.4 In the remaining cases, the ICA must be sacrificed to obtain an angiographic cure. The reasons for this undesired ICA occlusion were the following: (1) a large tear or complete transection of the ICA, (2) a fistulous orifice too small to allow balloon entry or inability to guide the balloon into the involved ICA segment because of intimal flaps, and (3) repeated balloon puncture by sharp bone fragments.4,7,8 Therefore, it is unlikely that the proportion of patients for which the ICA is preserved could be further increased by the traditional transarterial balloon embolization.
Since the introduction of detachable coils, they have been used to occlude DCCFs either by transvenous or transarterial means with promising results.9–12 However, there was still a need for parent artery occlusion via the transarterial route in approximately 15%–20% of the cases.9,10,13
N-butyl-2-cyanoacrylate (n-BCA) is a liquid adhesive and permanent embolizing agent. It has been used widely for embolization of intracranial arteriovenous malformations, indirect carotid cavernous fistulas, and, occasionally, cerebral aneurysms.14,15 n-BCA as an embolizing agent for endovascular embolization of traumatic DCCF has not been widely evaluated.
The purpose of this study was to report our experience of transarterial balloon-assisted n-BCA embolization for those traumatic DCCFs that failed to achieve angiographic cure and ICA preservation by detachable balloon and to evaluate the feasibility, efficacy, and safety of transarterial balloon-assisted n-BCA embolization.
Patients and Methods
From January 1992 to December 2004, we treated 176 patients with traumatic DCCFs. Of these, 141 patients (80%) had been successfully treated, with preservation of the ICA by transarterial embolization. Among 176 patients, 22 underwent transarterial balloon-assisted n-BCA embolization because we had failed to achieve occlusion by transarterial balloon occlusion. Of these 22 patients, 18 fistulas were successfully embolized by the transarterial balloon-assisted n-BCA method with preservation of the ICA on immediate postembolization angiography; 4 failed to achieve angiographic cure with ICA preservation presumably because of a large fistula or multiple tears of the ICA. The clinical data of the 18 patients are summarized in the Table. Seventeen patients had been involved in a motor vehicle crash; in one, the origin was iatrogenic. These patients were 13 men and 5 women whose ages ranged from 19 to 58 years (mean, 36 years). All patients had signs and symptoms related to the DCCFs, the most common being chemosis (n = 16), bruit (n = 16), proptosis (n = 12), decreasd visual acuity (n = 14), impairment of cranial nerve function (n = 6), or epistasis (n = 1).
Summary of 18 DCCF cases treated by transarterial balloon-assisted n-BCA embolization with internal carotid artery preservation
| Patient No./Sex/Age (y) | Indications for n-BCA | Embolizing Agents prior to n-BCA | Volume of n-BCA (mL) | Outcome | Follow-up (mo) |
|---|---|---|---|---|---|
| 1/M/49 | Residual DCCF | Balloon, 2 GDCs | 0.5 | Cure | 25 |
