Abstract
BACKGROUND AND PURPOSE: MR imaging has played an increasingly important role in the diagnosis of Creutzfeldt-Jakob disease (CJD) since basal ganglia abnormalities on T2-weighted images have been described; thus, the aim of our study was to compare the value of different MR images in the diagnosis of CJD.
METHODS: One hundred fifty-seven patients with CJD underwent MR imaging examinations. Ninety-two patients were neuropathologically confirmed, and 65 were clinically classified as having CJD through the CJD Surveillance Unit (probability of 95%). There was no standardized MR imaging protocol; thus, the examinations included 143 T2-weighted, 43 proton attenuation (PD)-weighted, 84 fluid-attenuated inversion recovery (FLAIR), and 44 diffusion-weighted images (DWI). The MR images were reviewed for pathologic changes of the basal ganglia, thalamus, and cerebral cortex.
RESULTS: Cortical abnormalities were present in 70 patients (45%) and were visible in 80% (35/44) of all available DWI examinations. The basal ganglia were affected in 94 patients (60%), in particular in the caudate nucleus; the most sensitive sequences were DWI (64%) and PD-weighted (63%). A thalamic involvement was more frequently diagnosed on PD-weighted images (19%) and DWI (14%) than on FLAIR or T2-weighted images.
CONCLUSION: PD-weighted images and DWI showed better results in the diagnosis of signal intensity changes in the basal ganglia compared with T2-weighted or FLAIR images; however, in the diagnosis of cortical changes, DWI was clearly superior. Our data suggest that DWI is the most sensitive MR imaging technique in the diagnosis of CJD.
Creutzfeldt-Jakob-Disease (CJD), a fatal neurodegenerative disorder, is diagnosed by the detection of an accumulation of an abnormal form of the human prion protein PrPSc in the brain.1,2 Brain biopsy or autopsy is required for a definitive diagnosis (definite CJD).3 In sporadic CJD (sCJD), diagnostic MR examinations are performed frequently and reveal typical findings.4–7 Hyperintense signal-intensity abnormalities, initially reported on T2-weighted and proton attenuation (PD)-weighted images, were also detectable on fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted images (DWI): the signal intensity in the head of the caudate nucleus and in the putamen was compared with that in the thalamus and the cerebral cortex to distinguish normal gray matter intensity from pathologic hyperintensity. These changes have a reported sensitivity of 67% and a specificity of 93% for the diagnosis of CJD.5
The comparison of the MR imaging signals of the basal ganglia with those in the thalamus and the cortex has become more and more questionable because hyperintensities were also found in the thalamus and, in some cases, also in the cerebral cortex. With the application of other sequences, such as FLAIR and DWI, these signal intensity changes are more easily identifiable.8 DWI shows a decrease of the apparent diffusion coefficient (ADC) in the affected areas, most probably because of the characteristic neuropathologic spongiform neuropil changes.9–11
The purpose of our study was to identify the most sensitive MR imaging technique in determining signal intensity abnormalities in patients with sCJD.
Methods
All patients suspected of having CJD in Germany are reported to the Surveillance Unit (CJD-SU) in Goettingen. An experienced neurologist from the CJD-SU supports the on-site clinician, personally examines the patient on the spot, and collects copies of the electroencephalogram (EEG) and MR imaging examinations as well as specimens from CSF. Thus, MR imaging scans were collected from every relevant patient being reported to the CJD-SU.
We conducted a retrospective analysis of 199 consecutively referred patients with sCJD. At this time, no variant CJD (vCJD) case has been identified in Germany; our sample only consists of sCJD patients. The patients were classified as probable sCJD from 1999 to 2003.
The quality of the MR images was evaluated (1, excellent; 6, not diagnostic) before the scans were rated for pathologic signal intensity alterations on the different available sequences. Forty-two examinations were considered not diagnostic (eg, because of motion artifacts); a sufficient MR imaging quality was present in 157 scans (mean ± SD, 2.7 ± 1.1).
