Abstract
BACKGROUND AND PURPOSE: Echo time (TE) can have a large influence on the spectra in proton MR spectroscopy (1H-MR spectroscopy). The purpose of this study was to comparatively assess the diagnostic value of 3T single-voxel 1H-MR spectroscopy with short or intermediate TEs in grading cerebral gliomas.
METHODS: Single voxel 1H-MR spectroscopy was performed at 3T in 35 patients with cerebral glioma. The spectra were obtained with both short (35 ms) and intermediate TEs (144 ms). Metabolite ratios of choline (Cho)/creatine (Cr), Cho/N-acetylaspartate (NAA), lipid and lactate (LL)/Cr and myo-inositol (mIns)/Cr were calculated and compared between short and intermediate TEs in each grade. After receiver operating characteristic curve analysis, diagnostic accuracy for each TE in differentiating high-grade glioma from low-grade glioma was compared.
RESULTS: At short TE, Cho/Cr and Cho/NAA ratios were significantly lower, and LL/Cr and mIns/Cr were significantly higher, compared with those at intermediate TE, regardless of tumor grade. Lactate inversion at intermediate TE was found in only 2 patients. At both TEs, there were significant differences in Cho/Cr and LL/Cr ratios between low- and high-grade gliomas. Diagnostic accuracy was slightly higher at short TE alone or combined with intermediate TE than intermediate TE alone (85.7% versus 82.9%).
CONCLUSION: Metabolite ratios were significantly different between short and intermediate TE. Cho/Cr and LL/Cr ratios at either TE were similarly useful in differentiating high-grade gliomas from low-grade gliomas. If only a single spectroscopic sequence can be acquired, short TE seems preferable because of poor lactate inversion at intermediate TE on 3T single-voxel 1H-MR spectroscopy.
Noninvasive preoperative prediction of cerebral glioma grade is important for treatment planning and prediction of prognosis. Although in vivo proton MR spectroscopy (1H- MR spectroscopy) at 1.5T has been attempted to predict the degree of malignancy of the gliomas, some studies have yielded the somewhat disappointing result that there was no reliable indicator for tumor grading.1-3 At both short and intermediate echo time (TE) sequences, different criteria have been argued in favor and against every option in the evaluation of the brain tumors.4 With the integration of 3T MR into clinical practice, there has been growing interest in the practical improvement of 1H-MR spectroscopy at 3T with respect to the established magnetic field strength of 1.5T, because both spectral and spatial resolutions depend linearly on the magnetic field.5-8 Here, we tried to assess the effect of various TEs at 3T single-voxel 1H-MR spectroscopy in low- and high-grade cerebral gliomas.
Materials and Methods
Patients
Thirty-five patients (23 men and 12 women; age range, 23–71 years; mean age, 47.3 years) with untreated gliomas were studied before surgical biopsy and/or resection. Histopathologic diagnosis obtained via either surgical biopsy (n = 14) or resection (n = 21) was graded by the World Health Organization II (WHO II) criteria. The biopsies were performed at the locations similar to the voxel placement in 1H- MR spectroscopy. Seven patients were grade II (3 astrocytomas and 4 oligodendrogliomas), 12 were grade III (5 anaplastic astrocytomas, 1 anaplastic oligoastrocytoma, 2 anaplastic gemistocytic astrocytomas, and 4 anaplastic oligodendrogliomas), and 16 were grade IV (glioblastoma). All patients provided informed consent for participation in the study, which was approved by our institutional review board.
1H-MR Spectroscopy Methods
All 1H-MR spectroscopy studies were performed using the automated single-voxel MR spectroscopy package Proton Brain Examination/Single Voxel (PROBE/SV) with 3T units (GE Medical Systems, Milwaukee, Wis) equipped with circularly polarized head coil. We acquired the data using point-resolved spectroscopy (PRESS) pulse sequences at both short TE (2000/35) (repetition time [TR]/TE) and intermediate TE (1500/144) sequences in all patients. MR imaging with T2-weighted fast spin-echo sequences (4000–5000/104–121, 24-cm field of view, 256 × 224 matrix, 5-mm sections) or fluid-attenuated inversion recovery (FLAIR) (9902/161, 24-cm field of view, 256 × 256 matrix, 5-mm sections) in axial or coronal planes preceded 1H-MR spectroscopy to define the volume of interest (VOI). The VOIs varied from 1.6 to 10.8 cm3 depending on the tumor size. The size and location of the voxel were carefully adjusted to include as much of the solid tumor portion as possible as determined from the previously obtained MR images. We intended to avoid inclusion in the voxel of obvious necrosis, cyst, hemorrhage, edema, calcification, and normal-appearing brain. All the 1H-MR spectroscopy spectra were obtained without contrast material administration.