| 2/M/40 | Residual DCCF | 2 balloons, 2 coils | 0.7, 0.4 | Cure | 24 |
| 3/M/25 | Residual DCCF | 4 balloons | 0.8, 0.4 | Pseudoaneurysm | 20 |
| 4/M/29 | Residual DCCF | 2 balloons, 2 GDCs | 0.7, 0.4 | Cure | 18 |
| 5/F/39 | Residual DCCF | Balloon, 3 GDCs | 0.5 | Cure | 25 |
| 6/M/30 | Residual DCCF | Balloon, 3 coils | 1.3, 0.7, 0.3 | Pseudoaneurysm, fatal subarachnoid hemorrhage | 1 |
| 7/F/38 | Recurrent DCCF | Balloon, 1 GDC | 0.7 | Pseudoaneurysm | 12 |
| 8/M/19 | Recurrent DCCF | Balloon, 2 GDCs | 0.6 | Cure | 20 |
| 9/M/53 | Recurrent DCCF | 2 ballons, 2 coils | 0.5 | Cure | 36 |
| 10/F/24 | Recurrent DCCF | Balloon, 2 GDCs | 0.7, 0.3 | Pseudoaneurysm | 6 |
| 11/M/39 | Recurrent DCCF | 2 balloons, 2 GDCs | 0.6, 0.4 | Cure | 12 |
| 12/M/29 | Recurrent DCCF | 2 balloons, 3 GDCs | 0.6 | Cure | 5 |
| 13/M/28 | Recurrent DCCF | Balloon, 3 GDCs | 0.5 | Cure | 24 |
| 14/M/50 | Puncture balloon | Coil | 0.6, 0.4 | Cure | 12 |
| 16/M/22 | Puncture balloon | 3 GDCs | 0.7 | Pseudoaneurysm | 13 |
| 16/F/58 | Puncture balloon | 2 coils | 0.7 | Cure | 26 |
| 17/M/41 | Puncture balloon | 2 GDCs | 0.7, 0.3 | Cure | 12 |
| 18/F/38 | Puncture balloon | 2 coils | 0.9 | Recurrence | 13 |
Note:—DCCF indicates direct carotid cavernous fistula; n-BCA, n-butyl-2-cyanoacrylate; GDC, Guglielmi detachable coil.
The first indication for transarterial balloon-assisted n-BCA embolization was a residual fistula after balloon detachment (n = 6, Figs 1 and 2); the cause of failure to achieve an angiographic cure was presumably related to multiple tears or a large tear and/or a large cavernous sinus. The second indication was a recurrent fistula after balloon treatment because of premature balloon deflation and/or migration (n = 7) (Fig 3); navigation of additional detachable balloons into the cavernous sinus failed because of blockade by previously detached balloons. The third indication was repeated puncture of the detachable balloons by the bony fragment of the cavernous sinus during or after balloon inflation (n = 5) (Fig 4).
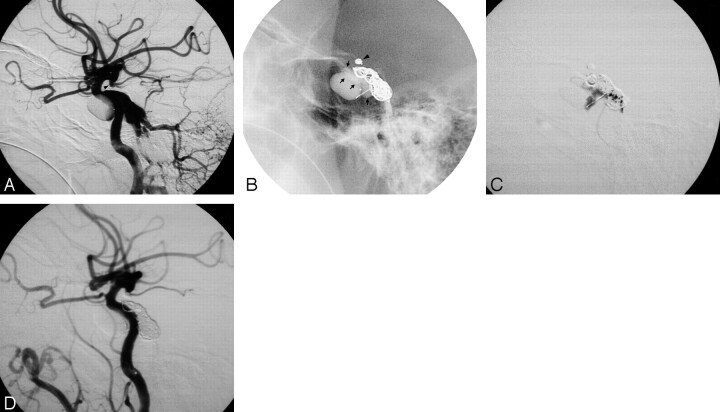
Fig 1.
Images of a 49-year man with a traumatic DCCF and a traumatic aneurysm at the left supraclinoid ICA. A, Left lateral carotid angiogram reveals a residual fistula after a detachable balloon embolization. B and C, Two GDCs were placed into the cavernous sinus. Further coil embolization to achieve angiographic cure failed because of recoil of the microcatheter, and the traumatic aneurysm was occluded by GDC (arrowhead). Under a protective balloon (arrows) at the cavernous portion of the ICA, a total of 0.5 mL of n-BCA mixture was infused into the cavernous sinus. D, Postembolization angiogram reveals total obliteration of the residual fistula with preservation of the ICA flow.
Fig 2.
Images of a 40-year-old man with traumatic DCCF. A, Right lateral carotid angiogram shows a residual fistula after 2 detached balloons and 1 coil embolization. B, A total of 1.1 mL n-BCA mixture was slowly deposited to the cavernous sinus in 2 attempts. C, Postembolization angiogram shows total occlusion of the fistula with ICA preservation.