Because there was no standardized protocol, the examinations included 143 T2-weighted scans, 43 PD-weighted scans, 84 FLAIR scans, and 44 DWIs (Fig 1). The observations were documented separately for caudate nucleus (CN), putamen, thalamus, and cerebral cortex. The results were evaluated by using the χ2 test.
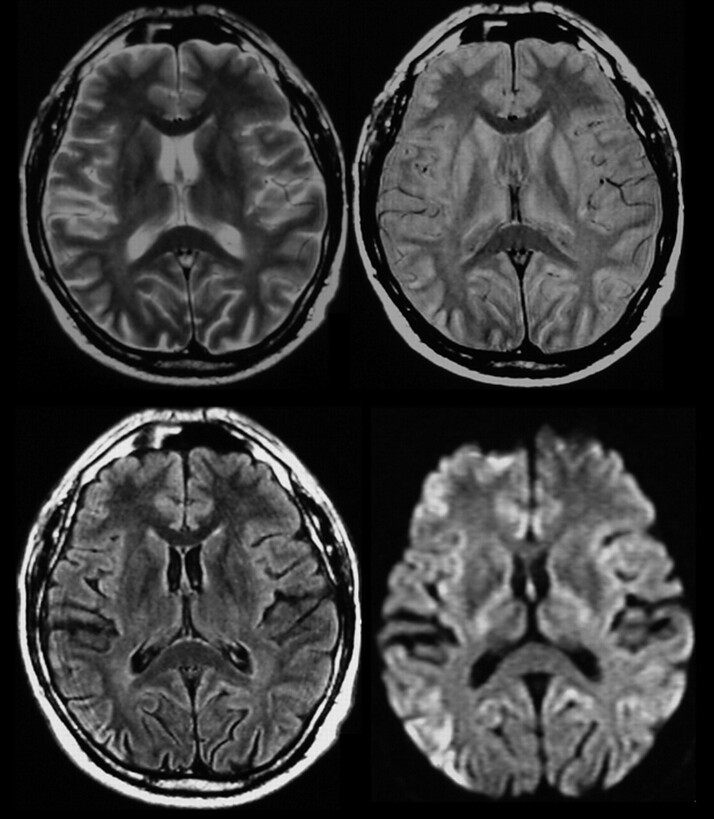
Fig 1.
T2-weighted, PD-weighted, FLAIR, and DWI images showing cortical abnormalities in the right parietal lobe; FLAIR and DWI also show abnormalities in the left hemisphere and the dorsal part of the cingulate gyrus.
Among the 157 patients, definite sCJD was proved by autopsy in 92 cases; in 65 patients, there was no consent for biopsy or autopsy by the patient or the patient’s family.
Results
In 112 (71%) of the 157 patients, typical signal intensity alterations were present on MR imaging (Fig. 2).
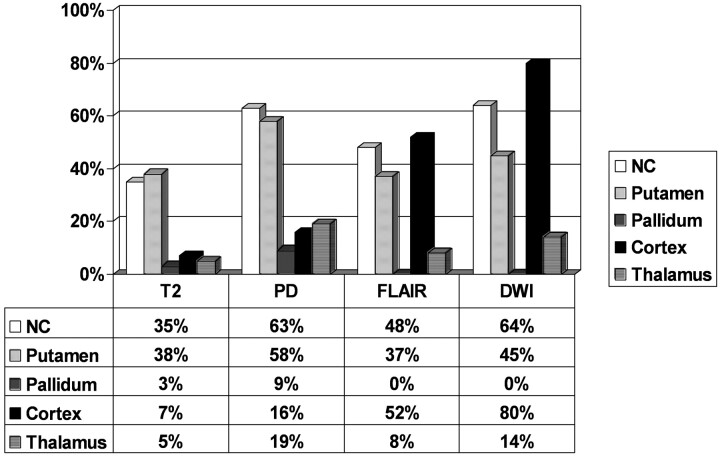
Fig 2.
