Abstract
PURPOSE: We describe a technique for functional MR imaging (fMRI) with high spatial and temporal resolution using a long intravascular half-life gadolinium-based contrast agent, MS-325.
METHODS: All fMRI measurements used a rat model of sensory cortex activation with forepaw electrical stimulation under α-chloralose anesthesia. Standard blood oxygen level–dependent (BOLD) fMRI measurement was initially performed. MS-325 was then intravenously administered and a MS-325 fMRI measurement was performed by using a 3D gradient-echo sequence.
RESULTS: We found that a dose of 0.1 mmol/kg MS-325 produced adequate signal intensity changes in rat sensory cortex to demonstrate activations. Using a boxcar stimulation pattern with a standard correlation analysis, the locations of the most significantly activated voxels (ie, highest Z score) in the MS-325 and BOLD fMRI measurements were not significantly different.
CONCLUSIONS: MS-325 fMRI has the advantage of using a T1-weighted sequence, rather than the highly T2*-weighted sequences used in other common fMRI techniques. This could reduce the susceptibility artifacts associated with fMRI.
Functional MR imaging (fMRI) techniques have gained widespread application in neuroscience research and clinical medicine. The most widely used fMRI technique, blood oxygen level–dependent (BOLD) fMRI, relies on changes in the magnetic susceptibility reflecting altered oxygen levels in response to increased neural activity. BOLD fMRI pulse sequences are typically heavily T2*-weighted, which results in areas of significant signal intensity loss as a result of susceptibility differences at air-tissue interfaces, such as near the mastoid air cells and paranasal sinuses. In addition, the BOLD signal intensity depends on a complex interaction between many processes, including cerebral blood flow, vascular permeability, and rate of oxygen consumption.1–5
Mandeville et al6 have shown that a long intravenous half-life contrast agent, monocrystalline iron oxide nanoparticles (MION) can be used for functional imaging based on changes in regional cerebral blood volume that occur in response to neural activity. As arterioles and capillaries dilate in response to neural activity, cerebral blood volume increases in these active regions, increasing the amount of MION in active portions of brain. The increased MION, with its high magnetic susceptibility, causes proton dephasing, decreasing signal intensity in active portions of the brain on T2*-weighted imaging sequences. MION is an ideal agent for this purpose because of its very long plasma half-life.
With the advent of new, long intravenous half-life gadolinium-based contrast agents, such as MS-325 (EPIX Medical Systems, Cambridge, Mass), we hypothesized that it would be possible to perform functional imaging by using T1-weighted pulse sequences with a gadolinium-based agent. T1-weighted sequences are potentially much less prone to the artifacts commonly seen with heavily T2*-weighted sequences, such as signal intensity dropout near the paranasal sinuses and mastoid air cells or from metallic objects, such as surgical clips.
The purpose of this study was to determine the feasibility of using a long intravenous half-life gadolinium agent (MS-325) with a T1-weighted sequence for obtaining fMRI measurements in a rat system of cortical activation by using forepaw stimulation.
Methods
Animal Preparation
The University of Washington Animal Care Committee approved all animal procedures. Our experimental preparation is based on a well-characterized rat model of cerebrovascular autoregulation7 that uses a forepaw stimulation paradigm similar to that of Hyder et al8 and has been described previously.9 In brief, adult, male, 350- to 450-g Sprague-Dawley rats (B & K, Kent, Wash) were anesthetized with 4% halothane (Halocarbon Laboratories, River Edge, NJ) and then maintained on 1%–2% halothane and 100% O2. Surgical incision sites were further anesthetized with subcutaneous injections of 2% mepivacaine hydrochloride (Astra USA, Inc, Westborough, Mass). Then, a femoral artery and vein were catheterized with the use of 24-gauge catheters (AngioCatheter; BD Instruments, Franklin Lakes, NJ), and continuous monitoring of arterial blood pressure and heart rate were initiated by using an MR-compatible monitor (BPA-2000 analyzer; Micro-Med, Inc, Louisville, Ky). A 22-gauge intraperitoneal catheter was then placed through a small, anterior midline abdominal incision. Next, tiny subcutaneous needle electrodes (made from 30-gauge copper transformer wire) were placed in the radial and ulnar aspects of the left forepaw. Finally, a tracheotomy was performed, and the trachea was cannulated with a 14-gauge catheter. Immediately after initiation of mechanical ventilation, the animal was paralyzed with d-tubocurarine chloride (1 mg/kg, IV; Abbott Laboratories, North Chicago, Ill).