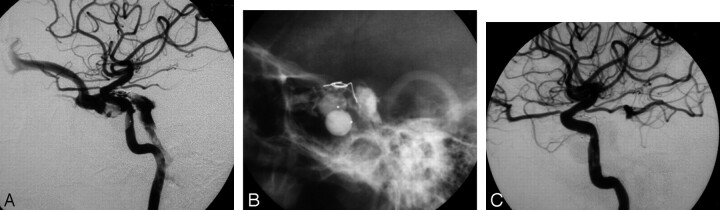
Fig 3.
Images of a 38-year-old woman with a DCCF; the DCCF was successfully occluded by a detached balloon with patency of the ICA. A, Premature balloon deflation causes recurrent DCCF and drains to the superior ophthalmic vein. Navigation of the additional balloon into the cavernous sinus failed because of blockage by a previously detached balloon (arrow). B and C, One GDC and 0.7 mL of n-BCA mixture were injected into the cavernous sinus under a protective balloon at the cavernous portion of the ICA (arrows), resulting in an angiographic cure of the recurrent DCCF with ICA preservation. Note a small asymptomatic false sac (arrowhead) at the cavernous sinus owing to insufficient NBCA filling.
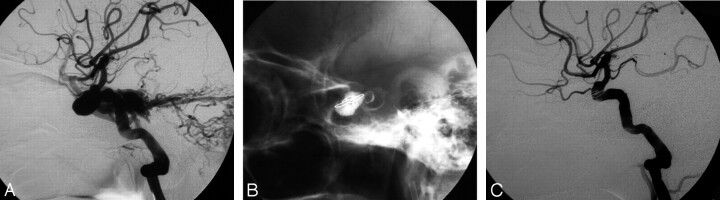
Fig 4.
Images of a 50-year-old man in whom the DCCF failed to occlude because of repeated puncture of the balloons. A, Left lateral angiogram shows a DCCF. B and C, The fistula was successfully obliterated with ICA preservation by a microcoil and 1 mL of n-BCA mixture.
All procedures were performed with the patient under local anesthesia by using a femoral artery approach. Systemic heparinization was achieved by administering an intravenous bolus of heparin (3000 IU) and maintained by a continuous intravenous infusion of heparin (1500 IU/hour). Activated clotting time measurements were performed every half hour during the procedure, and clotting time was maintained at a value that was approximately twice the baseline value. A 7F–9F guiding catheter was positioned in the ICA. A microcatheter (Renegade; Boston Scientific Cork, Cork, Ireland) was introduced and navigated to coaxially pass through the fistula into the cavernous sinus. Selective angiograms were obtained through the microcatheter to delineate the anatomy of the cavernous sinus and its drains. After the position of the microcatheter tip was secured in the cavernous sinus, microcoils (Vortx; Boston Scientific Cork) or Guglielmi detachable coils (GDCs, Target Therapeutics, Fremont, Calif) were introduced in 17 patients through the microcatheter into the cavernous sinus for partial blockage and reduction of the fistulous flow and as a framework for polymerization of the subsequent n-BCA embolization. In 1 patient, the fistula flow was partially blocked by 4 detached balloons. Therefore, a microcoil was not used as a second embolizing agent before transarterial balloon-assisted n-BCA embolization.
A mixture of 60% n-BCA/Lipiodol (iodized oil, Andre Guerbet, Aulnay-sous-Bois, France) was prepared. Before injection of the n-BCA, a second microcatheter (GVB) with a protective detachable balloon with 8 × 21 mm in maximal size and 0.8-mL volume was navigated and inflated in the ICA at the fistula site to occlude the fistula hole. An angiogram through the microcatheter was obtained to evaluate the adequacy of the situation to prevent reflux of the n-BCA mixture into the ICA. The injection of the n-BCA was preceded by a small amount of 5% dextrose to clean the microcatheter under temporary balloon protection (Figs 1B and 3B). After the n-BCA mixture reached the orifice of the fistula or advanced to the superior ophthalmic vein or inferior petrous sinus, the injection of the n-BCA was stopped and the microcatheter was withdrawn immediately. The protective balloon was deflated and removed a few seconds later to allow polymerization of the n-BCA mixture at the cavernous sinus and to prevent the escape of liquid n-BCA mixture into the ICA. The effect of heparin was reversed at the end of the procedure.