Relative distribution of signal abnormalities of particular MR sequences in specified anatomical regions.
Cortex
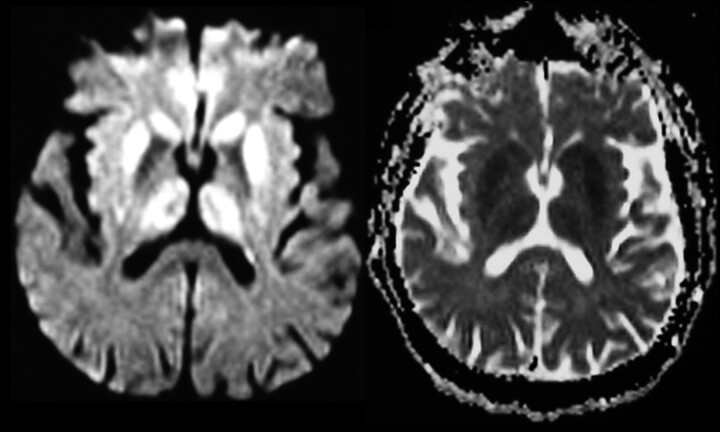
Abnormal signal intensity in the cortex (Fig 1) was found in 70 cases (45%). On FLAIR images, pathologic changes were diagnosed in 52% (44/84) and in 16% (7/43) on PD-weighted images. Findings on T2-weighted images were rated as abnormal in regard to cortical changes in only 6% (8/143). Cortical changes were most sensitively detectable on DWI: 80% (35/44) of the MR imaging examinations showed cortical areas with restricted water diffusibility, confirmed by ADC mapping (Fig 3).
Fig 3.
DWI and ADC showing decreased diffusivity in the basal ganglia and the thalamus as well as in the cortex.
In 17 cases, DWI was the only MR imaging technique showing the cortical pathology. Thus, in 39% of all patients whose examination included DWIs, the diagnosis was based exclusively on this technique.
Basal Ganglia
The basal ganglia (Fig 4) were involved in 94 patients (ie, 60% of all patients with CJD). In those cases with any abnormalities on MR imaging (112 patients), an abnormal signal intensity in the basal ganglia was found in 84%. The CN was affected most frequently (85 [90%] of the 94 patients with basal ganglia abnormalities). Putaminal involvement was found in 79 patients (84%), and 7 patients (7%) had an abnormal signal intensity in the pallidum. A singular pathology in just 1 of the nuclei was found in 24 cases (26%): 15 in the CN (16%) and 9 in the putamen (10%). A combination of CN involvement and putaminal involvement was detected in 63 patients (67%). An involvement of the pallidum was found only if both CN and putamen were affected as well.
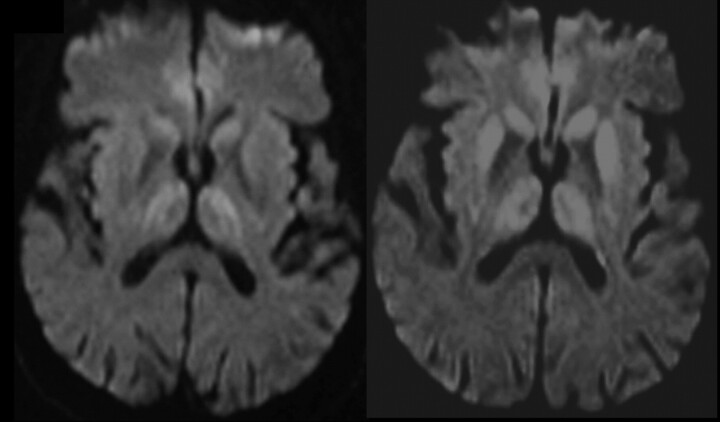
Fig 4.
Progression of decreased diffusivity in the basal ganglia (especially the putamen), thalamus, and cortex (time course, 3 months).