All the spectra were processed using Mrdx (CAD Impact, Seoul, Korea), a software program for MR data postprocessing, based on IDL software (Research Systems, Boulder, Colo). Typical full widths at half-maximum of 3–6 Hz were achieved in most examinations. A frequency-selective saturation pulse at the water resonance suppressed the water signal intensity. A sweep width of 1000 Hz was used with a data size of 1024 points. Only the second half of the echo was acquired. After the zero-filling of 8192 points in all free induction-decay data, an exponential line broadening (center, 0 ms; half time, 150 ms) was done before Fourier transformation. A zero-order phase correction was applied to all spectra.
Evaluation of the Spectra and Statistical Analysis
We estimated the levels of myo-inositol (mIns) at 3.6 ppm, choline compounds (Cho) at 3.2 ppm, creatine phosphocreatine (Cr) at 3.0 ppm, and N-acetylaspartate (NAA) at 2.0 ppm as heights of the peaks at both TEs. Concerning lipid and lactate, we defined and estimated the sum of the peak heights between 0.9 and 1.3 ppm as lipid and lactate (LL) at short TE. At intermediate TE, we defined and estimated the sum of the upright lipid peak around 0.9–1.3 ppm and the absolute value of the inverted lactate peak at 1.3 ppm as LL.
The metabolite ratios of Cho/Cr, Cho/NAA, LL/Cr, and mIns/Cr were calculated (mean ± SD) from both TE spectra and compared for short and intermediate TEs in 3 grades of gliomas and in 2-tiered classification (low [II] versus high [II + IV] grade). In addition, to assess the lactate inversion at intermediate TE, we evaluated the presence of the definite upright doublet peak at 1.3 ppm of short TE spectrum and the inverted lactate peak at 1.3 ppm of intermediate TE spectrum.
Statistical analysis was performed with the use of SPSS software for Windows (ver. 11.0; SPSS, Chicago, Ill). A P value less than 0.05 was considered to indicate a statistically significant difference. Mann-Whitney U tests were used to evaluate the significance in the differences of the metabolite ratios among the 3 grades and in 2-tiered classification of the gliomas. The paired t tests for the matched pairs were performed to compare metabolite ratios between short and intermediate TE.
Receiver operating characteristic (ROC) curve analysis was done to evaluate the performance of the Cho/Cr and LL/Cr ratios of both TEs in differentiating high grade (III + IV) from low grade (II) of the gliomas using the ROCKIT algorithm (available through the Internet from C. E. Metz, University of Chicago, Ill). The area under the ROC curve (Az) was calculated to summarize the performance of each Cho/Cr and LL/Cr ratio at both TEs in the task of differentiation of 2-tiered classification. We calculated sensitivity, specificity, positive predictive value, and negative predictive value using both Cho/Cr and LL/Cr ratios of the cutoff value obtained with minimum C1 error from the ROC analysis, where C1 equals 1 − (sensitivity + specificity)/2. When any ratio of either Cho/Cr or LL/Cr was larger than its cutoff value, we regarded it as high grade. Afterward, diagnostic accuracy was also calculated and compared at short TE, intermediate TE, and both TE sequences.
Results
Comparison of Metabolite Ratios between Short and Intermediate TE and Lactate Inversion at Intermediate TE
The metabolite ratios of cerebral gliomas at both TEs are given in Tables 1 and 2 and Fig 1. Mean Cho/Cr and Cho/NAA ratios of all tumors at short TE were significantly lower than those at intermediate TE, regardless of tumor grades (2.03 ± 0.80 at short TE versus 3.23 ± 2.87 at intermediate TE for Cho/Cr [P < .01]; 1.99 ± 0.93 at short TE versus 6.59 ± 6.32 at intermediate TE for Cho/NAA [P < .0001]). On the contrary, mean LL/Cr and mIns/Cr ratios of all tumors at short TE were significantly higher than those at intermediate TE, regardless of tumor grades (5.15 ± 7.71 at short TE versus 2.11 ± 3.83 at intermediate TE for LL/Cr [P < .01]; 1.12 ± 0.43 at short TE versus 0.63 ± 0.63 at intermediate TE for mIns/Cr [P < .0001]).
