Abstract
SUMMARY: Spinal cord arteries and veins are difficult to visualize and distinguish by MR angiographic techniques because of their small sizes, similar spatial course, and close vascular anatomy. Contrast-enhanced MR angiography was demonstrated to dynamically resolve the Adamkiewicz artery from the anterior radiculomedullary vein in the thoracolumbar spinal cord. The location of the Adamkiewicz artery and the anterior radiculomedullary vein could be validated in the postmortem specimen of a thoracoabdominal aortic aneurysm patient.
MR angiography (MRA) has recently emerged as an alternative imaging technique to digital subtraction angiography (DSA) for depiction of the spinal cord arteries, in particular the Adamkiewicz artery (AKA)1-8; however, because of the similar spatial course and close vascular anatomy of the spinal cord arteries and veins, separation of these vessels is rather difficult. To the best of our knowledge, no validation of the use of MRA for imaging the spinal cord arteries and veins has been provided. This case report describes a postmortem validation of the localization of the AKA and the anterior radiculomedullary vein by using preoperative contrast-enhanced MR angiography (CE-MRA) in a patient who underwent open thoracoabdominal aortic aneurysm (TAAA) repair.
Case Report
A 72-year-old woman was admitted to our hospital and scheduled for open surgical repair of a Crawford type III TAAA. Preoperative CE-MRA was performed to identify the segmental artery from which the AKA derived. Two consecutive dynamic phase CE-MRA scans (Fig 1), 40 seconds each, were acquired by using a 3D spoiled gradient-echo sequence with centrally ordered k-space filling, the start of which was synchronized with the arrival of 0.3 mmol/kg dose gadolinium contrast agent in the lower aorta.1 Voxel sizes were 0.8 × 0.8 × 1.2 mm at acquisition. Both phases were transferred to an image-processing workstation for multiplanar reformation (MPR). The AKA arose from the left 8th thoracic segmental artery (SA) according to the first-phase angiogram. The anterior median vein drained to the epidural space of the left 12th thoracic segmental vein according to the second-phase angiogram (Fig 1). The first-phase angiogram selectively depicted the arteries (anterior cord surface). The second-phase angiogram depicted the arteries (anterior cord surface) and veins (anterior and posterior cord surface; Figs 1 and 2). A curved MPR of the posterior cord surface showed the posterior median vein and 2 posterior radiculomedullary veins (Fig 3). The largest radiculomedullary vein draining the thoracolumbar cord is called the great radiculomedullary vein, and in this case it was located on the posterior cord surface (Fig 3). The following day the patient underwent surgery. Reconstruction of the aorta was performed from the 9th thoracic segmental artery down to the aortic bifurcation with reattachment of the renal and visceral arteries. No postoperative neurologic complications occurred. Unfortunately, the patient died of pulmonary complications 2 weeks after surgery. At autopsy, the spinal cord was exposed to unravel the course and origin of the AKA and the anterior radiculomedullary vein. The AKA was found at the left T8 vertebral level and the anterior radiculomedullary vein at the left T12 vertebral level both in agreement with the preoperative CE-MRA (Fig 1).