A postembolization angiogram was obtained immediately after the completion of the procedure to check for occlusion of the fistula. In 10 patients, residual fistulas were found. In 2 of these 10, the procedure was discontinued because of small and slow residual fistula flow and because spontaneous thrombosis was expected. The other 8 patients underwent a second (n = 7) or a third (n = 1) injection of the n-BCA mixture to achieve angiographic cure; the procedure was the same as the one we described previously. A total of 27 procedures were performed with an average of 1.5 per patient, and the volume of n-BCA mixture varied from 0.5 to 2.3 mL with a mean of 0.83 mL in each patient.
All 18 patients were followed clinically for an average of 17 months (range, 7 days to 36 months). Eight had follow-up angiograms either at 1 week (n = 4), 2 weeks (n = 2), 1 month (n = 1), or 3 months (n = 1) after embolization.
Results
Results and follow-up findings are listed in the Table. Complete fistula closures with preservation of the ICA were documented on immediate postembolization angiograms in 16 patients, whereas small residual flow remained in the other 2. In both of these 2 patients, spontaneous thrombosis was confirmed by follow-up angiograms 1 week later. Of the 16 patients with complete closure of the fistula seen on initial angiograms, 1 patient with a giant cavernous sinus varix developed a fatal subarachnoid hemorrhage on day 2 after embolization. Another patient with a DCCF after trans-sphenoid hypophysectomy had a recurrent fistula on day 3 after treatment and presented with massive epistaxis; the recurrent fistula was eventually treated by balloon occlusion of both the ICA and the fistula to save the patient’s life. In 4 patients, n-BCA mixtures migrated distally to the superior ophthalmic vein (n = 3) or the inferior petrous sinus (n = 1), but these patients remained asymptomatic. Five patients had an asymptomatic small false sac or pseudoaneurysm in the cavernous ICA because of shallow herniation of the protective balloon into the cavernous sinus through a large tear (n = 3) or insufficient n-BCA filling at the cavernous sinus (n = 2) (Fig 3C). Three patients experienced temporary impairment of cranial nerve function; this resolved completely within 6 months. No evidence of adhesion of the n-BCA mixture to the protective balloon or the microcatheter was observed, nor did we see reflux of the n-BCA mixtures into the cerebral or ophthalmic circulation.
All patients had complete resolution of symptoms related to their fistulas on postembolization imaging. Long-term angiography was not performed in most of these patients because the resolution of clinical symptoms was evident in each patient and clinical follow-up showed no recurrence of clinical symptoms.
Discussion
A traumatic DCCF results when the integrity of the cavernous portion of the ICA is disrupted, creating an abnormal communication between the high-pressure arterial systems and the low-pressure venous system. By this significant pressure gradient, a detachable balloon can be guided by fistula flow into the cavernous sinus through the fistula tract; after proper balloon inflation at the cavernous sinus, the inflated balloon can seal the fistula with occlusion of the shunt. Therefore, the size of the fistula hole and the cavernous sinus may affect the success rate for detachable balloon embolizations of DCCFs with preservation of the parent artery.16 Ideally, the size of the fistula hole should be larger than that of the deflated balloon (to allow access) and smaller than the inflated balloon (to prevent protrusion into the ICA). In addition, the cavernous sinus must be large enough to accommodate the detachable balloon for sealing the fistula. In some instances, the fistulous holes are too small to allow balloon entry and/or the cavernous sinuses are not big enough to accommodate the inflated balloon.