Next, a 1:10 (by weight) mixture of α-chloralose and urethane (both from Sigma, St. Louis, Mo) was intraperitoneally administered (40/400 mg/kg α-chloralose/urethane), and the halothane anesthesia was gradually discontinued over the ensuing 15 minutes. The inspired gas was then changed to a mixture of oxygen and room air producing a fraction of inspired oxygen (Fio2) of 60%. Finally, the animal was placed in an MR-compatible stereotactic head holder. MR imaging was delayed at least 1 hour after discontinuation of halothane anesthesia to assure adequate washout.
Body temperature was maintained using a circulating water warming blanket (Gaymar Industries, Orchard Park, NY). Supplemental doses of d-tubocurarine chloride (1 mg/kg) were given every 30 minutes for the duration of the experiment. Supplemental doses of α-chloralose/urethane (10/100–15/150 mg/kg, IP) were given approximately every 60 minutes for the duration of the experiment.
Once gradient shimming, radiofrequency (RF) transmitter, and RF receiver gains were set, these parameters and the animal’s position within the magnet remained unchanged for the duration of the experiment. At the end of each experiment, the rat was euthanized under anesthesia, according to an approved protocol.
BOLD fMRI
We used a previously described rat model of BOLD fMRI with sensory cortex activation in response to forepaw stimulation.9 All experiments were performed on a 1.5T GE Horizon EchoSpeed whole-body MR scanner (GE Medical Systems, Milwaukee, Wis) in an RF-shielded room. A 2.5-cm transmit-receive surface coil (designed in our laboratory by Cecil Hayes, PhD) was used for all measurements.
We used a standard multisection, 2D, single-shot, gradient-echo, echo-planar pulse sequence for all BOLD functional imaging. BOLD fMRI pulse sequence parameters were as follows: repetition time (TR) = 2000 ms, echo time (TE) = 50 ms, number of excitations (NEX) = 1, flip angle = 90°, field of view (FOV) = 7 × 4 cm, matrix = 70 × 40, with 1-mm section thickness and 1.1-mm section spacing. The frequency axis and the 7-cm FOV axis were oriented left/right. Both fat saturation and ramp sampling were enabled. These parameters resulted in 1-mm3 voxels. Ten different section locations were imaged at each time point, with 51 image sets in each BOLD fMRI measurement. Each measurement lasted 102 seconds and generated 510 images. After extensive testing,9 we found that a flip angle of 90° consistently optimized signal intensity-to-noise ratio (SNR) for this pulse sequence, reflecting the very long TR.
Forepaw electrical stimulation was accomplished with 5-V, 0.3-ms, 3-Hz pulses generated by a Grass pulse generator (Grass Instruments, Quincy, Mass). Maximal current delivered during each pulse was 0.75–1.0 mA. A boxcar pattern of forepaw stimulation was used (off/on/off/on/off for 11/10/10/10/10 image data points, respectively). The pulse generator was controlled by a PowerMac 8100/100 running PsyScope10 via a hardware interface (PsyScope Button Box; New Micros, Dallas, Tex). A monostable multivibrator (Texas Instruments) was used to shape the “scope trigger” output from the Integrated Pulse Generator (IPG) Module of the MR scanner. This allowed PsyScope to count each image in the fMRI acquisition as it was obtained and use this count to enable and disable the pulse generator at precise, predetermined times during the acquisition. We ensured that the forepaw stimulation pulses would not interfere with the MR measurements by using bipolar forepaw electrodes constructed from twisted-pair insulated copper wire, minimizing RF emissions.