Table 1:
Metabolite ratios of 3 grades of cerebral gliomas at both echo time (TE) sequences
| Short TE |
Intermediate TE |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cho/Cr | Cho/NAA | LL/Cr | mIns/Cr | Cho/Cr | Cho/NAA | LL/Cr | mIns/Cr | |
| Grade II (n = 7) | 1.52 ± 0.66 | 1.88 ± 0.96 | 1.07 ± 0.46 | 0.86 ± 0.19 | 1.92 ± 0.89 | 4.57 ± 4.35 | 0.30 ± 0.21 | 0.36 ± 0.11 |
| Grade III (n = 12) | 2.09 ± 0.82 | 2.51 ± 1.20 | 1.62 ± 0.80 | 1.23 ± 0.37 | 2.64 ± 1.39 | 8.14 ± 9.52 | 0.38 ± 0.23 | 0.49 ± 0.19 |
| Grade IV (n = 16) | 2.21 ± 0.79 | 1.66 ± 0.46 | 10.31 ± 10.22 | 1.15 ± 0.52 | 4.25 ± 3.83 | 6.31 ± 3.54 | 4.20 ± 4.95 | 0.74 ± 0.75 |
Note:—Cho indicates choline, Cr, creatine; NAA, N-acetylaspartate; LL, lipid and lactate; MI, myo-inositol.
Table 2:
Metabolite ratios of low-grade (II) and high-grade (III + IV) cerebral gliomas at both echo time (TE) sequences
| Short TE |
Intermediate TE |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cho/Cr | Cho/NAA | LL/Cr | mIns/Cr | Cho/Cr | Cho/NAA | LL/Cr | mIns/Cr | |
| Low grade (II) (n = 7) | 1.52 ± 0.66 | 1.88 ± 0.96 | 1.07 ± 0.46 | 0.86 ± 0.19 | 1.92 ± 0.89 | 4.57 ± 4.35 | 0.30 ± 0.21 | 0.36 ± 0.11 |
| High grade (III + IV) (n = 28) | 2.17 ± 0.79 | 2.02 ± 0.94 | 6.17 ± 8.33 | 1.18 ± 0.45 | 3.56 ± 3.10 | 7.09 ± 6.69 | 2.56 ± 4.17 | 0.63 ± 0.59 |
| P value | .0152 | NS | <.001 | NS | .0111 | NS | .0152 | NS |
Note:—Cho indicates choline, Cr, creatine; NAA, N-acetylaspartate; LL, lipid and lactate; mIns, myo-inositol; NS, not significant.
Fig 1.
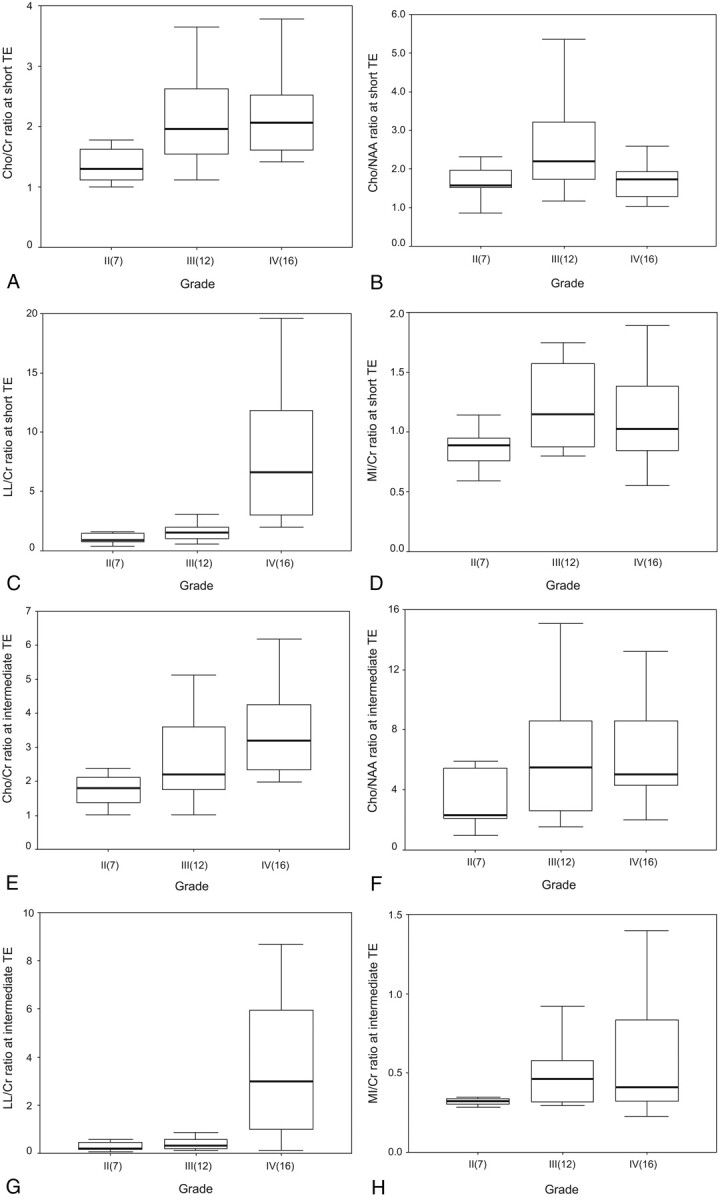
Box plots of the ratios of the metabolites at both TE sequences in the patients with cerebral gliomas. The horizontal thick line is the median, the upper and lower ends of the boxes are the 3rd and 1st quartiles, and the vertical lines show the full range of values in the data. The extremes and outliers were omitted.