Apart from these drawbacks, some DCCFs may have a large tear and/or a dilated cavernous sinus with variable dimensions because of long-standing high-flow fistulas or multiple tears. For those DCCFs, it may be difficult to seal the fistulas by detachable balloon and may result in residual fistula. In approximately 25% of DCCFs, the anatomy of the fistula precludes obliteration with preservation of the parent artery.4 Recurrent fistulas may also occur, primarily because of easy migration of detached balloons in large cavernous sinuses. Furthermore, balloons may protrude or shift into parent arteries through large tears and thus create a risk of thromboembolism.
For DCCFs in which we failed to achieve angiographic cure with ICA preservation by using detachable balloons, transarterial GDC embolization is an alternative. In 1992, Guglielmi et al17 reported that a DCCF was successfully occluded by transvenous GDC embolization. Since then, there have been several reports regarding the transarterial GDC embolization of DCCFs with promising results.9–12 Recently, Moron et al12 used stent-assisted coil embolization to occlude 6 severe injury high-flow DCCFs with preservation of the ICA. The advantage of the GDC is that it is retrievable and can be precisely placed into the cavernous sinus and its proximal outlets. GDC is a good embolizing material for those DCCFs with small fistulas and/or small cavernous sinuses because a limited amount of GDCs may be enough to fill up the cavernous sinus and its outlets, resulting in an angiographic cure.11,13,17
For a DCCF with a large tear and/or a large cavernous sinus, GDC is not an appropriate embolizing material, largely because many GDCs are needed to densely pack the cavernous sinus and to occlude the fistula. The mass effect of the coils in the cavernous sinus may also cause long-term cranial nerve palsies. In addition, coil mass may easily protrude into the parent artery in the large tear or may obscure the relationship between cavernous sinus and the nearby parent artery, with increased risk of occluding the parent artery. However, these problems can be solved by recently developed stent-assisted GDC embolization.12
The n-BCA is in the monomeric liquid adhesive. The polymerization time of n-BCA is controlled by the addition of Lipiodol. The advantages of n-BCA are good penetration, dispersion that is freer than that for other embolizing materials, and rapid induction of thrombosis and permanent occlusion after polymerization. Also, it is relatively easy to deliver through a microcatheter. Kerber et al18 reported the successful occlusion of 3 DCCFs by using liquid iso-butyl-cyanoacrylate as an embolic agent; however, all 3 patients had iso-butyl-cyanoacrylate cerebral embolization, though the symptoms were reversed thereafter. Evans et al19 reported that transarterial balloon-assisted n-BCA embolization successfully obliterated a traumatic DCCF that deposition of 5 GDCs had failed to cure.
Theoretically, there are 3 main potential disadvantages when n-BCA is used as an embolizing agent for treating DCCFs. The first is that highly concentrated n-BCA polymerizes very rapidly and tends to stick to the microcatheter and protective balloons. Debrun et al20 reported 29 (3%) glued microcatheters among a total of 936 feeders of brain arteriovenous malformation embolizations, with the use of 25% glue. This complication rate is very low; only 2 patients developed neurologic symptoms. To reduce the frequency of glued microcatheters, one must inject the glue very slowly, with simultaneous good quality road-mapping digital subtraction, which can show any reflux of glue. In addition, the microcatheter should be pulled out as quickly as possible. In our experience, we used a more concentrated n-BCA mixture (60% n-BCA) for occlusion of fistulas because this allows adequacy of opacity and proper polymerization. Even at this high concentration, we did not have any occasions of microcatheters or protective balloons being glued, because small amounts of the n-BCA mixture were used in each procedure, with short injection times in most cases.