Fig 1.
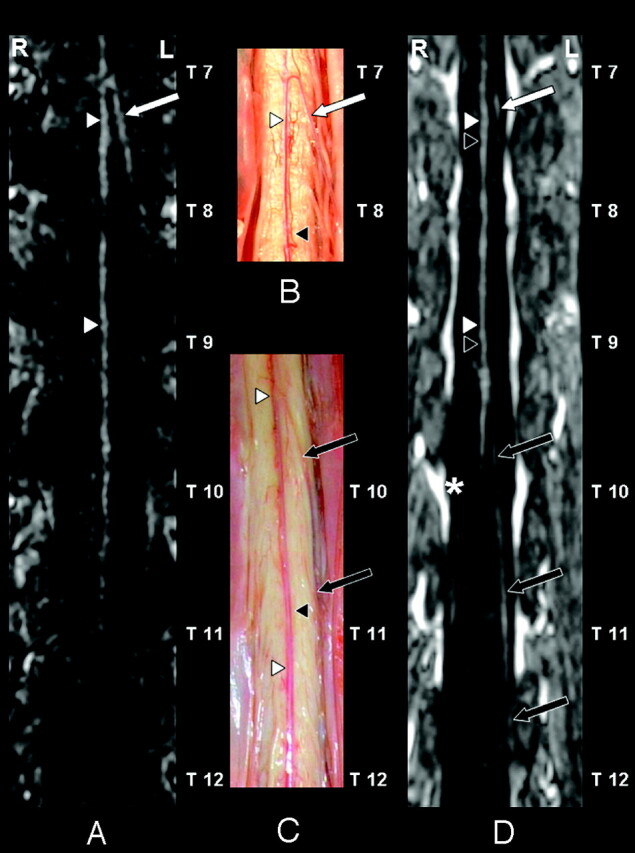
Coronal curved multiplanar reformation of the first dynamic phase of the CE-MRA examination (A), postmortem spinal cord specimens (B and C), and the second dynamic phase (D) of the anterior spinal cord surface. In the first (ie, arterial) dynamic phase (A) only the anterior spinal artery (ASA) (white arrowheads) and the AKA (white arrow) are depicted. The AKA derived from the left 8th thoracic segmental artery (SA) according to the CE-MRA (A) in agreement with the postmortem specimen (B). On the anterior cord surface of the postmortem specimens (B and C) both the ASA (white arrowheads) and anterior median vein (AMV) (black arrowheads) are visualized. Furthermore, the anterior radiculomedullary vein (black arrows) could be identified. This vein entered the epidural space at the left 12th thoracic vertebral level (C). Note the close anatomical relation (B and C) between the ASA (white arrowheads) and the AMV (black arrowheads), which explains that these vessels are not spatially resolved in the second-phase image (D). The second dynamic phase (D) shows a diminished signal intensity of the AKA (white arrow). In contrast to the first-phase angiogram (A), the anterior radiculomedullary vein (black arrows) is visualized and localized in the second-phase angiogram (D) and was in agreement with the postmortem specimen (C). In the midline the combination of the ASA (white arrowheads) and AMV (black arrowheads) are visualized. Note the strong enhancement of the epidural venous plexus (asterisk) in the second phase (D).
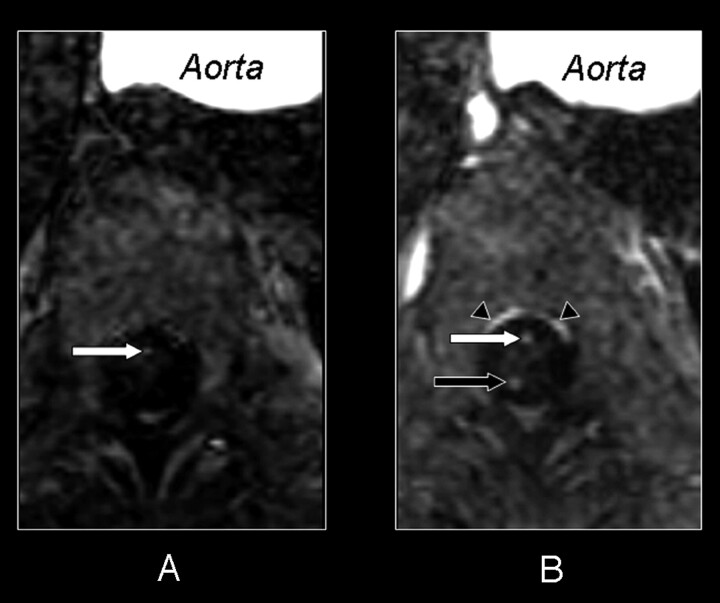
Fig 2.
Axial section of the first (A) and second (B) dynamic phase of the CE-MRA examination. In both phases a cross-section at the 10th thoracic vertebral level shows the aorta and the intra- and extradural vessels. In the first phase (A) there is only enhancement on the midline of the anterior side of the spinal cord. This enhancement corresponds with the cross-section of the ASA (white arrow). The second phase (B) shows enhancement in the midline of both the anterior (white arrow) and posterior surface (black arrow) of the spinal cord. As there usually is only one vessel running along the midline of the posterior surface, the posterior median vein (black arrow), there must also be venous enhancement on the anterior surface. Therefore both the ASA and AMV are displayed on the midline of the anterior surface of the spinal cord in the second phase (B). Because of their close anatomic relation, no separation can be achieved between the ASA and AMV in the second phase (B). Again note the strong enhancement of the epidural venous plexus (black arrowheads) in the second phase (B).
Fig 3.