MS-325 fMRI
All experiments were performed on a 1.5T GE Horizon EchoSpeed whole-body MR scanner in an RF-shielded room with the same 2.5-cm transmit-receive surface coil used for BOLD fMRI measurements. Each animal received an IV injection of MS-325 (0.1 mmol/kg) 1 minute before performing the fMRI measurement.
We used a 3D volume acquisition gradient-recalled echo (GRE) pulse sequence for all MS-325 functional imaging. Pulse sequence parameters were as follows: TE = 2.1 ms, TR = 8.5 ms, NEX = 1, flip angle = 25°, FOV = 12 × 12 cm, matrix = 128 × 128, and 1.1-mm section thickness. Each volume acquisition required approximately 10 seconds. Ten different section locations were imaged at each time point (ie, 10 sections in each volume), but 2 sections were discarded to yield a usable set of 8 images per volume, with 31 volumes acquired for each MS-325 fMRI measurement. Each fMRI measurement lasted approximately 320 seconds and generated 248 images.
A boxcar pattern of forepaw stimulation was used (off/on/off/on/off for 8/6/6/6/5 image sets, respectively). Other than timing of the boxcar function, forepaw stimulation parameters were identical to the parameters used in BOLD fMRI measurements.
Experimental Design
The test group consisted of 9 experiments in 9 different animals in which both BOLD fMRI and MS-325 fMRI measurements were performed. In each of these experiments, a BOLD fMRI measurement was first performed. After a rest period of approximately 15 minutes, the animal was given 0.1 mmol/kg MS-325, IV. One minute after MS-325 injection to allow for contrast agent distribution and intravascular equilibration, an MS-325 fMRI measurement was performed.
The control group consisted of 4 experiments in 4 different animals. These experiments were identical to those done in the test group except that an equivalent volume of normal saline was given instead of MS-325. This was done to control against the possibility that the imaging pulse sequence used for MS-325 fMRI was detecting activations through a mechanism unrelated to gadolinium enhancement.
Data Analysis
Preliminary analysis to qualitatively detect areas of functional activation in both BOLD and MS-325 fMRI measurements was performed during the experiment (FuncTools; GE, Milwaukee, Wis) on a UNIX-based workstation. Images were then extracted into DICOM format (xginx; GE, Milwaukee, Wis). Detailed quantitative analysis was then performed on the extracted images by using MEDx (Sensor Systems, Sterling, Va) running on a LINUX-based computer. Z scores were generated for each individual voxel by using an unpaired t test statistic referenced to a boxcar waveform matching the pattern of forepaw stimulation with a 4-second hemodynamic delay (BOLD data) or no delay (MS-325 data). Activation maps were generated by thresholding these Z score maps. Motion correction and linear detrending correction were not used for either the BOLD or MS-325 fMRI analysis. A uniform value of Z = 3 was used for BOLD activation maps. A uniform value of Z = 2.5 was used for MS-325 activation maps.