The second potential disadvantage of the transarterial balloon-assisted n-BCA embolization is penetration of the n-BCA mixture into its venous drainages (eg, the superior ophthalmic vein, superior petrous sinus, or cerebral or cerebellar veins) and/or the pulmonary venous system, leading to venous occlusion or pulmonary complications. In our series, the high-flow fistula was temporarily blocked by the protective balloon in the ICA at the fistula site. Therefore, the penetration of the n-BCA mixture is largely dependent on the force and pressure of the hand injection. To decrease the risk of distal penetration of the n-BCA mixture toward the venous side, we use a 60% n-BCA mixture to allow early polymerization. In addition, the initial injection should be performed slowly; after the n-BCA mixture reaches the superior ophthalmic vein/inferior petrous sinus, the microcatheter should be removed. Before injection of the n-BCA mixture, a small amount of dextrose is recommended to clean the microcatheter, because a large amount of dextrose filling in the cavernous sinus may cause nonpolymerization of the n-BCA mixture in the cavernous sinus and may be brought to distal venous drainages by fistula flow while the protective balloon is deflated. However, asymptomatic penetration and casting of n-BCA mixture to the proximal portion of superior ophthalmic vein and inferior petrous sinus were identified in 4 patients.
Third, and the most feared, drawback is the potential risk of leakage of n-BCA mixture into the parent artery, resulting in cerebral infarction and/or ophthalmic artery occlusion. Therefore, before n-BCA injection, one must ensure reliable protection of the cerebral and ophthalmic circulation. To avoid uncontrolled escape of the n-BCA mixture into the ICA, Mericle et al21 and Moret et al22 described the technique of using an endoluminal balloon to protect and preserve the ICA for the embolization of intracranial aneurysms. In our series, we used a temporarily protective balloon in the ICA at the level of the fistula site. Regarding the selection of the protective balloon, a soft balloon is mandatory. In this series, we preferred to use the detachable balloon because it is soft enough to adjust the shape to the tortuous cavernous portion of the ICA. Besides, it can be used to sacrifice the ICA when the transarterial balloon-assisted n-BCA embolization fails to occlude the fistula. Before deflation of the protective balloon, we usually wait a few seconds to achieve n-BCA polymerization and forming of the glue case, thus reducing the risk of the liquid n-BCA mixture leaking into the ICA. Furthermore, the injection of the n-BCA mixture can be divided into 2 or 3 steps in each session to avoid excessive injection. In our series, a total of 27 procedures were performed with an average of 1.5 per patient, and the volume of n-BCA mixture varied from 0.5 to 2.3 mL with a mean of 0.83 mL.
Because the transarterial balloon-assisted n-BCA embolization requires training, we do not recommend using it as a first line of treatment. n-BCA is the embolizer of choice only for those procedures failing to achieve occlusion and ICA preservation by balloons. In our series, the first indication for transarterial balloon-assisted n-BCA embolization was the failure of an angiographic cure and ICA preservation after treatment with detachable balloons. The failure presumably was related to a large tear and/or a large cavernous sinus or to multiple tears. A high-flow DCCF with a complete steal phenomenon does not mean that the ICA is separated and cannot be preserved after closure of the fistula.23 A large tear may be associated with a complete or severe steal phenomenon with an extremely fast-flow DCCF. For those patients in whom the fistula failed to seal with detachable balloons, we prefer the use of transarterial balloon-assisted n-BCA embolization. Nevertheless, if the transarterial balloon-assisted n-BCA embolization does not significantly reduce the fistula flow after 2 or 3 attempts, the ICA should be sacrificed in favor of treating the fistula, because the ICA is nearly transected or has multiple tears or the tear is too large to be preserved.
The second indication for transarterial balloon-assisted n-BCA embolization is a recurrent fistula due to premature balloon deflation and/or migration, in which the endovascular team did not introduce additional balloons into the cavernous sinus through the fistula owing to the presence of prematurely deflated and/or migrated balloons partially blocking the fistula orifice. In our series, all except one of the recurrent fistulas were cured by the n-BCA mixture. Therefore transarterial balloon-assisted n-BCA embolization is a good option for those recurrent fistulas that have been successfully treated by detached balloons. It is rarely necessary to sacrifice parent arteries to achieve angiographic cure.