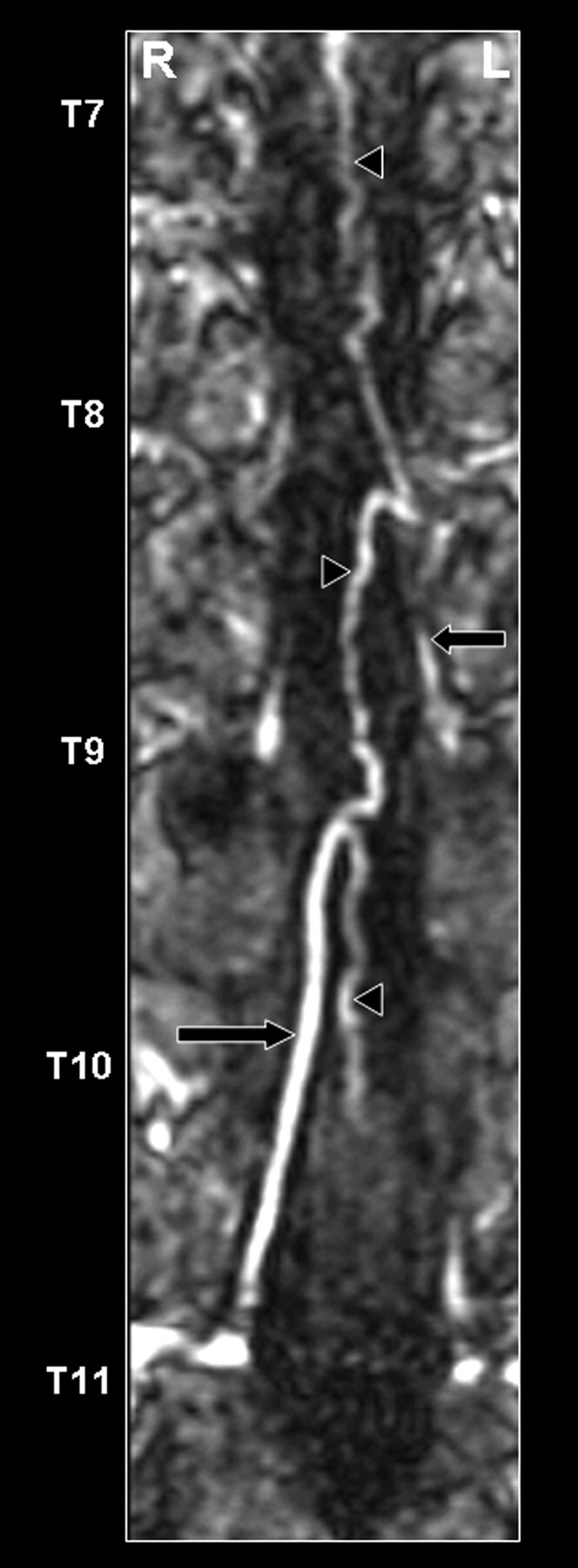
Coronal curved multiplanar reformation of the second dynamic phase of the CE-MRA examination of the posterior spinal cord surface. The posterior median vein (black arrowheads), as well as 2 draining posterior radiculomedullary veins (thoracic vertebral levels 9 and 11), are displayed (black arrows). The great radiculomedullary vein, which is defined as the largest of the radiculomedullary veins, entered the epidural space at the level of the 11th thoracic segmental vein on the right side.
Discussion
The major challenge in spinal CE-MRA is to selectively depict the very small arteries of the spinal cord. This is difficult to realize because (1) spinal cord arteries and veins are spatially close and have very small calibers that may only (partly) fill in one imaging voxel, (2) spinal cord veins are usually thicker but have a similar configuration that easily may lead to misinterpretation between arteries and veins, and (3) the AKA may originate from a strongly variable craniocaudal location (T5–L2). So far, several MR angiography techniques have been attempted. Blood flow–dependent techniques such as 3D phase contrast angiography9-11 and 3D CE time-of-flight imaging12,13 were the first techniques to be used. These flow-dependent techniques are unfortunately not able to fulfill all 3 requirements. Two-phase MR imaging featuring rapid contrast medium injection with a high-speed sophisticated acquisition sequence seems to be more capable of simultaneously providing the necessary features.1-8,14-17 The spinal CE-MRA protocol used here offers (1) sufficient spatial resolution and (2) effective temporal resolution and (3) covers a large field of view, to overcome these conflicting requirements, respectively. Nevertheless, validation of this technique is necessary. The obvious validation technique is DSA. DSA catheterization can be performed only by specialists and is not without risks in TAAA patients,18 has a variable and limited sensitivity,18,19 and is hampered by the occlusion of orifices of possibly many segmental arteries, which explains why spinal DSA is not performed in the work-up of our TAAA patients. CE-MRA is an attractive alternative imaging technique to localize the important AKA before TAAA surgery.2-8
The current postmortem specimen provided the opportunity to validate the location of the AKA and the anterior radiculomedullary vein as obtained by preoperative CE-MRA. This case demonstrated that CE-MRA is an imaging technique that is able to depict the AKA and anterior spinal artery (ASA) correctly and temporarily separate these arteries from the intradural veins in a TAAA patient.