Definition of Terms
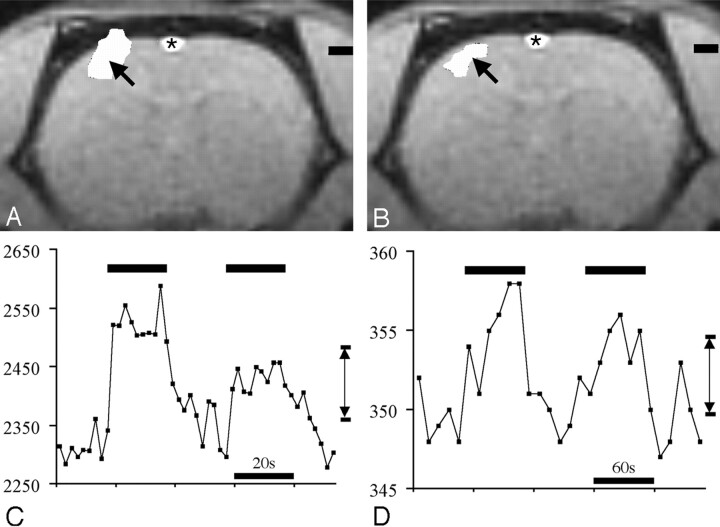
Using data from the most significantly activated voxel (ie, highest Z score) within the cluster of activated voxels, the activation amplitude was defined to be the difference between the average signal intensity in this voxel during forepaw stimulation and at rest (Fig 1; bottom row). The percentage of signal intensity change was defined to be the activation amplitude divided by the average signal intensity in the most significantly activated voxel times 100%. The activation volume was determined by counting the number of voxels in the cluster of activated voxels and multiplying by the voxel volume.
Fig 1.
BOLD fMRI (A) and MS-325 fMRI (B) response to forepaw stimulation. All data are from the same experiment in the same animal. BOLD fMRI was performed first. Then 0.1 mmol/kg MS-325 was administered intravenously and the MS-325 fMRI measurement was then performed. Top row, activation maps for BOLD fMRI (left) and MS-325 fMRI (right). Arrows indicate the voxel with the highest Z score in each measurement. Bottom row, time series data from the most active voxels (indicated by arrows in top row) for BOLD (left) and MS-325 (right) fMRI. Double arrow lines (C and D) indicate activation amplitude for BOLD and MS-325 data in the most significantly active voxel. Asterisk indicates flow-related enhancement in the superior sagittal sinus that is not related to activation.
Comparing Location of BOLD and MS-325 fMRI Activations
Animals were not moved once an experiment had begun. Location of the most significantly activated voxel in the BOLD and MS-325 fMRI measurements for each animal were determined as a Cartesian coordinate (x, y, z) relative to the landmark (ie, zero reference of the MR scanner) defined at the start of the experiment. For each animal, the difference between the location of the most significantly active voxel in the BOLD and MS-325 measurements was determined for each direction (ie, Δx = xBOLD − xMS-325, etc). For the 9 animals that underwent both BOLD and MS-325 fMRI measurements, a t test statistic was used to determine whether the difference in each direction was significantly different from zero.
Results
BOLD fMRI
As shown in Fig 1A, we observed a focus of BOLD activation in the expected location of the contralateral forepaw sensory cortex in all animals of this study, consistent with previously reported results for this preparation.9
MS-325 fMRI
In the test group in which both BOLD and MS-325 fMRI measurements were performed, all 9 animals demonstrated MS-325 fMRI activations in the expected location of the rat forepaw sensory cortex (Fig 1B).
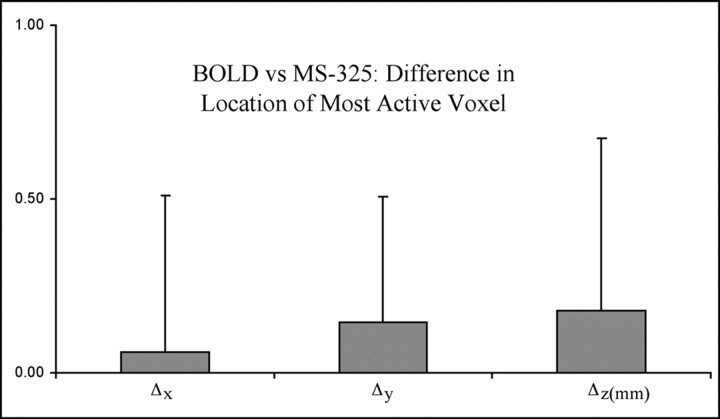
We found that the difference in the location of the most significantly activated voxel in BOLD and MS-325 fMRI measurements was not significantly different from zero (Fig 2). In addition, the difference was never more than 1 mm in any animal (ie, for each animal, the separation between the most active voxel in BOLD and MS-325 measurements never exceeded the spatial resolution of the measurements).