The third and final indication for transarterial balloon-assisted n-BCA embolization is failure to occlude the fistula by using detachable balloon embolization because of sharp objects such as bony fragments that are repeatedly puncturing the balloon during or after inflation. In our series, before the injection of the n-BCA mixtures, several microcoils were placed into the cavernous sinus alongside the previously detached balloons in the expectation that these embolizing materials could be retained. Balloons and/or microcoils not only have the capability to slow down the flow patterns within fistulas but also serve as a framework for polymerization of the n-BCA and may form a physical barrier to the further movement of the n-BCA mass to the venous side.
One patient with a giant cavernous sinus varix had a fatal subarachnoid hemorrhage on day 2 after treatment. The cause of the hemorrhage was presumed to be a direct impact of the presence of embolizing materials in the giant varix. Another patient with a DCCF after trans-sphenoid hypophysectomy had a recurrent fistula after balloon occlusion, presenting with massive epistaxis on day 3 after embolization. Here, the recurrent fistula was eventually treated by balloon occlusion of both ICA and fistula to save the patient’s life. The recurrence was presumed to be a large fistula with insufficient filling by the n-BCA mixture into the cavernous sinus and fistula because of blood clots between the cavernous sinus and the sphenoid sinus. Lysis or absorption of old blood can result in recanalization of the fistula tract associated with massive epistaxis. No other severe complications such as sticking of the microcatheter or protective balloon were observed, nor did we find reflux of the n-BCA mixture into the parent artery in our series. A small pseudoaneurysm or sac may also form because of shallow herniation of the protective balloon into the fistula/cavernous sinus or insufficient filling of the cavernous sinus by the n-BCA mixture.
Conclusions
Transarterial detachable balloon embolization with preservation of the ICA can be achieved in most patients with DCCF. However, in some instances, it may be difficult to seal the fistula because of a residual fistula after detached balloon treatment, a recurrent fistula with failure to introduce additional balloons into cavernous sinus, or repeated balloon puncture by bony fragments. In our series, transarterial balloon-assisted n-BCA embolization allowed the treatment of 16 DCCFs that were not treatable without sacrificing the ICA. This strategy proved feasible, efficient, and safe and should be considered as an alternative treatment for those fistulas in which the use of detachable balloons fails to occlude the fistula and preserve the ICA.
Footnotes
This work was supported by grants TVGH-94–398 and, in part, NSC91–2314-B-075–121 from Taipei Veterans General Hospital.
References
- 1.Debrun GM, Vinuela F, Fox AJ, et al. Indications for treatment and classification of 132 carotid-cavernous fistulas. Neurosurgery 1988;22:285–89 [DOI] [PubMed] [Google Scholar]
- 2.Goto K, Hieshima GB, Higashida RT, et al. Treatment of direct carotid cavernous sinus fistulae: various therapeutic approaches and results in 148 cases. Acta Radiol Suppl 1986;369:576–79 [PubMed] [Google Scholar]
- 3.Kahara VJ, Seppanen S, Kuurne T, et al. Endovascular treatment of carotid-cavernous fistulae. Acta Neurol Scand 1998;98:254–58 [DOI] [PubMed] [Google Scholar]
- 4.Lewis AI, Tomsick TA, Tew JM. Management of 100 consecutive direct carotid-cavernous fistulas: result of treatment with detachable balloons. Neurosurgery 1995;36:239–45 [DOI] [PubMed] [Google Scholar]
- 5.Luo CB, Teng MMH, Yen DH, et al. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma 2004;56:1214–20 [DOI] [PubMed] [Google Scholar]