Fifty-four percent (85/157) of the acquired MR imaging examinations presented abnormal signal intensities in the CN, but there was a big difference regarding the MR techniques. PD-weighted imaging and DWI detected these alterations significantly more frequent than T2-weighted images (P < .01): 64% (28/44) on DWI and 63% (27/43) on PD-weighted imaging compared with only 35% (50/143) on T2-weighted imaging.
Thirty-five percent (78/157) of the MR imaging examinations showed an abnormal signal intensity in the putamen, which was visible on T2-weighted images in 38% (54/143), whereas hyperintensities were detected in 58% (25/43) on PD-weighted and in 37% (31/84) on FLAIR imaging. Findings of DWI were considered pathologic in 45% (20/44). Statistically, there was a significant difference between PD-weighted imaging on one side and T2-weighted and FLAIR imaging on the other side, indicating a higher sensitivity of PD-weighted imaging (P < .05). There was no significant difference between PD-weighted imaging and DWI.
Thalamus
Signal intensity abnormalities of the thalamus (Fig. 4) were relatively rare but still present in 21 cases (13% of all 157 patients with MR imaging scans). The abnormalities were more frequently detected on DWI (14%, 6/44) and on PD-weighted imaging (19%, 8/43) than on T2-weighted imaging (5%, 7/143; P < .05 and P < .01, respectively).
Discussion
The clinical diagnosis of sCJD is based upon the combination of rapidly progressive dementia, myoclonus, and multifocal neurologic dysfunction. Additionally, generalized periodic sharp wave complexes (PSWC) in the EEG12 or the detection of 14–3-3 proteins in the CSF amend the diagnostic accuracy up to 98% in cases of sCJD (probable sCJD).2,13,14 Patients with the clinical signs of sCJD but without the EEG and CSF abnormalities are clinically classified as possible sCJD according to World Health Organization (WHO) criteria.14
PSWC in the EEG4 and a positive 14–3-3 test (CSF)13,15 are part of the WHO criteria in the diagnosis of CJD, whereas MR imaging changes were, until now, included only in the clinical definition of vCJD. Patients with vCJD, most probably caused by the transmission of the bovine spongiform encephalopathy agent to humans,16 show bilateral increased signal intensity on MR imaging in the pulvinar nuclei of the thalamus (relative to the gray matter of other basal ganglia and the cerebral cortex), with a reported sensitivity of 78% and a specificity of 100%.17 This MR imaging finding serves as a necessary condition to classify a patient as probable vCJD.18
DWI has recently been described by several authors as being highly sensitive in CJD. Studies reported a sensitivity of up to 100%.19,20 In (together) 16 (n = 419 and n = 1220) patients, signal intensity alterations in the basal ganglia and the cortex were described. In some patients, there was also an involvement of the thalamus.11 The underlying pathomechanism explaining the decrease in isotropic water diffusibility indicated by DWI is not yet fully understood. One study by Mittal et al21 compared autopsy data with DWI findings and showed hyperintense signal-intensity changes in DWIs, corresponding to spongiform changes and nerve cell loss but not to astrocytic gliosis. In contrast, Haik et al22 reported that high signal intensity on DWIs correlated with the accumulation of the pathologic form of PrPSc in the brain but not with spongiform changes or gliosis. Our data confirm a high sensitivity of DWI in the diagnosis of CJD; however, we could not reproduce the reported excellent sensitivity. Because the MR images in our study were partially collected retrospectively, some images may represent very early stages of the disease and may account for a number of false-negative cases, but even some of the patients with autopsy-proved sCJD and long-standing history did not (or no longer) show abnormal signal intensities on MR imaging.
An involvement of the thalamus on MR images is considered a diagnostic key feature of vCJD. Some authors suggest a sensitivity of 78% and a specificity of 100%.17 Few published reports address signal intensity changes in the thalamus of patients with sCJD.6,19 In contrast, neuropathology almost always shows the thalamus to be involved in sCJD. A recent study by Tschampa et al11 confirmed thalamic involvement in patients with sCJD on the basis of quantitative analyses of diffusion changes. Hyperintensities of the thalamus are a diagnostic key feature in vCJD but may also be visible in the brains of patients with sCJD. Thus, hyperintensity is not considered a specific sign for vCJD in general. In line with the current literature, none of our patients with sCJD presented with an isolated thalamic pathology on MR imaging, and generally signal intensity changes in the thalamus were of an intensity similar to that of other affected brain regions.