A, Cho/Cr at short TE; B, Cho/NAA at short TE; C, LL/Cr at short TE; D, mIns/Cr at short TE; E, Cho/Cr at intermediate TE; F, Cho/NAA at intermediate TE; G, LL/Cr at intermediate TE; H, mIns/Cr at intermediate TE.
Focusing on the lactate signal intensity, of the 6 patients who showed the definite doublet peak at 1.3 ppm of short TE spectrum, 4 patients failed to show the lactate inversion at intermediate TE spectrum (Figs 2, 3), whereas other 2 patients revealed the lactate inversion with decreased LL/Cr ratio (Fig 4). All the other cases showed neither doublet peak at 1.3 ppm in short TE spectrum nor lactate inversion in intermediate TE spectrum. Baseline of the spectra seemed to be slightly noisier in intermediate TE than in short TE in most patients.
Fig 2.
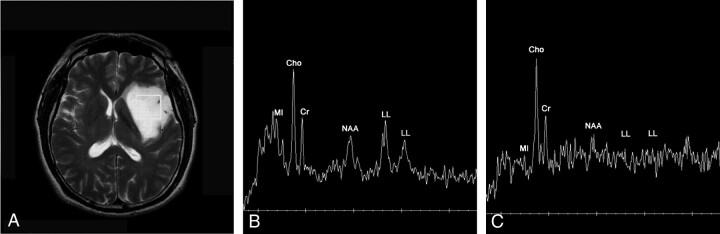
A 35-year-old man with low-grade (grade II) astrocytoma.
A, Axial T2-weighted image reveals diffuse infiltrating glioma involving left basal ganglia and frontotemporal area with a square voxel (6.2 cm3).
B, 1H-MR spectrum obtained at short TE (2000/35) shows increased Cho/Cr ratio (1.9:1), increased Cho/NAA ratio (2.3:1) and increased lipid-lactate (LL)/Cr ratio (1.6:1).
C, 1H-MR spectrum obtained at intermediate TE (1500/144) shows more increased Cho/Cr ratio (2.4:1), more increased Cho/NAA ratio (4.9:1) and hardly discernible peak of lipid-lactate between 0.9 and 1.3 ppm (LL/Cr ratio: 0.3:1). The baseline of the spectrum is noisier than B.
Fig 3.
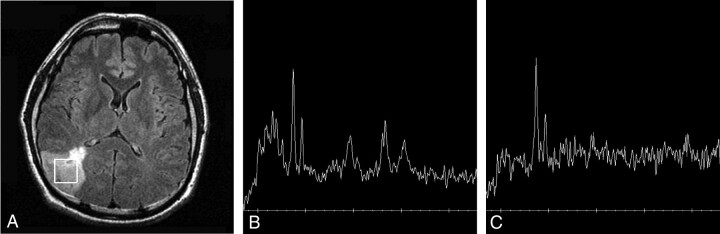
A 38-year-old man with anaplastic (grade III) oligodendroglioma.
A, Axial FLAIR image reveals a infiltrating tumor in the right parietal lobe with a square voxel (4.2 cm3).
B, 1H-MR spectrum obtained at short TE (2000/35) shows increased Cho/Cr ratio (2.4:1), increased Cho/NAA ratio (4.1:1), and increased lipid-lactate (LL)/Cr ratio (2.1:1).
C, 1H-MR spectrum obtained at intermediate TE (1500/144) shows more increased Cho/Cr ratio (2.9:1), more increased Cho/NAA ratio (5.7:1), and much smaller peaks of lipid-lactate at 1.3 ppm (LL/Cr: 0.8:1). The baseline of the spectrum is noisier than B.
Fig 4.
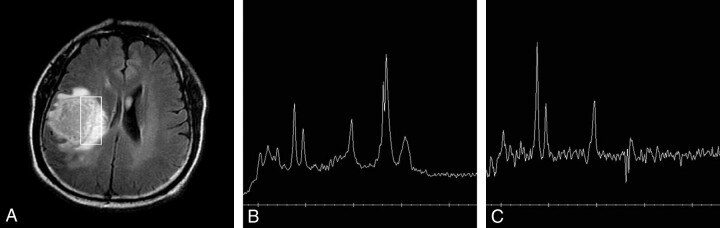
A 61-year-old man with glioblastoma (grade IV).