Fig 2.
Average distance (millimeters) between the most active voxel in BOLD fMRI measurements versus the location of the most active voxel in MS-325 fMRI in the same animal (n = 9). The distances (Δx, Δy, and Δz) between the most active voxels were determined for each animal. Data are displayed as mean millimeters ± SD.
Using the test group animals (n = 9), we looked at the percentage signal intensity change in the most significantly activated voxel for the set of BOLD experiments and compared this with the MS-325 experiments. The average percentage signal intensity change in the BOLD measurements was 2.9 ± 1.2%. In the same animals, the average percentage signal intensity change in the MS-325 measurements was 1.9 ± 0.9%. Using a 2-tailed, paired t test, the difference between BOLD activation amplitude and MS-325 fMRI activation amplitude was not significantly different (P = .10).
Using the test group animals, we determined the volume of activation for both BOLD and MS-325 fMRI by counting the number of activated voxels and multiplying by the voxel volume. We found the volumes to be 9.8 ± 8.6 and 3.2 ± 2.0 mm3 for BOLD and MS-325 fMRI, respectively. The volume of BOLD activation was significantly larger (P = .019, 2-tailed, paired t test).
Four control experiments were performed in which intravenous normal saline was administered instead of MS-325. In each of these experiments, BOLD activations were observed. However, no activations were observed when saline was substituted for MS-325.
Discussion
We have demonstrated a fMRI technique based on a gadolinium-based blood pool contrast agent, MS-325, with a long intravenous half-life. This technique has the advantage of using a T1-weighted pulse sequence, instead of a heavily T2*-weighted sequence, thus potentially reducing areas of susceptibility-related signal intensity loss observed with BOLD fMRI. We have shown that the locations of the most significantly active voxels, as determined by BOLD and MS-325 fMRI are not significantly different; therefore, the activation areas compare very favorably with both techniques in this experimental model.
MS-325 is an investigational, IV, extracellular gadolinium chelate currently undergoing phase III clinical trials for human use in MR angiography measurements. It reversibly binds to albumin (80%–90% bound to human serum albumin [HSA],11–13), prolonging intravascular half-life and minimizing extravasation into tissue interstitial spaces and resulting in higher relaxivity values. It is potentially useful as a T1-enhancing contrast agent for fMRI based on changes in regional blood volume with activation because of 2 major characteristics. Average effective half-life in humans is approximately 2 hours (versus 20 minutes for gadolinium-diethylene-triaminepentaacetic acid [Gd-DTPA]),11–13 which means that a relatively constant, usable blood concentration of the contrast agent will remain during the entire time currently required for a typical fMRI experiment, which generally requires between 1 and 1.5 hours. The second major characteristic of MS-325 is that it has a markedly increased relaxivity (48 [mmol/L]−1s−1) in human plasma, more than 10 times the relaxivity of standard extracellular gadolinium contrast agents such as Gd-DTPA (3.8 [mmol/L]−1s−1).14
Biochemical research has also shown that MS-325 is less tightly bound to rat serum albumin (RSA) compared with HSA (estimated binding to RSA, 45%–50%; to HSA, 80%–90%11), resulting in a shorter intravascular half-life, estimated to be approximately 30 minutes.11 Relaxivity in rat vascular space is not known, but is also believed to be significantly less than that in human blood. For these reasons, it was not known whether MS-325 would be effective in measuring changes in regional blood volume associated with functional brain activation in our rat model. However, we reasoned that, if feasible, it was likely to be more sensitive, per unit of injected contrast dose in a human subject, though this remains to be demonstrated. MS-325 is currently an investigational new drug undergoing phase III clinical trials in the United States, so testing our protocol in human subjects cannot currently be done without an approved protocol. Thus, we tested our hypothesis in a rat model. Based on our results, we anticipate that the technique should work in human fMRI experiments, perhaps with increased activation amplitude because of the higher relaxivity of MS-325 in humans as a result of a higher degree of serum protein binding as discussed above. The optimal T1 pulse sequence for use in human subjects also remains to be determined. Efficacy of this method remains to be tested in human subjects if MS-325 receives FDA approval for clinical use. Obviously, the major disadvantage of this technique is the addition of an intravenously injected contrast agent, which then makes this type of fMRI an invasive technique. However, if the activation amplitude increases and the signal intensity loss adjacent to the paranasal sinuses is minimized then, at least for selected types of experiments that require visualization of those areas, this may prove to be a useful alternative.