Kovanen et al23 described gray matter involvement in early stages of CJD recognized on CT or MR imaging scans and a progressive atrophy of cortical structures. Whereas several instances of patients with CJD with cortical hyperintensities have been reported, to our knowledge, no analysis of the frequency of cortical involvement exists. The comprehensiveness of this phenomenon seems to be very important because the present state-of-the-art evaluation of the signal intensity of the basal ganglia is performed by comparing it to that of the cortex. However, in our opinion, the current rule of thumb of comparing signal intensities of cortex and basal ganglia when diagnosing CJD, with basal ganglia being classified as pathologic only when exhibiting a signal intensity higher than that of the cortex, needs reevaluation. Because cortical involvement is common in sCJD—detected in up to 80% in our study, depending on the MR imaging technique—comparing the signal intensity of the basal ganglia to that of the normal cortical gray matter on PD-weighted, T2-weighted, and FLAIR images is a real challenge because the cortical signal intensity may be misinterpreted. The pathologies are more easily depicted on DWI because of the better contrast between areas with normal and restricted water diffusibility.
Our data emphasize that this comparison has to be done extremely carefully because cortical involvement seems to be more frequent than hyperintensities of the basal ganglia—a hypothesis supported by the results from DWI. This phenomenon has been underestimated in the past, before MR imaging techniques like FLAIR and DWI were used. The advantage of FLAIR and DWI, compared with T2-weighted and PD-weighted imaging, in evaluating the cortex can easily be explained by a partial volume effect on T2-weighted and PD-weighted imaging and by the contrast between gray matter and CSF in these sequences. The signal intensity of involved cortex is high on all sequences (except ADC), and only on FLAIR and DWI is the adjacent CSF dark. In addition, DWI is a very fast sequence if performed using the echo-planar technique, reducing motion artifacts in restless patients.
Conclusion
Typical signal intensity abnormalities in the brain were detected most frequently with DWI, particularly regarding the cerebral cortex, compared with PD-weighted, FLAIR, and T2-weighted imaging (P < .01). DWI is a fast technique and, therefore, considered to be robust, whereas conventional sequences carry a higher risk of motion artifacts. DWI should be part of the MR imaging protocol in the examination of patients with dementia, especially if young patients are involved and/or the symptoms progress rapidly.
In addition to the protein 14-3-3 and EEG changes, MR imaging may be a helpful paraclinical tool and should be incorporated in the WHO diagnostic criteria for sCJD.