A, Axial FLAIR image reveals a large necrotic tumor in the right frontal lobe with a rectangular voxel (8.0 cm3).
B, 1H-MR spectrum obtained at short TE (2000/35) shows increased Cho/Cr ratio (1.5:1), increased Cho/NAA ratio (1.2:1), and markedly increased LL/Cr ratio (3.8:1) at 1.3 ppm.
C, 1H-MR spectrum obtained at intermediate TE (1500/144) shows more increased Cho/Cr ratio of (2.2:1), more increased Cho/NAA ratio (2.1:1), and a smaller inverted lactate peak with smaller lipid peaks at 1.2–1.4 and 0.9 ppm (LL/Cr: 0.9:1). The baseline of the spectrum is noisier than B.
Comparison of Metabolite Ratios among Three Grades
The medians of Cho/Cr and LL/Cr ratios had increasing tendency with grade at both TEs. But the medians of Cho/NAA and mIns/Cr ratios did not tend to increase with grade at both TEs (Fig 1). Except for LL/Cr of grade IV at both TEs, all the other metabolite ratios had much overlap with the adjacent grade. Cho/Cr ratio had significant difference between grade II and IV (1.52 ± 0.66 versus 2.21 ± 0.79 at short TE [P < .05], 1.92 ± 0.89 versus 4.25 ± 3.83 at intermediate TE [P < .05]), but not between grade II and III and not between III and IV. LL/Cr ratio had significant differences between grade II and IV (1.07 ± 0.46 versus 10.31 ± 10.22 at short TE [P < .001], 0.30 ± 0.21 versus 4.20 ± 4.95 at intermediate TE [P < .01]), and between grade III and IV (1.62 ± 0.80 versus 10.31 ± 10.22 at short TE [P < .001] and 0.38 ± 0.23 versus 4.20 ± 4.95 at intermediate TE [P < .01]), but not between grades II and III. Representative cases are shown in Figs 2–4.
Comparison of Metabolite Ratios in Two-Tiered Classification
The mean metabolite ratios at both TEs in 2-tiered classification, low (grade II) and high (grade III + IV) grades, are given in the Table 1. Cho/Cr and LL/Cr ratios demonstrated significant differences between low- and high-grade gliomas at both TEs (Cho/Cr, 1.52 ± 0.66 versus 2.17 ± 0.79 at short TE [P < .05] and 1.92 ± 0.89 versus 3.56 ± 3.10 at intermediate TE [P < .05]; LL/Cr, 1.07 ± 0.46, versus 6.17 ± 8.33 at short TE [P < .001] and 0.30 ± 0.21 versus 2.56 ± 4.17 at intermediate TE [P < .05]) but both mIns/Cr and Cho/NAA did not show any significant difference.
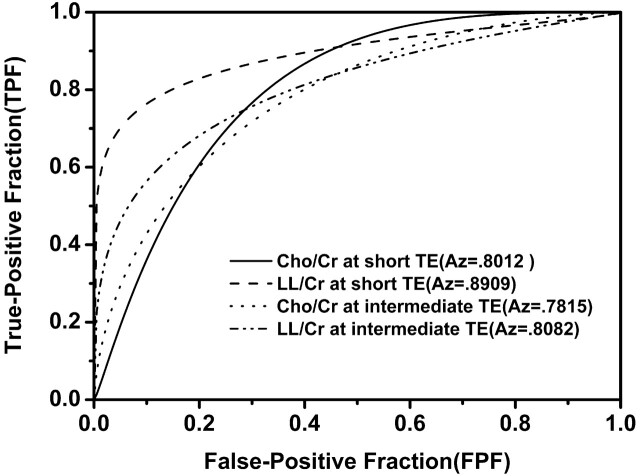
In the evaluation of the performance differences of Cho/Cr and LL/Cr ratios at both TEs in the differentiation between low- and high-grade tumors using ROC analysis, there were no significant differences in the Az values of Cho/Cr and LL/Cr ratios between short and intermediate TEs (P < .05), even though the Az values of Cho/Cr and LL/Cr at short TE were slightly larger than those at intermediate TE. The corresponding ROC curves and the Az values for each metabolite ratio are shown in Fig 5.
Fig 5.
Graph shows 4 ROC curves of Cho/Cr and LL/Cr ratios at both TEs for differentiation of high-grade glioma from low-grade glioma. Az value (area under the ROC curve) is the highest in LL/Cr ratio at short TE and lowest in Cho/Cr ratio at intermediate TE. But there are no significant differences among all the Az values.