The optimal dosing of MS-325 for fMRI measurements has not been determined. Preclinical studies in nonhuman mammals11 report MS-325 doses ranging from 0.025–0.2 mmol/kg. Preclinical human studies report dosing in the 0.01–0.05 mmol/kg range.15
There are several important differences between the MS-325 and BOLD fMRI pulse sequences. The slightly smaller MS-325 fMRI voxel size (0.97 mm3 for MS-325, 1 mm3 for BOLD) minimally decreased the amplitude of MS-325 activations. Because we used a surface transmit/receive coil for both MS-325 and BOLD measurements, the primary noise and signal intensity sources were similar in both measurements and relatively insensitive to differences in FOV. The MS-325 measurement used a 128 × 128 matrix, whereas the BOLD measurement used a 40 × 70 matrix. This increased SNR in the MS-325 measurements by spreading noise over a greater number of voxels. Finally, the 3D GRE sequence used for MS-325 fMRI repeatedly stimulated the entire volume of interest (VOI), decreasing the available pool of resonating protons through partial saturation, decreasing relative SNR. The BOLD sequence used a single-shot GRE-EPI sequence that stimulated each section in the VOI once per image, maximizing the pool of resonating protons and SNR.
We found that the percentage signal intensity change for BOLD and MS-325 fMRI measurements were not significantly different in our preparation. The purpose of this study was to prove feasibility of an MS-325 based technique. Thus, the pulse sequence and MS-325 dose have not been extensively optimized. Further refinements of this technique may improve the percentage signal intensity change associated with MS-325 fMRI activation.
The activation volume was significantly larger for the BOLD fMRI measurements in our preparation by using our predetermined Z thresholds. However, making this comparison is problematic because the Z threshold for both BOLD and MS-325 could potentially be set to achieve almost any desired activated voxel count. In addition, the MS-325 imaging technique has not been extensively optimized. With further optimization, the MS-325 activation volumes may increase. Finally, the activation signals associated with functional imaging using long intravenous half-life agents, such as MS-325 and MION, are related to changes in relative cerebral blood volume and do not depend on changes in blood oxygenation.6 Thus, the decreased MS-325 activation volume could also represent decreased contribution from venules and draining cortical veins compared with BOLD fMRI. This possibility is supported by the observation that several of our BOLD measurements showed activations extending into the superior sagittal sinus. However, none of the MS-325 measurements showed this artifact. Finally, the 2 techniques use different pulse sequences with very different contrast weighting. The BOLD technique uses a T2* sequence with heavy susceptibility weighting that results in known “blooming” of activation signal intensity that extends beyond the capillary and venular vascular space. The MS-325 technique, on the other hand, uses a T1-weighted sequence that identifies activation signal intensity only in the vascular compartment, where there is enhancement from the blood pool contrast agent. This may result in more localized signal intensity changes that may partly account for the reduced voxel volume of activation and is illustrated by the extension of apparent “activation” signal intensity beyond the intracerebral tissue and into the skull in the BOLD image in Fig 1A.
Functional MR imaging measurements based on long intravenous half-life blood-pool agents can provide insights into the mechanisms underlying the control of cerebral blood flow and how cerebral blood flow changes in response to neural activation. The use of blood-pool agents has the advantage of not reflecting the susceptibility-weighted signal intensity changes associated with a hemoglobin oxygenation state that are believed to be the major source of contrast in BOLD fMRI.