References
- 1.Prusiner SB. Prions. Proc Natl Acad Sci U S A 1998;95:13363–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters CL, Harris JO, Gajdusek DC, et al. Creutzfeldt-Jakob disease: patterns of worldwide occurrence and the significance of familial and sporadic clustering. Ann Neurol 1979;5:177–88 [DOI] [PubMed] [Google Scholar]
- 3.Kretzschmar HA, Ironside JW, DeArmond SJ, et al. Diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Arch Neurol 1996;53:913–20 [DOI] [PubMed] [Google Scholar]
- 4.Finkenstaedt M, Szudra A, Zerr I, et al. MR imaging of Creutzfeldt-Jakob disease. Radiology 1996;199:793–98 [DOI] [PubMed] [Google Scholar]
- 5.Schroter A, Zerr I, Henkel K, et al. Magnetic resonance imaging in the clinical diagnosis of Creutzfeldt-Jakob disease. Arch Neurol 2000;57:1751–57 [DOI] [PubMed] [Google Scholar]
- 6.Urbach H, Paus S, Tschampa HJ, et al. Creutzfeldt-Jakob disease: value of MRI [in German]. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 2001;173:509–14. Erratum in 2001;173:847 [DOI] [PubMed] [Google Scholar]
- 7.Urbach H, Klisch J, Wolf HK, et al. MRI in sporadic Creutzfeldt-Jakob disease: correlation with clinical and neuropathological data. Neuroradiology 1998;40:65–70 [DOI] [PubMed] [Google Scholar]
- 8.Kropp S, Finkenstaedt M, Zerr I, et al. Diffusion-weighted MRI in patients with Creutzfeldt-Jakob disease. Nervenarzt 2000;71(2):91–95 [DOI] [PubMed] [Google Scholar]
- 9.Bahn MM, Kido DK, Lin W, et al. Brain magnetic resonance diffusion abnormalities in Creutzfeldt-Jakob disease. Arch Neurol 1997;54:1411–15 [DOI] [PubMed] [Google Scholar]
- 10.Sellars RJ, Collie DA, Will RJ. Progress in understanding Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2002;23:1070–72 [PMC free article] [PubMed] [Google Scholar]
- 11.Tschampa HJ, Murtz P, Flacke S, et al. Thalamic involvement in sporadic Creutzfeldt-Jakob disease: a diffusion-weighted MR imaging study. AJNR Am J Neuroradiol. 2003;24:908–15 [PMC free article] [PubMed] [Google Scholar]
- 12.Steinhoff BJ, Racker S, Herrendorf G, et al. Accuracy and reliability of periodic sharp wave complexes in Creutzfeldt-Jakob disease. Arch Neurol 1996;53:162–66 [DOI] [PubMed] [Google Scholar]
- 13.Zerr I, Bodemer M, Gefeller O, et al. Detection of 14–3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease. Ann Neurol 1998;43:32–40 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Human transmissible spongiform encephalopathies. Wkly Epidemiol Rec 1998;73:361–65 [PubMed] [Google Scholar]
- 15.Zerr I, Pocchiari M, Collins S, et al. Analysis of EEG and CSF 14–3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology 2000;55:811–85 [DOI] [PubMed] [Google Scholar]
- 16.Bruce ME, Will RG, Ironside JW, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 1997;389:498–501 [DOI] [PubMed] [Google Scholar]
- 17.Zeidler M, Sellar RJ, Collie DA, et al. The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt-Jakob disease. Lancet 2000;355:1412–18 [DOI] [PubMed] [Google Scholar]
- 18.Will RG, Zeidler M, Stewart GE, et al. Diagnosis of new variant Creutzfeldt-Jakob disease. Ann Neurol 2000;47:575–82 [PubMed] [Google Scholar]
- 19.Bahn MM, Parchi P. Abnormal diffusion-weighted magnetic resonance images in Creutzfeldt-Jakob disease. Arch Neurol 1999;56:577–83 [DOI] [PubMed] [Google Scholar]
- 20.Demaerel P, Sciot R, Robberecht W, et al. Accuracy of diffusion-weighted MR imaging in the diagnosis of sporadic Creutzfeldt-Jakob disease. J Neurol 2003;250:222–25 [DOI] [PubMed] [Google Scholar]
- 21.Mittal S, Farmer P, Kalina P, et al. Correlation of diffusion-weighted magnetic resonance imaging with neuropathology in Creutzfeldt-Jakob disease. Arch Neurol 2002;59:128–34 [DOI] [PubMed] [Google Scholar]
- 22.Haik S, Dormont D, Faucheux BA, et al. Prion protein deposits match magnetic resonance imaging signal abnormalities in Creutzfeldt-Jakob disease. Ann Neurol 2002;51:797–99 [DOI] [PubMed] [Google Scholar]
- 23.Kovanen J, Erkinjuntti T, Iivanainen M, et al. Cerebral MR and CT imaging in Creutzfeldt-Jakob disease. J Comput Assist Tomogr 1985;9:125–28 [DOI] [PubMed] [Google Scholar]