Cutoff values with minimum C1 error of Cho/Cr and LL/Cr at short TE were 1.47 and 1.63, respectively, whereas those at intermediate TE were 1.84 and 0.59, respectively. Diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of Cho/Cr and/or LL/Cr ratios at both TEs for differentiating high-grade (III + IV) from low-grade (II) are given in Table 2. Sensitivity at short TE, intermediate TE, and both TE sequences was 92.9%, 89.3%, and 96.4%, respectively. Overall diagnostic accuracy at short TE, either alone or combined with intermediate TE, is slightly superior to that at intermediate TE alone, but the difference was not statistically significant among short TE, intermediate TE, and both TE sequences (85.7%, 82.9%, and 85.7%, respectively).
Discussion
In the present study, the Cho/Cr and Cho/NAA ratios were significantly lower at short TE compared with those at intermediate TE. It can be explained by that T2 relaxation time of Cho changes to be longer than those of Cr and NAA in cerebral gliomas. This is in contrast to the reports that in normal brain NAA showed the longest T2 relaxation time, followed by Cho, and Cr in descending order.6,9-12 At 3T, the T2 relaxation times of the metabolites are shortened compared with 1.5T, but the descending order of them is not changed in normal brain.6,12 Our results are supported by a study by Isobe et al13 in which T2 relaxation time of NAA is significantly shorter in glioma than in healthy subjects. Differences in the T2 relaxation times of metabolites between glioma and normal brain may result from the changeable cellular environments, including cellular energy metabolism, pH, oxygen pressure, paramagnetic substances, such as microscopic hemorrhage, and susceptibility effect.13
Our results of significantly higher mIns/Cr and LL/Cr ratios at short TE compared with those of intermediate TE might be attributed to the shorter T2 relaxation times of mIns and lipid relative to T2 relaxation time of Cr.11,14,15
The optimal pulse sequence parameters for 1H-MR spectroscopy in glioma grading are yet to be investigated at 3T. In general, using short TE, it is possible to detect metabolites with short T2 relaxation times, and there is little need for T2 correction, but there are such disadvantages as the distortions of baseline of spectrum by the effect of the eddy current and the overlapped lipids and lactate peaks in short TE. Intermediate or longer TE may be chosen to detect the metabolites of relatively longer T2 relaxation times with little or no contamination of lipid or fat tissue with underlying undistorted baselines. For precise detection of lactate at 1.3 ppm, intermediate TE sequence is necessary because the doublet lactate peak at 1.3 ppm is superimposed with lipid at short TE but separated from lipid peak with inversion at intermediate TE because of J coupling at 1.5T.4,8,13 In the present study with 3T, the lactate inversion at intermediate TE was found in only 2 of 6 patients with doublet peak at 1.3 ppm on short TE spectra, suggesting lactate. This result is contrary to that of a previous report at 1.5T in which there was inverted lactate peak with intermediate TE in 10 of 11 patients who showed doublet peak at 1.3 ppm with short TE.14 According to our study16 dealing with intraindividual comparison between 1.5T and 3T in 13 patients, only 3 patients showed inverted lactate at 3T, whereas 7 patients revealed lactate inversion at 1.5T. The mean signal-to-noise ratio (SNR) of the inverted lactate at intermediate TE decreased down to 49.2 ± 18.5% at 3T compared with 1.5T.16 According to the studies by Dydak et al17 and Kelley et al,18 poor lactate inversion in intermediate TE at 3T is due to chemical shift misregistration effect caused by the narrow bandwidth of the refocusing pulses used with 3T. This is a shortcoming of clinical 3T units at present. In theory, increasing the bandwidth of the radiofrequency pulses can reduce this effect. Kelley et al18 suggested a new technique, BASING, to reduce the chemical shift misregistration effect. Dydak et al17 advised to acquire with TE of 288 ms when lactate is a metabolite of concern using a high field unit.