References
- 1.Nijenhuis RJ, Leiner T, Cornips EM, et al. Spinal cord feeding arteries at MR angiography for thoracoscopic spinal surgery: feasibility study and implications for surgical approach. Radiology 2004;233:541–47 [DOI] [PubMed] [Google Scholar]
- 2.Hyodoh H, Kawaharada N, Akiba H, et al. Usefulness of preoperative detection of artery of Adamkiewicz with dynamic contrast-enhanced MR angiography. Radiology 2005;236:1004–09. [DOI] [PubMed] [Google Scholar]
- 3.Kawaharada N, Morishita K, Fukada J, et al. Thoracoabdominal or descending aortic aneurysm repair after preoperative demonstration of the Adamkiewicz artery by magnetic resonance angiography. Eur J Cardiothorac Surg 2002;21:970–74 [DOI] [PubMed] [Google Scholar]
- 4.Kawaharada N, Morishita K, Hyodoh H, et al. Magnetic resonance angiographic localization of the artery of Adamkiewicz for spinal cord blood supply. Ann Thorac Surg 2004;78:846–51 [DOI] [PubMed] [Google Scholar]
- 5.Yamada N, Okita Y, Minatoya K, et al. Preoperative demonstration of the Adamkiewicz artery by magnetic resonance angiography in patients with descending or thoracoabdominal aortic aneurysms. Eur J Cardiothorac Surg 2000;18:104–11 [DOI] [PubMed] [Google Scholar]
- 6.Yamada N, Takamiya M, Kuribayashi S, et al. MRA of the Adamkiewicz artery: a preoperative study for thoracic aortic aneurysm. J Comput Assist Tomogr 2000;24:362–68 [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka K, Niinuma H, Ohira A, et al. MR angiography and CT angiography of the artery of Adamkiewicz: noninvasive preoperative assessment of thoracoabdominal aortic aneurysm. Radiographics 2003;23:1215–25 [DOI] [PubMed] [Google Scholar]
- 8.Nijenhuis RJ, Gerretsen S, Leiner T, et al. Comparison of 0.5-M Gd-DTPA with 1.0-M gadobutrol for magnetic resonance angiography of the supplying arteries of the spinal cord in thoracoabdominal aortic aneurysm patients. J Magn Reson Imaging 2005;22:136–44 [DOI] [PubMed] [Google Scholar]
- 9.Mascalchi M, Bianchi MC, Quilici N, et al. MR angiography of spinal vascular malformations. AJNR Am J Neuroradiol 1995;16:289–97 [PMC free article] [PubMed] [Google Scholar]
- 10.Mascalchi M, Quilici N, Ferrito G, et al. Identification of the feeding arteries of spinal vascular lesions via phase-contrast MR angiography with three-dimensional acquisition and phase display. AJNR Am J Neuroradiol 1997;18:351–58 [PMC free article] [PubMed] [Google Scholar]
- 11.Mascalchi M, Ferrito G, Quilici N, et al. Spinal vascular malformations: MR angiography after treatment. Radiology 2001;219:346–53 [DOI] [PubMed] [Google Scholar]
- 12.Bowen BC, Fraser K, Kochan JP, et al. Spinal dural arteriovenous fistulas: evaluation with MR angiography. AJNR Am J Neuroradiol 1995;16:2029–43 [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen BC, DePrima S, Pattany PM, et al. MR angiography of normal intradural vessels of the thoracolumbar spine. AJNR Am J Neuroradiol 1996;17:483–94 [PMC free article] [PubMed] [Google Scholar]
- 14.Farb RI, Kim JK, Willinsky RA, et al. Spinal dural arteriovenous fistula localization with a technique of first-pass gadolinium-enhanced MR angiography: initial experience. Radiology 2002;222:843–50 [DOI] [PubMed] [Google Scholar]
- 15.Mascalchi M, Cosottini M, Ferrito G, et al. Contrast-enhanced time resolved MR angiography of spinal vascular malformations. J Comput Assist Tomogr 1999;23:341–45 [DOI] [PubMed] [Google Scholar]
- 16.Shigematsu Y, Korogi Y, Yoshizumi K, et al. Three cases of spinal dural AVF: evaluation with first pass, gadolinium-enhanced, three-dimensional MR angiography. J Magn Reson Imaging 2000;12:949–52 [DOI] [PubMed] [Google Scholar]
- 17.Binkert CA, Kollias SS, Valavanis A. Spinal cord vascular disease: characterization with fast three-dimensional contrast-enhanced MR angiography. AJNR Am J Neuroradiol 1999;20:1785–93 [PMC free article] [PubMed] [Google Scholar]
- 18.Kieffer E, Fukui S, Chiras J, et al. Spinal cord arteriography: a safe adjunct before descending thoracic or thoracoabdominal aortic aneurysmectomy. J Vasc Surg 2002;35:262–68 [DOI] [PubMed] [Google Scholar]
- 19.Williams GM, Roseborough GS, Webb TH, et al. Preoperative selective intercostals angiography in patients undergoing thoracoabdominal aneurysm repair. J Vasc Surg 2004;39:314–21 [DOI] [PubMed] [Google Scholar]