- 6.Norman D, Newton TH, Edwards MSB. Carotid cavernous fistula: closure with detachable silicone balloon. Radiology 1983;149:149–59 [DOI] [PubMed] [Google Scholar]
- 7.Bavinzski G, Killer M, Gruber A, et al. Treatment of post-traumatic carotid-cavernous fistulae using electrolytically detachable coils: technical aspects and preliminary experience. Neuroradiology 1997;39:81–85 [DOI] [PubMed] [Google Scholar]
- 8.Halbach VV, Higashida RT, Hieshima GB, et al. Direct puncture of the proximally occluded internal carotid artery for treatment of carotid cavernous fistulas. AJNR Am J Neuroradiol 1989;10:151–54 [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen Q, Dorfler A, Forsting M, et al. Endovascular therapy of arteriovenous fistulae with electrolytically detachable coils. Neuroradiology 1999;41:951–57 [DOI] [PubMed] [Google Scholar]
- 10.Nesbit GM, Barnwell SL. The use of electrolytically detachable coils in treating high-flow arteriovenous fistulas. AJNR Am J Neuroradiol 1998;19:1565–69 [PMC free article] [PubMed] [Google Scholar]
- 11.Siniluoto T, Seppanen S, Kuurne T, et al. Transarterial embolization of a direct carotid cavernous fistula with Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:519–23 [PMC free article] [PubMed] [Google Scholar]
- 12.Moron FE, Klucznik RP, Mawad ME, et al. Endovascular treatment of high-flow carotid cavernous fistulas by stent-assisted coil placement. AJNR Am J Neuroradiol 2005;26:1399–404 [PMC free article] [PubMed] [Google Scholar]
- 13.Halbach VV, Higashida RT, Barnwell SL, et al. Transarterial platinum coil embolization of carotid-cavernous fistulas. AJNR Am J Neuroradiol 1991;12:429–33 [PMC free article] [PubMed] [Google Scholar]
- 14.Cognard C, Weill A, Tovi M, et al. Treatment of distal aneurysms of the cerebellar arteries by intraaneurysmal injection of glue. AJNR Am J Neuroradiol 1999;20:780–84 [PMC free article] [PubMed] [Google Scholar]
- 15.Teng MM, Chen CC, Lirng JF, et al. N-butyl-2-cyanoacrylate for embolization of carotid aneurysm. Neuroradiology 1994;36:144–47 [DOI] [PubMed] [Google Scholar]
- 16.Teng MMH, Chang CY, Chiang JH, et al. Double-balloon technique for embolization of carotid cavernous fistulas. AJNR Am J Neuroradiol 2000;21:1753–56 [PMC free article] [PubMed] [Google Scholar]
- 17.Guglielmi G, Vinulela F, Briganti F, et al. Carotid-cavernous fistula caused by a ruptured intracavernous aneurysm: endovascular treatment by electrothrombosis with detachable coils. Neurosurgery 1992;31:54–56 [DOI] [PubMed] [Google Scholar]
- 18.Kerber CW, Bank WO, Cromwell LD. Cyanoacrylate occlusion of carotid-cavernous fistula with preservation of carotid artery flow. Neurosurgery 1979;4:210–15 [DOI] [PubMed] [Google Scholar]
- 19.Evans AJ, Jesen ME, Mathis JM, et al. The Guglielmi detachable coil in the treatment of arteriovenous fistulae. Interv Neuroradiol 1996;2:201–07 [DOI] [PubMed] [Google Scholar]
- 20.Debrun GM, Aletich VA, Shownkeen H, et al. Glued catheters during embolization of brain AVMs with acrylic glue. Interv Neuroradiol 1997;3:13–19 [DOI] [PubMed] [Google Scholar]
- 21.Mericle RA, Wakhloo AK, Rodriquez R, et al. Temporary balloon protection as an adjunct to endosaccular coiling of wide-neck cerebral aneurysms: technical note. Neurosurgery 1997;41:975–78 [DOI] [PubMed] [Google Scholar]
- 22.Moret J, Cognard C, Weil A, et al. The remodeling technique in the treatment of wide neck intracranial aneurysms: angiographic results and clinical follow up in 56 cases. Interv Neuroradiol 1996;3:21–35 [DOI] [PubMed] [Google Scholar]
- 23.Debrun GM. Angiographic workup of a carotid cavernous sinus fistula or what information does the interventionalist need for treatment. Surg Neurol 1995;44:35–39 [DOI] [PubMed] [Google Scholar]