The metabolite ratios of Cho/Cr, LL/Cr, Cho/NAA, and MI/Cr at 3T in the present study were not significantly different from those at 1.5T as reported in the literature.3,19-22 Significant increase of Cho/Cr and LL/Cr ratios in high-grade gliomas (III + IV) compared with those of low-grade (II) at either short TE or intermediate TE was in accordance with many other studies.3,19-21 However, LL/Cr ratios of high grade tumors (III + IV) were much lower at intermediate TE than those at short TE (2.56 ± 4.17 versus 6.17 ± 8.33, in respect), as seen in Table 2. It could be explained by poor inversion of lactate caused by chemical shift misregistration effect at intermediate TE with 3T as described earlier, in addition to relatively short T2 relaxation time of lipids. The LL/Cr ratio is higher in the necrotic portion of the tumor. As compared with grade III tumors, grade IV tumors have more necrotic portions and less solid (nonnecrotic) portions within them. In a relatively small-sized voxel, more necrotic portions are inevitably contained in grade IV tumors than in grade III tumors. Thus, inclusion of more necrotic portions within the voxel in grade IV tumors results in a higher LL/Cr ratio compared with those of grade III tumors. MIns/Cr ratio tended to increase with grade in the present study with 3T, which was similar to the results of a study at 1.5T by Fan et al23 but disagreed with an article by Castillo et al.22
It is not always clear what criteria should be used in determining the optimal cutoff values (threshold values) in grading gliomas. To obtain potentially useful cutoff values of Cho/Cr and LL/Cr in differentiating high-grade from low-grade glioma, we chose to minimize C1 error, which maximizes the average of the observed sensitivity and specificity from the ROC analysis. Alternatively, one may choose to minimize the C2 error (fraction of total number of misclassified tumors). The sensitivities of Cho/Cr and/or LL/Cr ratios at short TE, intermediate TE, and both TEs of 92.9%, 89.3%, and 96.4%, respectively, in the present study indicates that the metabolite ratios can be useful in determining glioma grade.
Metabolite ratios of gliomas and their threshold values for grading varied among many papers. These variations are caused by the differences in the methods of acquisition parameters, voxel size and location, number of patients, tumor’s own heterogeneity, and extrinsic factors such as MR field strength. In the present study, the ranges of all the metabolite ratios including Cho/Cr and LL/Cr were wide and overlapped among each grade, particularly between grades II and III and between grades III and IV. These wide ranges might be due mainly to the partial volume-averaging effect from the heterogeneous tumor tissue, including cyst, necrosis, hemorrhage, etc. Even small amounts of extraneous fat tissue, such as subcutaneous or retroorbital fat, may contaminate the spectra when the voxel is located adjacent to those tissues.24 Although attempts were made to avoid such contaminations, they were inevitable in many cases. In some patients, intraindividual differences in the spectra of a tumor between different locations may be often larger than differences between the spectra of tumors with different grades.1
Our results suggest that single-voxel 1H-MR spectroscopy even at 3T with higher SNR also is of limited value for the grading of cerebral gliomas like 1.5T. To overcome the limitations of single-voxel technique, more precisely refined chemical shift imaging technique with reduced voxel size should be developed and applied to the evaluation of large heterogenous tumors to more accurately predict the tumor grade.
Conclusion
Metabolite ratios were significantly different between short and intermediate TEs. Cho/Cr and LL/Cr ratios at either TE were similarly useful in differentiating high-grade from low-grade gliomas. Combined short and intermediate TEs are more desirable, even though either TE alone showed no significant difference in diagnostic accuracy in grading cerebral gliomas. If only a single spectroscopic sequence can be acquired, short TE seems preferable, because of some limitations at intermediate TE including poor lactate inversion, increased baseline noise, and slightly lower diagnostic accuracy and sensitivity at 3T single-voxel 1H-MR spectroscopy.
Table 3:
Diagnostic accuracies of Cho/Cr and/or LL/Cr ratios at short echo time (TE), intermediate TE, and both TEs in differentiation between low-grade (II) and high-grade (III + IV) gliomas using the cutoff (threshold) values obtained with minimum C1 error
| Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|
| Grading with Cho/Cr and/or LL/Cr at short TE | 85.7 | 92.9 | 57.1 | 89.7 | 66.7 |
| Grading with Cho/Cr and/or LL/Cr at intermediate TE | 82.9 | 89.3 | 57.1 | 89.3 | 57.1 |
| Grading with Cho/Cr and/or LL/Cr at both TEs | 85.7 | 96.4 | 42.9 | 87.1 | 75.0 |
Note:—Cho indicates choline, Cr, creatine; NAA, N-acetylaspartate; LL, lipid and lactate; mIns, myo-inositol; PPV, positive predictive value; NPV, negative predictive value.
Footnotes
This work was supported by research grant 04-2003-031-0 from Seoul National University Hospital (2003).