Conclusions
We have demonstrated the feasibility of fMRI by using a gadolinium-based blood-pool agent, MS-325, with a long intravenous half-life. This technique produced strong activations in a rat model in response to forepaw stimulation. We found that the location of MS-325 fMRI activations was not significantly different from the location of activations measured by BOLD fMRI in the same animal and the magnitude of the fMRI response was similar for the 2 techniques. MS-325 fMRI does not require the use of highly T2*-weighted sequences, potentially reducing the susceptibility-related artifacts associated with BOLD fMRI techniques.
References
- 1.Hyder F, Kida I, Behar KL, et al. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed 2001;14:413–31 [DOI] [PubMed] [Google Scholar]
- 2.Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 2002;25:27–31 [DOI] [PubMed] [Google Scholar]
- 3.Arthurs OJ, Williams EJ, Carpenter TA, et al. Linear coupling between functional magnetic resonance imaging and evoked potential amplitude in human somatosensory cortex. Neuroscience 2000;101:803–06 [DOI] [PubMed] [Google Scholar]
- 4.Glover GH, Lemieux SK, Drangova M, et al. Decomposition of inflow and blood oxygen level-dependent (BOLD) effects with dual-echo spiral gradient-recalled echo (GRE) fMRI. Magn Reson Med 1996;35:299–308 [DOI] [PubMed] [Google Scholar]
- 5.Hoge RD, Atkinson J, Gill B, et al. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med 1999;42:849–63 [DOI] [PubMed] [Google Scholar]
- 6.Mandeville JB, Marota JJ, Kosofsky BE, et al. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med 1998;39:615–24 [DOI] [PubMed] [Google Scholar]
- 7.Morii S, Ngai AC, Winn HR. Reactivity of rat pial arterioles and venules to adenosine and carbon dioxide: with detailed description of the closed cranial window technique in rats. J Cereb Blood Flow Metab 1986;6:34–41 [DOI] [PubMed] [Google Scholar]
- 8.Hyder F, Behar KL, Martin MA, et al. Dynamic magnetic resonance imaging of the rat brain during forepaw stimulation. J Cereb Blood Flow Metab 1994;14:649–55 [DOI] [PubMed] [Google Scholar]
- 9.Morton DW, Maravilla KR, Meno JR, et al. Clinically relevant rat model for testing BOLD functional MR imaging techniques by using single-shot echo-planar imaging at 1.5 T. Radiology 2001;218:598–601 [DOI] [PubMed] [Google Scholar]
- 10.Macwhinney B, Cohen J, Provost J. The PsyScope experiment-building system. Spat Vis 1997;11:99–101 [DOI] [PubMed] [Google Scholar]
- 11.Parmelee DJ, Walovitch RC, Ouellet HS, et al. Preclinical evaluation of the pharmacokinetics, biodistribution, and elimination of MS-325, a blood pool agent for magnetic resonance imaging. Invest Radiol 1997;32:741–47 [DOI] [PubMed] [Google Scholar]
- 12.Caravan P, Cloutier NJ, Greenfield MT, et al. The interaction of MS-325 with human serum albumin and its effect on proton relaxation rates. J Am Chem Soc 2002;124:3152–62 [DOI] [PubMed] [Google Scholar]
- 13.Lauffer RB, Parmelee DJ, Dunham SU, et al. MS-325: albumin-targeted contrast agent for MR angiography. Radiology 1998;207:529–38 [DOI] [PubMed] [Google Scholar]
- 14.Lauffer RB, Parmalee DJ, Ouellet HS, et al. MS-325: a small-molecule vascular imaging agent for MR imaging. Acad Radiol 1996;3:S356–58 [DOI] [PubMed] [Google Scholar]
- 15.Bluemke DA, Stillman AE, Bis KG, et al. Carotid MR angiography: phase II study of safety and efficacy for MS-325. Radiology 2001;219:114–22 [DOI] [PubMed] [Google Scholar]