References
- 1.Ott D, Hennig J, Ernst T. Human brain tumor: assessment with in vivo proton MR spectroscopy. Radiology 1993;186:745–52 [DOI] [PubMed] [Google Scholar]
- 2.Barker PB, Glickson JD, Bryan N. In vivo magnetic resonance spectroscopy of human brain tumors. Top Magn Reson Imaging 1993;5:32–45 [PubMed] [Google Scholar]
- 3.Shimizu H, Kumabe T, Tominaga T, et al. Noninvasive evaluation of malignancy of brain tumors with proton MR spectroscopy. AJNR Am J Neuroradiol 1996;17:737–47 [PMC free article] [PubMed] [Google Scholar]
- 4.Majos C, Julia-Sape M, Alonso J, et al. Brain tumor classification by proton MR spectroscopy: comparison of diagnostic accuracy at short and long TE. AJNR Am J Neuroradiol 2004;25:1696–704 [PMC free article] [PubMed] [Google Scholar]
- 5.Gonen O, Gruber S, Li BS, et al. Multivoxel 3D proton spectroscopy in the brain at 1.5 versus 3.0 T: signal-to-noise ratio and resolution comparison. AJNR Am J Neuroradiol 2001;22:1727–31 [PMC free article] [PubMed] [Google Scholar]
- 6.Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med 2001;45:765–69 [DOI] [PubMed] [Google Scholar]
- 7.Gruber S, Mlynarik V, Moser E. High-resolution 3D proton spectroscopic imaging of the human brain at 3T: SNR issues and application for anatomy-matched voxel sizes. Magn Reson Med 2003;49:299–306 [DOI] [PubMed] [Google Scholar]
- 8.Kantarci K, Reynolds G, Petersen RC, et al. Proton MR spectroscopy in mild cognitive impairment and Alzheimer disease: comparison of 1.5 and 3T. AJNR Am J Neuroradiol 2003;24:843–49 [PMC free article] [PubMed] [Google Scholar]
- 9.Rutgers DR, van der Grond J. Relaxation times of choline, creatine and N-acetyl aspartate in human cerebral white matter at 1.5T. NMR Biomed 2002;15:215–21 [DOI] [PubMed] [Google Scholar]
- 10.Jiru F, Dezortova M, Burian M, et al. The role of relaxation time corrections for the evaluation of long and short echo time 1H MR spectra of the hippocampus by NUMARIS and LCModel techniques. MAGMA 2003;16:135–43 [DOI] [PubMed] [Google Scholar]
- 11.Tong Z, Yamaki T, Harada K, et al. In vivo quantification of the metabolites in normal brain and brain tumors by proton MR spectroscopy using water as an internal standard. Magn Reson Imaging 2004;22:1017–24 [DOI] [PubMed] [Google Scholar]
- 12.Traber F, Block W, Lamerichs R, et al. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging 2004;19:537–45 [DOI] [PubMed] [Google Scholar]
- 13.Isobe T, Matsumuraa A, Anno I. Quantification of cerebral metabolites in glioma patients with proton MR spectroscopy using T2 relaxation time correction. Magnetic Resonance Imaging 2002;20:343–49 [DOI] [PubMed] [Google Scholar]
- 14.Kaminogo M, Ishimaru H, Morikawa M, et al. Diagnostic potential of short echo time MR spectroscopy of gliomas with single-voxel and point-resolved spatially localised proton spectroscopy of brain. Neuroradiology 2001;43:353–63 [DOI] [PubMed] [Google Scholar]
- 15.Sacktor N, Skolasky RL, Ernst T, et al. A multicenter study of two magnetic resonance spectroscopy techniques in individuals with HIV dementia. J Magn Reson Imaging 2005;21:325–33 [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Chang KH, Na DG, et al. Comparison of 1.5T and 3T 1H MR spectroscopy in human brain tumors. Korean J Radiol (in press). [DOI] [PMC free article] [PubMed]
- 17.Dydak U, Lange T, Alexander AL, et al. Pitfalls in lactate measurements with MR spectroscopy at high field strength, presented American Society of Radiology 43rd Annual Meeting; May 21–27,2005; Toronto, Canada.
- 18.Kelley DAC, Wald LL, Star-Lack JM. Lactate detection at 3T: compensating J coupling effects with BASING. J Magn Reson Imaging 1999;9:732–37 [DOI] [PubMed] [Google Scholar]
- 19.Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 2003;49:223–32 [DOI] [PubMed] [Google Scholar]
- 20.Moller-Hartmann W, Herminghaus S, Krings T, et al. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology 2002;44:371–81 [DOI] [PubMed] [Google Scholar]
- 21.Poptani H, Gupta RK, Roy R, et al. Characterization of intracranial mass lesions with in vivo proton MR spectroscopy. AJNR Am J Neuroradiol 1995;16:1593–603 [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo M, Smith JK, Kwock L. Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am J Neuroradiol 2000;21:1645–49 [PMC free article] [PubMed] [Google Scholar]
- 23.Fan G, Sun B, Wu Z, et al. In vivo single-voxel proton MR spectroscopy in the differentiation of high-grade gliomas and solitary metastases. Clin Radiol 2004;59:77–85 [DOI] [PubMed] [Google Scholar]
- 24.Kwock L, Brown MA, Castillo M. Extraneous lipid contamination in single-volume proton MR spectroscopy: phantom and human studies. AJNR Am J Neuroradiol 1997;18:1349–57 [PMC free article] [PubMed] [Google Scholar]